Abstract
Purpose
To determine the impact of chemotherapy or external beam radiotherapy (EBRT) on pediatric anophthalmic sockets.
Design
A retrospective, nonrandomized, interventional cohort study.
Participants
A total of 135 sockets of 133 children undergoing enucleation from late 1999 to early 2009 at the St. Jude Children’s Research Hospital were included.
Methods
A retrospective chart review of outcomes after enucleation in patients treated with systemic chemotherapy or orbital EBRT either before or after removal of the eye compared with patients who received no other treatment.
Main Outcome Measures
Incidence of implant exposure, migration, extrusion, socket contracture, and pyogenic granuloma formation.
Results
Retinoblastoma was the primary diagnosis in 128 eyes (95%). Median follow-up was 3.6 years (range, 0.1–9.3 years). Event-free course was observed in 94 sockets (69.6%). Complications included implant exposure (n = 28, 20.7%), socket contracture (n = 16, 11.9%), pyogenic granuloma (n = 9, 6.7%), implant extrusion (n = 3, 2.2%), and migration (n = 2, 1.5%). Exposure resolved in 21 sockets (77.8%) and improved in 2 sockets (11.1%); 1 patient with exposure died. Use of prior, adjuvant, or subsequent chemotherapy increased the long-term risk of exposure (odds ratio [OR] = 3.7; 95% confidence interval [CI], 1.4–9.4), and contracture (OR could not be calculated, P<0.0001). External beam radiotherapy greatly increased the risk of contracture (OR 24.0; 95% CI, 6.9–82.8) and exposure (OR 2.89; 95% CI, 1.1–7.9).
Conclusions
In this unique pediatric population with cancer, chemotherapy and EBRT had an additive effect, significantly increasing the incidence of exposure and socket contracture.
Pediatric anophthalmic sockets have a higher incidence of complications than adult anophthalmic sockets.1–3 Traumatic injury or terminal blind painful eye remains the most likely indication for enucleation in adults.4,5 Retinoblastoma remains a major diagnosis leading to removal of an eye in a child,1,6 despite advances in eye-sparing therapies, such as chemoreduction,7 plaque brachytherapy,8,9 and, recently, super-selective intra-arterial chemotherapy.10
Enucleation remains the definitive therapy for children with advanced unilateral retinoblastoma.11 In the absence of high-risk histopathologic features, enucleation is curative without additional therapies. Otherwise, adjuvant chemotherapy or external beam radiotherapy (EBRT) may be required. Secondary enucleation may be needed for eyes with retinoblastoma that fail conservative cancer therapies.
The effect of antecedent or subsequent retinoblastoma therapies and particularly of chemotherapy on an anophthalmic socket has been examined in only a limited number of patients.2,12,13 This study reviews all enucleations performed over the past 10 years at St. Jude Children’s Research Hospital, assessing the impact of radiation and chemotherapy on anophthalmic sockets with hydroxyapatite (HA) implants.
Materials and Methods
This was a single-institution, retrospective interventional study approved by the institutional review board of St. Jude Children’s Research Hospital with all research adhering to the tenets of the Declaration of Helsinki. A patient database compliant with the Health Information Portability and Accountability Act was queried for a list of consecutive patients undergoing primary or secondary enucleation between November 1999 and February 2009. Patients with a follow-up period less than 30 days, those enucleated before arrival at the hospital, and those receiving no orbital implant were excluded. The data extracted from the medical records included gender, race, diagnosis, eye involved, laterality of the disease, age at the time of enucleation, time to enucleation, implant characteristics, history and timing of chemotherapy, and EBRT. The type and timing of various complications and presence of an orbital implant at the last follow-up were recorded. Event-free course was defined as absence of exposure, socket contracture, pyogenic granuloma formation, implant migration, or extrusion.
For the purposes of statistical analyses, sockets were treated as independent variables. Descriptive statistics were used to assess the baseline patient and eye characteristics, implant features, and incidence of complications. Chi-square analyses with Pearson coefficients were used to compare the incidence of complications in sockets receiving various types of retinoblastoma therapies. Analysis of variance and individual t tests were performed comparing outcomes in the treatment groups. Product-limit analysis (Kaplan–Meier survival curve) was used to compare the incidence of exposure among treatment groups. Multivariate models were built by using stepwise introduction of variables with the probability of 0.15 required to enter the model. Nominal logistic fit was then performed identifying the contributing variables with P<0.05. The mean of quantitative variables is reported with the standard deviation. Two-tailed P values are reported where appropriate, with P<0.05 considered statistically significant. Odds ratios (ORs) are reported with 95% confidence intervals (CIs). Statistical analyses were performed with JMP (v. 7.0 for Windows, SAS Inc., Cary, NC).
Literature Review
By searching both PubMed and Embase, references containing the terms “enucleation” and “implant” were selected without any limit on date or language of publication. Citations containing in their title, abstract, or keywords the terms “child,” “pediatric,” or “retinoblastoma” were then reviewed. All articles reporting the outcomes in at least 15 pediatric sockets or children with retinoblastoma are presented. Articles in French and Polish were reviewed with speakers of respective languages.
Surgical Technique
All cases underwent enucleation using a standard approach. Lateral canthotomy was performed in young children when safe removal of an eye would be compromised by a narrow palpebral fissure. A 360-degree conjunctival peritomy was performed around the corneal limbus. The 4 recti muscles were cautiously isolated, placed on a double-locking 5-0 Vicryl suture, and disinserted. Inferior and superior oblique muscles were disinserted from the globe. After gentle outward rotation of the globe, the optic nerve was carefully cut using curved Metzenbaum scissors to obtain a longer section of the nerve. The globe was removed, and fresh tumor tissue was extracted. Hemostasis was maintained with digital pressure while polymer-coated hydroxyapatite (PC-HA) or wrapped hydroxyapatite (W-HA) orbital implants were prepared. Once the implant was inserted into the orbital space, the recti muscles were attached to the anterior surface 3 to 4 mm behind the anticipated anterior pole of the sphere. A 3-layered closure was performed using 5-0 Vicryl suture on a spatulated needle to close deep Tenon’s fascia, superficial Tenon’s fascia, and conjunctiva. A medium conformer was placed, followed by a temporary tarsorrhaphy. Finally, a pressure patch dressing was applied. All patients received intraoperative intravenous and postoperative oral antibiotics, and were seen in follow-up the next day. Ocular prosthesis fitting was performed 8 weeks subsequent to enucleation in sockets free of active complications. Patients with retinoblastoma were examined under general anesthesia at frequent intervals until the age of 5 years; thereafter, they were examined in the clinic.
Results
During the study interval, 140 eyes of 135 patients were enucleated by 2 surgeons (M.W.W. and B.G.H.) using a similar technique. Sufficient follow-up for inclusion into the study was available on 135 orbits of 133 patients, whose baseline demographic features are summarized in Table 1. Briefly, the median age at surgery was 2.2 years (range, 0.17–9 years). Retinoblastoma was the reason for enucleation in 128 eyes (95.0%), medulloepithelioma in 3 eyes (2.2%), persistent fetal vasculature in 2 eyes (1.4%), and retinal dysplasia in 1 eye (0.7%). Enucleation was performed a median of 5 days after the diagnosis (range, 0 days to 7.7 years). Implant size was 20 mm in 92 sockets (68.1%) and 18 mm in 43 sockets (32.9%). Polymer-coated hydroxyapatite was placed in 85 orbits (63.0%) and W-HA was placed in 50 orbits (37.0%), including 31 with dermal allograft, 16 with polyglactin mesh, and 3 with retroauricular muscle. Median follow-up was 3.6 years (range, 0.1–9.3 years).
Table 1.
Baseline Patient Demographics and Follow-up Period
| Demographic | No. (% of Total)* |
|---|---|
| Eyes included | 135 eyes of 133 patients |
| Left: 64 (47.4) | |
| Right: 73 (54.1) | |
| Diagnosis, eyes | Retinoblastoma 128 (94.8) |
| Medulloepithelioma 3 (2.2) | |
| Other (non-trauma) 3 (2.2) | |
| Disease process, patients | Unilateral 91 (68.4) |
| Bilateral 42 (31.6) | |
| Age at enucleation, yrs | Median 2.2, range 0.2–9 |
| Mean 2.5±1.8 | |
| Race, patients | Caucasian: 75 (56.4) |
| African American: 39 (29.3) | |
| Hispanic: 15 (11.3) | |
| Gender, patients | Male 71 (53.4) |
| Female 62 (46.6) | |
| Time, diagnosis to enucleation | Median: 5 days, range 0–7.7 yrs |
| Mean: 0.5±1.0 yrs | |
| Follow-up | Median: 3.6, range 0.1–9.3 yrs |
| Mean: 3.9±2.4 yrs |
Unless otherwise indicated.
Event-free course was observed in 94 sockets (69.6%). Observed complications included implant exposure (n = 28, 20.7%), socket contracture (n = 16, 11.9%), purulent socket infection (n = 8, 5.9%), pyogenic granuloma formation (n = 9, 6.7%), implant migration (n = 1, 0.7%), and extrusion (n = 3, 2.2%). Six of 8 cases of infection were associated with exposure. Infection preceded exposure in 3 of the 8 cases. At the last follow-up, 133 sockets (99%) maintained a deep implant, exposure resolved in 21 sockets (77.8%) and improved in 2 sockets (11.1%), and 1 patient died of metastatic retinoblastoma before any repair.
Effect of Chemotherapy and External Beam Irradiation
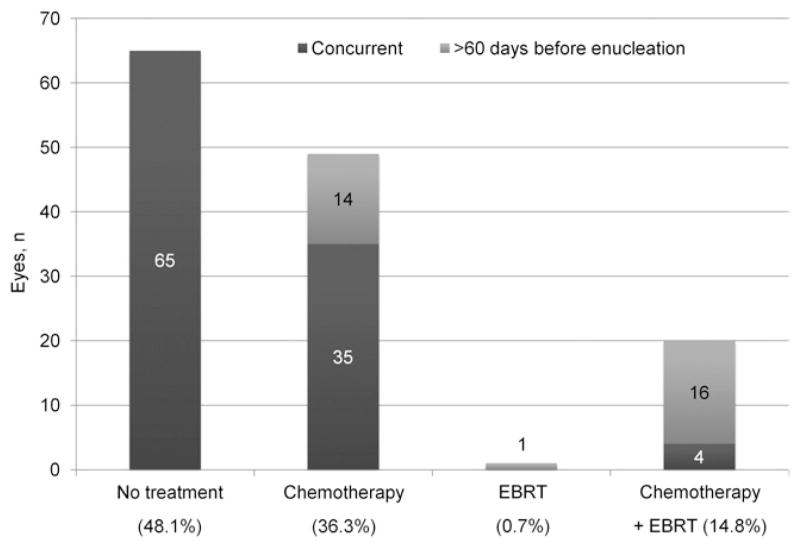
Seventy sockets (51.9%) were exposed to additional therapy before, during, or after enucleation. Chemotherapy was the only additional treatment in 49 sockets (36.3%), whereas 20 sockets (14.8%) were exposed to both chemotherapy and EBRT, and 1 socket received EBRT alone (Fig 1).
Figure 1.
Distribution and timing of additional therapies. Percent is calculated on the basis of the total of 135 sockets. EBRT = external beam radiotherapy.
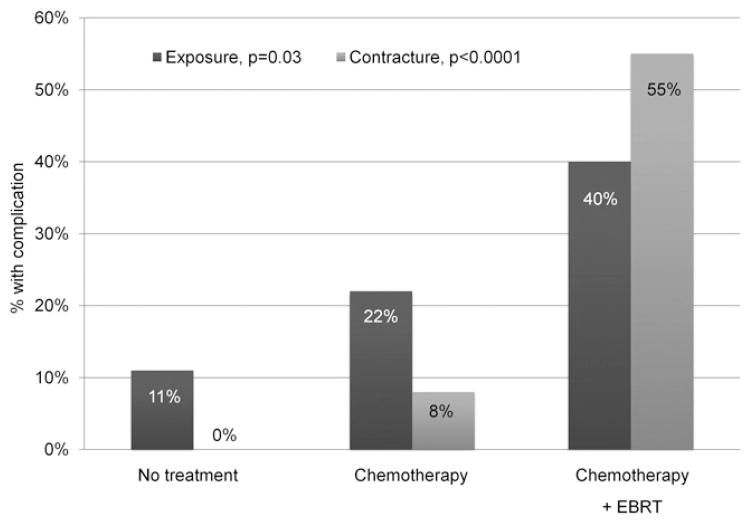
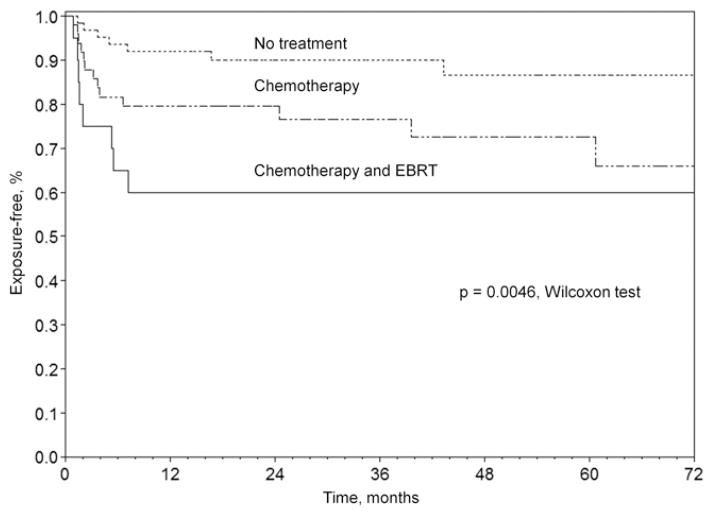
Table 2 summarizes the characteristics of the eyes in each treatment group and the outcomes observed. With greater therapy load, the number of sockets with event-free course decreased from 57 (87.7%) in primarily enucleated eyes to 30 (61.2%) in sockets exposed to chemotherapy and to 6 (30%) in sockets receiving both chemotherapy and EBRT (P<0.0001). In univariate analyses, chemotherapy increased the risk of exposure (OR = 3.7; 95% CI, 1.4–9.4) and contracture (OR cannot be calculated, P<0.0001) with a trend for increased socket infections (OR = 7.3; 95% CI, 0.9–61.4). External beam radiotherapy greatly increased the risk of contracture (OR 24.0; 95% CI, 6.94–82.8) and exposure (OR 2.89; 95% CI, 1.1–7.9). Incidence of exposure increased from 7 (10.8%) in the treatment-free group to 13 (26.5%) in the chemotherapy group and to 8 (40%) in the group receiving a combination of EBRT and chemotherapy (P = 0.009). The combination of chemotherapy and EBRT had a similar additive effect on the probability of developing contracture (P<0.0001) (Fig 2). Furthermore, by product-limit analysis, the initial rate of exposure development increased with a greater treatment load (P<0.005) (Fig 3).
Table 2.
Patient Demographics and Incidence of Complications in the Three Treatment Groups
| Primary n (%)* | Chemotherapy n (%)* | Chemotherapy and EBRT n (%)* | P Value | |
|---|---|---|---|---|
| Eyes | 65 (48.1) | 49 (36.3) | 20 (14.8) | |
| Patients | 65 (48.9) | 49 (36.8) | 19 (14.3) | |
| Age at enucleation,† yrs | 2.3 (0.3–9), 2.7±1.8 | 1.7 (0.2–9), 1.9±1.5 | 2.6 (1.4–8.3), 3.1±1.6 | 0.02 |
| Time, diagnosis to enucleation,† mos | 0.2 (0–0.9), 0.13±0.12 | 3.4 (0–34.3), 5.7±7.8 | 20.5 (0–125.6), 24.3±21.7 | <0.0001 |
| 20-mm implant | 49 (75.4) | 35 (71.4) | 7 (35) | 0.003 |
| PC-HA | 47 (72.3) | 30 (61.2) | 8 (40) | 0.03 |
| Event-free course | 57 (87.7) | 30 (61.2) | 6 (30) | <0.0001 |
| Exposure | 7 (10.8) | 13 (26.5) | 8 (40.0) | 0.009 |
| Maximum size,† mm | 5.0 (1–11), 5.9±4.0 | 6 (1–14), 6.8±4.2 | 8 (2–15), 7.6±4.4 | 0.73 |
| Time to exposure,† mos | 5.0 (1.4–43.3), 11.3±15.0 | 3.2 (0.9–60.7), 11.7±18.6 | 1.8 (0.9–7.2), 3.2±2.4 | 0.42 |
| Contracture | 0 (0) | 5 (10.2) | 11 (55.0) | <0.0001 |
| Extrusion | 1 (1.5) | 2 (4.1) | 0 (0) | 0.51 |
| Migration | 0 (0) | 1 (2.0) | 0 (0) | 0.42 |
| Pyogenic granuloma | 0 (0) | 8 (16.3) | 1 (5.0) | 0.003 |
| Further surgeries | 6 (9.2) | 11 (22.5) | 8 (40.0) | 0.006 |
| Exposure resolved | 5 (71.4) | 9 (81.8) | 6 (75.0) | 0.87 |
EBRT = external beam radiotherapy; PC-HA = polymer-coated hydroxyapatite.
Significant differences indicated by bolded P values. External beam radiotherapy alone is not included in the table because only 1 eye was in that group.
Unless otherwise indicated.
Reported as median (range), mean ± standard deviation.
Figure 2.
Incidence of exposure and socket contracture in different treatment groups. Percent is calculated for each treatment group. EBRT = external beam radiotherapy.
Figure 3.
Product-limit test of exposure by treatment group, Wilcoxon test, P = 0.0046. EBRT = external beam radiotherapy.
In addition to retinoblastoma therapy, other possible factors relating to implant exposure and contracture were explored. Smaller 18-mm implants were associated with increased risk of contracture (OR 4.3; 95% CI, 1.46–12.9), whereas the use of a larger 20-mm sphere did not increase the exposure rate (OR 1.2; 95% CI, 0.5–3.0). Postoperative purulent socket infection correlated significantly with subsequent exposure (OR 7.2; 95% CI, 1.1–45.4) but not contracture (P > 0.5). History of secondary surgery, excluding fornix-deepening procedures, was associated with an increased risk of subsequent socket contracture (OR 6.79; 95% CI, 1.82–25.4). No significant effect on the rate of exposure or contracture was observed according to race, eye involved, gender, age at enucleation, and the coating or wrapping of the implant (P > 0.07).
Subgroup analyses were performed on 69 sockets receiving chemotherapy. Of these, chemoreduction failed to control the disease in 32 eyes leading to secondary enucleation. For the remaining 37 sockets, adjuvant chemotherapy was started within 30 days of enucleation for high-risk histopathology or bilateral involvement. Event-free recovery occurred at the same rate regardless of the timing of chemotherapy (OR 0.85; 95% CI, 0.3–2.2). Contracture occurred more frequently in sockets treated with prior chemoreduction (OR 3.4; 95% CI, 1.02–11.0). This effect of prior chemotherapy persisted even after exclusion of sockets additionally treated by EBRT. The risk of exposure, however, tended to increase in sockets receiving secondary adjuvant chemotherapy versus those treated with primary chemoreduction (OR 1.6; 95% CI, 0.6–4.6), although not reaching statistical significance.
Effects of EBRT timing were examined in the subgroup of 21 sockets treated with radiation. The likelihood of event-free course was similar between orbits receiving EBRT before and after enucleation (OR 1; 95% CI, 0.1–13.4). Although not reaching statistical significance, socket contracture was more likely when EBRT preceded enucleation (OR 2.5; 95% CI, 0.2–32.8), and implant exposure was more likely when radiation followed eye removal (OR 4; 95% CI, 0.3–53.5). Neither implant migration nor extrusion was observed in irradiated sockets.
Multivariate logistic fit analysis identified both the history of chemotherapy (regardless of timing) and radiation therapy as significant predictors of event-free course and contracture (P<0.03 for each variable). Implant size, however, did not remain in the model. By analyzing exposure, while chemotherapy remained in the model (P<0.02), EBRT had only borderline significance (P<0.07).
Management of Complications
At least 1 of the aforementioned complications occurred in 41 (30.4%) of the 135 sockets. Sixteen orbits (39%), including 7 with small exposures, required no further surgical intervention. Twenty-five sockets required at least 1 additional surgery, including treatment of exposure in 18 sockets, management of contracture with fornix deepening sutures in 5 sockets, and removal of pyogenic granuloma in 2 sockets.
Implant exchange was required in 6 sockets, including 1 with a primary extrusion, 2 with extrusion after implant exposure, and 3 with severe exposure but no extrusion. A median of 1 additional implant was attempted (mean 1.3±1, range 0–3). Two of 6 sockets received secondary 18-mm PC-HA, which subsequently had to be replaced with 18-mm silicone spheres because of repeat exposure. Two sockets received 16-mm silicone spheres as the initial secondary implant, and the remaining 2 sockets could not maintain a secondary implant because of an ongoing infection.
Seventy-one additional surgeries were required in 25 sockets, a median of 3 surgeries per socket (mean 2.9±1.6, range 1–6) at a median of 1.9 months after enucleation (mean 3.9±3.9, range 0–15.2). The number of subsequent surgeries was similar for sockets treated solely by enucleation and those exposed to additional treatments (3.2±1.8 vs. 2.8±1.5, P = 0.66).
Discussion
This is the largest study of cancer therapies and orbital implants in a pediatric population reported to date. Table 3 (available at http://aaojournal.org) summarizes the reported outcomes of pediatric orbital implants with particular focus on retinoblastoma, chemotherapy, and EBRT drawing on published reports that included at least 15 pediatric sockets.1,2,4,6,13–24 Our cohort, composed predominantly of patients with retinoblastoma, represents one of the few series in which the majority of children received additional cancer treatments.2,20 Event-free course, defined by absence of exposure, socket contracture, implant migration, implant extrusion, and pyogenic granuloma formation, was seen in 69% of sockets. Implant exposure of any size was the most common complication, affecting >20% of sockets. At the final follow-up, 99% of sockets had an implant in place.
Several reports have looked at outcomes of orbital implants in patients with retinoblastoma.4,15,16,18,19,21–24 However, only a few have examined specifically the effect of chemotherapy and radiation on rates of exposure2,13,14,20 and socket contracture14 with various implant types. Higher exposure rates are reported with a greater proportion of sockets receiving chemotherapy (22%–50%)2,13,19,22 and radiation (15%–35%)2,14,16 compared with studies with few sockets receiving additional therapies (0%–6.1%).6,15,17,24 Previous reports often failed to reach statistical significance, perhaps because of a small number of patients treated with chemotherapy or EBRT.6,14,15 Many of these studies were limited by the use of multiple types of implants and wrappings, making it difficult to separate the impact of the implant type from the effect of therapy.
In our study using only HA implants, more than one half of anophthalmic sockets received cancer therapies preceding, concurrent with, or after the enucleation. More than one third were treated with chemotherapy alone, and approximately 15% received combined radiation and chemotherapy. The size of the exposure and the time to the onset of exposure did not differ between treatment modalities. Chemotherapy and EBRT had a significant additive effect on the incidence of exposure. Compared with untreated sockets, exposure increased more than 2.5-fold to 26.5% in sockets exposed to chemotherapy alone and approximately 4-fold to 40% in sockets receiving chemotherapy and EBRT.
Chemotherapy and radiation may delay tissue healing and implant biointegration. The fact that the time to exposure was similar among the treatment groups (median 3.4 months) suggests that other factors also play a role. Magnetic resonance imaging of HA implants demonstrates a “cold” zone of failed fibrovascular ingrowth anteriorly for up to 2 months.25,26 Several other studies observed similar timing of 3.8 to 4 months.2,15 The resorption of polymer necessary for the biointegration of an implant exposes the abrasive anterior face of HA to the overlying tissues. Exposure may ensue if overlying tissues are compromised by socket irritation with prosthesis, seasonal allergies, or inflammatory reaction with associated upper respiratory infection. Periorbital cellulitis may be associated with upper respiratory infections in up to 86% of cases.27,28 In fact, postoperative purulent socket infections significantly increased the rate of exposure in our study. Sinusitis may complicate up to 8% of upper respiratory tract infections,29 leading to orbital cellulitis.28 Allergic sinusitis may further increase the rate of orbital involvement.30 The majority of our patients reside in the southern United States, where pollen season is significantly longer,31 and upper respiratory infections occur up to 5 times per year in a healthy child between 6 months and 3 years.32 Geographic patterns of referral with resulting environmental differences may explain, in part, the slightly higher rate of exposure observed in this study compared with that seen in coastal states.6
Soft tissue contracture of the socket, including forniceal shortening, was solely seen in sockets exposed to additional cancer therapies. Radiation was the most important factor in development of contracture, with an incidence of 55%. Chemotherapy alone demonstrated a significant rate of contracture, affecting 10% of sockets. Contracture was also more common in sockets undergoing additional surgery. Larger implant size appeared protective against the development of contracture, but this finding was not statistically significant in multivariate analysis. Of note, the rate of exposure was not significantly increased with the 20-mm implant. Several studies point to the importance of adequate volume replacement in the enucleated socket for orbital development and cosmesis in adulthood.33,34 In fact, Kaltreider et al35 found that in childhood enucleations, less than 25% of adult-size volume is replaced, and they advocated for the use of the largest implant that a socket can maintain. Even in adults, a smaller implant size may “offer a false sense of security against implant extrusion.”36 A larger implant in a child may, therefore, provide better orbital size augmentation into adulthood, potentially avoiding the need for a secondary implant exchange in the future. The size of implants used in the current study may be adequate for adult implantation. The rate of exposure was not increased in our study with larger size implants, and the majority of exposures were successfully managed medically or surgically.
Management of Exposure
Exposures, if small, could be managed conservatively with vaulting of the prosthesis and antibiotics.1 In our series, more than 20% of exposures resolved without surgical intervention. When surgery was necessary, multiple procedures, often with grafts, were typically required to maintain proper implant coverage. Implant exchange was performed in 18% of the sockets with exposure, which was similar to the observed rate of 16.7% seen in the pooled adult data.37 Cancer therapies did not increase the severity of exposure or the number of surgeries required for its correction.
We advise caution in proceeding with subsequent surgeries or socket revisions unless indicated by significant exposure or implant complications. The risk of contracture increased with additional surgeries, which may be unnecessary because medical therapy can lead to stabilization or resolution of exposure and pyogenic granuloma.
Should HA implant removal be necessary, silicone spheres offered the best chance of retention as a secondary implant according to the limited number of cases in our study. Porous materials, including HA, may be less suitable than solid spheres as replacement implants in infected sockets, because the porous channels may create a hospitable environment for infectious agents.37 Alternatively, in the setting of infection, a planned secondary implant or dermis fat graft at a later date may be considered.
A multitude of implant shape and composition options are available for volume augmentation in an anopthalmic orbit. Most commonly used implants37 are porous, including naturally occurring and artificial HA,1,4 porous polyethylene,2,14,24,38 and aluminum oxide.39 Solid implants are also used and include acrylic and silicone.2,6 Pediatric sockets appear more prone to inflammation, infection, and contracture. Implants used in children with cancer should allow good tissue resolution on imaging and provide adequate volume replacement into adulthood without compromising the tolerability in the socket. Despite the potential for increased infections with porous implants, HA appears to offer good biointegration and retention in children with retinoblastoma, with few attendant complications when chemotherapy or EBRT is not required.6,15
Our study was limited by its retrospective design. It was not posed to compare different implant materials. The effects of radiation were closely linked to chemotherapy, because primary EBRT is rarely performed in the current management of retinoblastoma. However, we report on multiple types of complications, including contracture, not previously studied in sockets receiving chemotherapy. Further strengths of our study include a single-institution design, a predominance of patients with retinoblastoma, the most likely pediatric group to require enucleation in developed countries,6,15,23 a large number of sockets receiving chemotherapy or EBRT, and the consistency of surgical technique and implant used.
In conclusion, in one of the largest reported study of HA implants in pediatric sockets, chemotherapy and additional EBRT appeared to incrementally increase the risk of exposure and socket contracture. With close follow-up and surgical intervention, implant retention is possible in the majority of these sockets.
Supplementary Material
Acknowledgments
Grant Support: St. Giles Foundation, New York, New York; Research to Prevent Blindness, Inc., New York, New York.
Footnotes
Financial Disclosure(s): The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Presented in part at: the American Society of Ophthalmic Plastic and Reconstructive Surgery 40th Annual Fall Scientific Symposium, October 21–22, 2009, San Francisco, California.
References
- 1.Shields CL, Shields JA, De Potter P, Singh AD. Problems with the hydroxyapatite orbital implant: experience with 250 consecutive cases. Br J Ophthalmol. 1994;78:702–6. doi: 10.1136/bjo.78.9.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee V, Subak-Sharpe I, Hungerford JL, et al. Exposure of primary orbital implants in postenucleation retinoblastoma patients. Ophthalmology. 2000;107:940–6. doi: 10.1016/s0161-6420(00)00016-6. [DOI] [PubMed] [Google Scholar]
- 3.Li T, Shen J, Duffy MT. Exposure rates of wrapped and unwrapped orbital implants following enucleation. Ophthal Plast Reconstr Surg. 2001;17:431–5. doi: 10.1097/00002341-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Yoon JS, Lew H, Kim SJ, Lee SY. Exposure rate of hydroxyapatite orbital implants: a 15-year experience of 802 cases. Ophthalmology. 2008;115:566–72. doi: 10.1016/j.ophtha.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Setlur VJ, Parikh JG, Rao NA. Changing causes of enucleation over the past 60 years. Graefes Arch Clin Exp Ophthalmol. 2010;248:593–7. doi: 10.1007/s00417-009-1262-8. [DOI] [PubMed] [Google Scholar]
- 6.Christmas NJ, Van Quill K, Murray TG, et al. Evaluation of efficacy and complications: primary pediatric orbital implants after enucleation. Arch Ophthalmol. 2000;118:503–6. doi: 10.1001/archopht.118.4.503. [DOI] [PubMed] [Google Scholar]
- 7.Cohen VM, Kingston J, Hungerford JL. The success of primary chemotherapy for group D heritable retinoblastoma. Br J Ophthalmol. 2009;93:887–90. doi: 10.1136/bjo.2008.142679. [DOI] [PubMed] [Google Scholar]
- 8.Shields CL, Mashayekhi A, Sun H, et al. Iodine 125 plaque radiotherapy as salvage treatment for retinoblastoma recurrence after chemoreduction in 84 tumors. Ophthalmology. 2006;113:2087–92. doi: 10.1016/j.ophtha.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 9.Merchant TE, Gould CJ, Wilson MW, et al. Episcleral plaque brachytherapy for retinoblastoma. Pediatr Blood Cancer. 2004;43:134–9. doi: 10.1002/pbc.20094. [DOI] [PubMed] [Google Scholar]
- 10.Abramson DH, Dunkel IJ, Brodie SE, et al. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology. 2008;115:1398–404. doi: 10.1016/j.ophtha.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Linn Murphree A. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin North Am. 2005;18:41–53. viii. doi: 10.1016/j.ohc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Anteby I, Ramu N, Gradstein L, et al. Ocular and orbital complications following the treatment of retinoblastoma. Eur J Ophthalmol. 1998;8:106–11. doi: 10.1177/112067219800800210. [DOI] [PubMed] [Google Scholar]
- 13.Heimann H, Bechrakis NE, Zepeda LC, et al. Exposure of orbital implants wrapped with polyester-urethane after enucleation for advanced retinoblastoma. Ophthal Plast Reconstr Surg. 2005;21:123–8. doi: 10.1097/01.iop.0000152495.25263.61. [DOI] [PubMed] [Google Scholar]
- 14.Karcioglu ZA, al-Mesfer SA, Mullaney PB. Porous polyethylene orbital implant in patients with retinoblastoma. Ophthalmology. 1998;105:1311–6. doi: 10.1016/S0161-6420(98)97040-3. [DOI] [PubMed] [Google Scholar]
- 15.De Potter P, Shields CL, Shields JA, Singh AD. Use of the hydroxyapatite ocular implant in the pediatric population. Arch Ophthalmol. 1994;112:208–12. doi: 10.1001/archopht.1994.01090140084028. [DOI] [PubMed] [Google Scholar]
- 16.Lumbroso L, Levy C, Plancher C, et al. Complications of hydroxyapatite orbital implants in children: a series of 105 cases [in French] J Fr Ophtalmol. 2000;23:249–54. [PubMed] [Google Scholar]
- 17.Van Acker E, De Potter P. Porous polyethylene (Medpor) orbital implant: prospective study of 75 primary implantations [in French] J Fr Ophtalmol. 2001;24:1067–73. [PubMed] [Google Scholar]
- 18.Nolan LM, O’keefe M, Lanigan B. Hydroxyapatite orbital implant exposure in children. J AAPOS. 2003;7:345–8. doi: 10.1016/s1091-8531(03)00183-6. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Khwarg SI, Choung HK, Yu YS. Management of porous polyethylene implant exposure in patients with retinoblastoma following enucleation. Ophthalmic Surg Lasers Imaging. 2004;35:446–52. [PubMed] [Google Scholar]
- 20.Kim NJ, Choung HK, Khwarg SI, Yu YS. Free orbital fat graft to prevent porous polyethylene orbital implant exposure in patients with retinoblastoma. Ophthal Plast Reconstr Surg. 2005;21:253–8. doi: 10.1097/01.iop.0000170406.09927.c4. [DOI] [PubMed] [Google Scholar]
- 21.Hautz W, Gralek M, Karczmarewicz B, et al. Evaluation of results of enucleations with orbital implant in children and adolescents [in Polish] Klin Oczna. 2006;108:312–5. [PubMed] [Google Scholar]
- 22.Shields CL, Uysal Y, Marr BP, et al. Experience with the polymer-coated hydroxyapatite implant after enucleation in 126 patients. Ophthalmology. 2007;114:367–73. doi: 10.1016/j.ophtha.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 23.Wang JK, Liao SL, Lin LL, et al. Porous orbital implants, wraps, and PEG placement in the pediatric population after enucleation. Am J Ophthalmol. 2007;144:109–16. doi: 10.1016/j.ajo.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 24.Iordanidou V, De Potter P. Porous polyethylene orbital implant in the pediatric population. Am J Ophthalmol. 2004;138:425–9. doi: 10.1016/j.ajo.2004.04.062. [DOI] [PubMed] [Google Scholar]
- 25.Kim YD, Goldberg RA, Shorr N, Steinsapir KD. Management of exposed hydroxyapatite orbital implants. Ophthalmology. 1994;101:1709–15. doi: 10.1016/s0161-6420(94)31112-2. [DOI] [PubMed] [Google Scholar]
- 26.Galluzzi P, De Francesco S, Giacalone G, et al. Contrast-enhanced magnetic resonance imaging of fibrovascular tissue ingrowth within synthetic hydroxyapatite orbital implants in children. Eur J Ophthalmol. 2011 Jan 28; doi: 10.5301/EJO.2011.6298. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Ambati BK, Ambati J, Azar N, et al. Periorbital and orbital cellulitis before and after the advent of Haemophilus influenzae type B vaccination. Ophthalmology. 2000;107:1450–3. doi: 10.1016/s0161-6420(00)00178-0. [DOI] [PubMed] [Google Scholar]
- 28.Georgakopoulos CD, Eliopoulou MI, Stasinos S, et al. Periorbital and orbital cellulitis: a 10-year review of hospitalized children. Eur J Ophthalmol. 2010;20:1066–72. doi: 10.1177/112067211002000607. [DOI] [PubMed] [Google Scholar]
- 29.Revai K, Dobbs LA, Nair S, et al. Incidence of acute otitis media and sinusitis complicating upper respiratory tract infection: the effect of age [report online] [Accessed April 13, 2011];Pediatrics. 2007 119:e1408–12. doi: 10.1542/peds.2006-2881. Available at: http://pediatrics.aappublications.org/cgi/reprint/119/6/e1408. [DOI] [PubMed] [Google Scholar]
- 30.Holzmann D, Willi U, Nadal D. Allergic rhinitis as a risk factor for orbital complication of acute rhinosinusitis in children. Am J Rhinol. 2001;15:387–90. [PubMed] [Google Scholar]
- 31.Lewis WH, Dixit AB, Ward WA. Distribution and incidence of North American pollen aeroallergens. Am J Otolaryngol. 1991;12:205–26. doi: 10.1016/0196-0709(91)90121-u. [DOI] [PubMed] [Google Scholar]
- 32.Chonmaitree T, Revai K, Grady JJ, et al. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008;46:815–23. doi: 10.1086/528685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaste SC, Chen G, Fontanesi J, et al. Orbital development in long-term survivors of retinoblastoma. J Clin Oncol. 1997;15:1183–9. doi: 10.1200/JCO.1997.15.3.1183. [DOI] [PubMed] [Google Scholar]
- 34.Fountain TR, Goldberger S, Murphree AL. Orbital development after enucleation in early childhood. Ophthal Plast Reconstr Surg. 1999;15:32–6. doi: 10.1097/00002341-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Kaltreider SA, Peake LR, Carter BT. Pediatric enucleation: analysis of volume replacement. Arch Ophthalmol. 2001;119:379–84. doi: 10.1001/archopht.119.3.379. [DOI] [PubMed] [Google Scholar]
- 36.Liu D. A comparison of implant extrusion rates and postoperative pain after evisceration with immediate or delayed implants and after enucleation with implants. Trans Am Ophthalmol Soc. 2005;103:568–91. [PMC free article] [PubMed] [Google Scholar]
- 37.Custer PL, Kennedy RH, Woog JJ, et al. Ophthalmic Technology Assessment Committee Oculoplastics Panel. Orbital implants in enucleation surgery: a report by the American Academy of Ophthalmology. Ophthalmology. 2003;110:2054–61. doi: 10.1016/S0161-6420(03)00857-1. [DOI] [PubMed] [Google Scholar]
- 38.Blaydon SM, Shepler TR, Neuhaus RW, et al. The porous polyethylene (Medpor) spherical orbital implant: a retrospective study of 136 cases. Ophthalmic Plast Reconstr Surg. 2003;19:364–71. doi: 10.1097/01.IOP.0000083643.36461.84. [DOI] [PubMed] [Google Scholar]
- 39.Jordan DR, Klapper SR, Gilberg SM, et al. The bioceramic implant: evaluation of implant exposures in 419 implants. Ophthal Plast Reconstr Surg. 2010;26:80–2. doi: 10.1097/IOP.0b013e3181b80c30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.