Abstract
22q11.2 chromosomal deletions are recurrent copy number mutations that increase the risk of schizophrenia around thirty-fold. Deletion of the orthologous chromosomal region in mice offers an opportunity to characterize changes to neuronal structure and function that may account for the development of this disease. The hippocampus has been implicated in schizophrenia pathogenesis, is reduced in volume in 22q11.2 deletion carriers and displays altered neuronal structure in a mouse model of the mutation (Df(16)A+/− mice). Here we investigate hippocampal CA1 physiology, hippocampal-dependent spatial memory and novelty-induced hippocampal activation in Df(16) A+/− mice. We found normal spatial reference memory (as assayed by the Morris water maze test) as well as modest but potentially important deficits in physiology. In particular, a reduction in the level of inhibition of CA1 pyramidal neurons was observed, implying a decrease in interneuron activity. Additionally, deficits in LTP were observed using certain induction protocols. Induction of c-Fos expression by exploration of a novel environment suggested a relative sparing of CA1 and dentate gyrus function but showed a robust decrease in the number of activated CA3 pyramidal neurons in Df(16) A+/− mice. Overall, experiments performed in this 22q11.2 deletion model demonstrated deficits of various degrees across different regions of the hippocampus, which together may contribute to the increased risk of developing schizophrenia.
Keywords: Schizophrenia, 22q11.2 deletion, Hippocampus, Interneuron, c-Fos, Disinhibition, Synaptic plasticity, CA1, CA3
Introduction
Hemizygous deletion of the human chromosomal region 22q11.2 occurs in around 1/4000 live births and causes a complex syndrome in which 25–30% of mutation carriers develop schizophrenia (SCZ) (Pulver et al., 1994; Murphy et al., 1999; Scambler, 2000). Hence, 22q11.2 deletions likely account for around 1% of sporadic cases of SCZ (Bassett et al., 2008; Brunet et al., 2008; Xu et al., 2008) and this degree of penetrance makes 22q11.2 deletions the highest known genetic risk factor for developing SCZ (Karayiorgou et al., 2010, Drew et al., 2010). The mutations, which typically occur de novo, are most commonly approximately 3 Mb in size (Edelmann et al., 1999; Shaikh et al., 2000), however, deletions of around 1.5 Mb also occur at significant rates and are similarly associated with elevated rates of SCZ (Karayiorgou et al., 1995). Therefore, this region, which contains 28 functional genes, has been termed the “SCZ critical region” (Karayiorgou et al., 1995; 2010).
For SCZ (and other psychiatric illnesses), where the core molecular, cellular and neural circuit level pathologies remain largely unresolved, the generation of etiologically valid mouse models and the types of experiments afforded by such models offer immense potential for establishing key disease mechanisms (Arguello and Gogos, 2006). Remarkably, all but one of the genes in the 22q11.2 SCZ critical region are contained in the orthologous region of mouse chromosome 16 which has allowed the generation of mice with this SCZ-associated mutation (Drew et al., 2010) including the mice used in the present study (Stark et al., 2008; Df(16)A+/− mice). Multilevel characterization of these mouse models, and the comparison of their phenotypes with other valid models of SCZ, will likely lead towards a better understanding of this illness (Arguello and Gogos, 2006; Flint and Shifman, 2008).
Behavioral testing of Df(16)A+/− mice has shown them to have deficits in sensorimotor gating (prepulse inhibition), emotional learning (cued and contextual fear conditioning) and acquisition of a working memory task (delayed non-matched to place (DNTP) T-maze), as well as increased anxiety related behavior (open field and light/dark preference) (Stark et al., 2008). One candidate mechanism for the observed behavioral changes is a reduction in long-range synchrony between brain regions, as demonstrated for activity in the hippocampus and the prefrontal cortex during performance of the DNTP T-maze task (Sigurdsson et al., 2010).
The molecular and cellular bases, such as altered neuronal structure and synapse formation (Mukai et al., 2008), for physiological and behavioral changes are thought to be the result of complex, non-linear interactions between multiple loci within the deletion but with some loci making major contributions (Karayiorgou and Gogos, 2004; Paterlini et al., 2005; Drew et al., 2010). To date, substantial roles have been suggested for abnormalities in microRNA synthesis (due to haploinsufficiency at the DGCR8 locus; Stark et al., 2008), reduced palmitoylation of key neuronal proteins (due to haploinsufficiency at the ZDHHC8 locus; Mukai et al., 2008) and changes in proline and dopamine metabolism (from reduced expression of ProdH and Comt; Paterlini et al., 2005).
The hippocampus has been widely implicated in the pathophysiology of SCZ (Roberts, 1990), with reductions in hippocampal volume being one of the more consistent anatomical findings in SCZ patients (see Nelson et al., 1998). Interestingly, similar decreases in volume have been found in 22q11.2 deletion carriers (Debbané et al., 2006; Tan et al., 2009). Neuropathological studies of SCZ have given mixed results but overall point to subtle disease-associated changes in the expression of synaptic markers in this structure (see Harrison, 2004). Finally, functional imaging studies have shown hippocampal hyperactivity at rest in SCZ patients (Friston et al., 1992; Kawasaki et al., 1992; Heckers et al., 1998; Medoff et al., 2001 Schobel et al., 2009) and abnormal activation in cognitive tasks (Heckers et al., 1998; Fusar-Poli et al., 2007).
Despite this literature, it is unclear if changes in hippocampal structure and function are primary events in SCZ pathogenesis or secondary to changes in other systems (see Lisman et al., 2008). This study presents a number of assays of hippocampal function in Df(16)A+/− mice using electrophysiological, immediate early gene (IEG) expression and behavioral techniques, to assess the impact of a SCZ risk allele on this brain region. We show that a number of functional properties of this structure are preserved but that there are numerous modest changes that may affect hippocampal function in ways that are relevant to SCZ pathophysiology.
Material and Methods
All procedures were carried out in accordance with institutional guidelines. All recordings and the majority of data analysis were done blind to the genotype of the experimental subject. For behavioral and immunohistochemical experiments, the investigator was blind to genotype throughout the experimental procedures and data analysis. All reagents from Sigma-Aldrich unless otherwise stated.
The generation of the Df(16)A+/− mice used in this study has previously been described (Stark et al., 2008); all mice had been back-crossed to a C57Bl/6J background to at least F8. Only male mice were used and for all experiments littermate controls were used.
Slice preparation
Mice were anesthetized with isoflourane and then decapitated. The brain was removed and chilled in ice-cold dissection solution (in mM: sucrose 195, NaCl 10, KCl 2.5, NaH2PO4 1, NaHCO3 25, glucose 10, MgCl2 5, MgSO4 1, CaCl2 0.5). The cerebellum and the anterior portion of the brain were removed and horizontal brain sections cut on a vibratome (Leica VT1200S). For field recordings, 400 μm slices were cut and then immediately transferred to an interface chamber and allowed to recover for at least 2 hrs at 31–32°C. For whole-cell recordings, 350 μm brain slices were cut and recovered in a submerged chamber at 37°C for 45 min and then at room temperature until use in ACSF (in mM: NaCl 124, KCl 2.5, NaH2PO4 1, NaHCO3 25, Glucose 10, MgSO4 1, CaCl2 2). Experimental animals were 6–10 weeks old for field recordings (unless otherwise stated) and 5–6 weeks for whole-cell recordings.
Extracellular field recordings
Extracellular field recordings were made in an interface chamber (Fine Science Tools) at 31–32°C in ACSF (2 ml/min) with recording electrodes (≈ 2–3 MΩ) filled with ACSF. Stimulation was with a concentric bipolar stimulating electrode (tip diameter 0.2 mm, FHC). Data were acquired by pClamp10 software, using an extracellular amplifier (Cygnus Technologies, ER-1) and Axon Instruments Digidata 1440A. To record SC evoked fEPSPs in CA1, the recording electrode was positioned in stratum radiatum (SR) of CA1 and the stimulating electrode in CA1 SR close to CA2. For simultaneous population spike recordings a second recording electrode was placed in SP, at a similar depth to and perpendicular to the SR recording electrode. For recording TA pathway evoked fEPSPs, the recording electrode was positioned in CA1 SLM and the stimulating electrode in SLM at the border between CA1 and the subiculum. fEPSPs were quantified by measuring the slope of the linear part of the rising phase, fiber volleys were quantified by their amplitudes and the amplitude of population spikes was measured by subtracting their peak amplitudes from a point concurrent with the peak extrapolated from a line linking the prior and after positive components. At the beginning of the recording period input-output curves were acquired by stimulating in 3V increments. For subsequent assaying of PPR, LTP and LTD the stimulus intensity was adjusted to evoke a fEPSP 1/3 of the maximum and all test pulses and tetani were delivered at this intensity. 20–30 minute baselines were recorded and post-induction responses were normalized to the final 10 min. LTP was induced by a) 100 Hz: 2 trains of 1 sec at 100 Hz separated by a 30 sec interval b) Theta bursts: 5 stimuli at 100 Hz repeated 12 times at 200 msec intervals (5 Hz) repeated 3 times with 20 sec interval c) 200 Hz L-LTP: 40 stimuli at 200 Hz, repeated 10 times with 5 sec intervals. LTD and depotentiation were induced by 900 stimuli at 1 Hz. The average of the last 5 responses of monitoring period was taken as the effect size for each slice in each protocol.
Whole-cell, patch-clamp recordings
Whole-cell patch clamp recordings were made at 31–32°C in ACSF (see above) using boroscillate glass pipettes (initial resistance 4–5 MΩ). For assessing intrinsic properties of pyramidal neurons and all recordings in interneurons an internal solution was used that contained (in mM): KMeSO4 130, KCl 10, HEPES 10, NaCl 9, EGTA 0.1, MgATP 4, Na2GTP 0.3, phosphocreatine 10. For synaptic recordings in pyramidal neurons the internal solution contained (in mM): CsMeSO4 125, QX314.Cl 5, HEPES 10, NaCl 4, EGTA 1, MgATP 4, Na2GTP 0.3, phosphocreatine 10. Junction potentials were not corrected for. Input resistance was calculated as the slope of membrane polarization in response to current injection −50 to +25 pA at resting potential. Action potential thresholds were calculated by a stepwise 20 pA depolarizing current injection and then a 150 pA current ramp over 1 sec and the potential at which the membrane potential clearly deviated from the monotonic slope was recorded. SC stimulation was by a concentric electrode positioned in SR approximately 300 μm from the recording electrode. To measure AMPA and NMDA receptor components of eEPSCs SCs were stimulated, in the presence of the GABAA receptor antagonist SR95331, to give an EPSC of approximately 250 pA at −60 mV. Currents mediated solely by AMPA receptors were recorded −60 mV interleaved with glutamatergic currents at +60 mV that are carried by AMPA and NMDA receptors. For recording glutamatergic and GABAergic currents in the same cell, synaptic currents were recorded at 0 mV (glutamatergic reversal potential) and −60 mV (GABAergic reversal potential). To induce DSI, eIPSCs were recorded as the outward current at −40 mV in response to SC stimulation (i.e. feedforward IPSCs), the membrane was then stepped to 0 mV for 5 sec and then stepped back to −40mV and another eIPSC evoked 250 msec later. The voltage-gated sodium channel blocker TTX (500 nM), NMDA receptor antagonist AP-V (50 μM), AMPA/kainite receptor antagonist NBQX (5 μM) and GABAA receptor antagonist SR95531 (10 μM) (all Tocris) were used as indicated. All protocols were repeated 4–8 times for each cell and the average taken to represent that cell’s response.
Electrophysiological Data analysis
Data were analyzed using Clampfit, Minianalysis, Sigmaplot and Sigmastat software. Voltage sag was quantified as the steady-state amplitude of the hyperpolarization from resting potential over the peak hyperpolarization in response to a 500 msec, −100 pA current injection. The EPSC decay tau was derived from monoexponential curves fitted in Clampfit (Chebyshev method). To quantify AMPA:NMDA receptor ratios, decay tau was first fitted to the (AMPA receptor-mediated) current at −60 mV. Then, the AMPA component was measured as the +60 mV current amplitude at the time of the peak of the −60mV current and the NMDA receptor component as the +60 mV current amplitude 3 × the decay tau after this point. Spontaneous and miniature synaptic currents were analyzed using Minianalysis software. sEPSCs and mEPSCs were detected over 5 minute recording periods; the detection threshold was 5 pA. sIPSCs and mIPSCs were detected over 2 minute recording periods; the detection threshold was also 5 pA. Sigmoidal fits to fEPSP-PS curves were fitted using Sigmaplot: P = a/(1+exp− (E−E0/b)) where E0 = the value of E (fEPSP) that yields a 50% maximum PS and b = the slope of the relationship at that point. Data are presented as mean ± standard error of the mean, n indicates: number of slices/cells and P < 0.05 was taken as significant.
Morris Water Maze
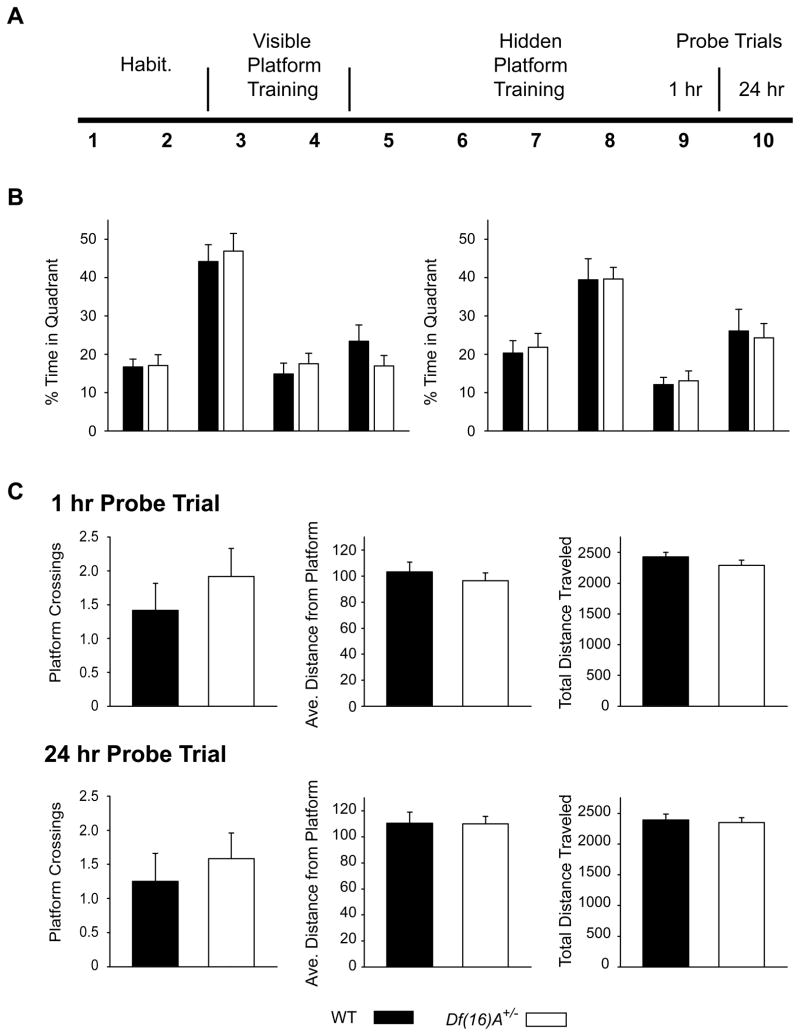
Adult male mice (5–7 months old) were tested in a water maze procedure as previously described (Kvajo et al., 2008; Meshi et al., 2006). It consisted of four phases: (1) habituation, (2) visible platform training, (3) hidden platform training, and (4) long-term memory probes (Fig. 5A). The pool used was a circular, white plastic tank, 1.7 m in diameter, which contained 21°C water, made opaque by the addition of non-toxic white paint. Mouse movement was recorded using a video tracking system (Chromotrack, San Diego Instruments) and distance traveled and percent time in each quadrant was calculated using custom software. In the first phase, mice were habituated to the water in a bucket, and trained to climb onto, and remain on, a submerged platform. This training was for 2 days, with 2 trials/day. During the visible platform phase, mice were placed into the pool of water at 1 of 8 locations around the perimeter. They were allowed 2 min to find the location of a submerged opaque circular platform (14.6 cm in diameter, ~ 1 cm below the surface of the water), whose location was marked by a visible cue above the water. The location of the platform was fixed, and mice received 2 days of training, with 3 trials/day. For the hidden platform phase, the training was as described above, except there was no visible marker above the platform. Mice were required to use extramaze cues located in the room. This took place for 5 days. On days 1–4 there were 3 trials/day. On the 5th day, there were 2 trials, followed by a probe trial, 1 hr after the last training session. 24 hrs later there was another probe trial. During the probe trials, the platform was removed from the pool, and mice were allowed 1 minute to search for it. During all probe trials, the mice were placed into the pool in the position opposite from where the platform had been located during the training. Analysis of the data was performed as a repeated-measures ANOVA with genotype as a factor, to assess the latency and distance to target during training (across trials), or percent time spent in the quadrants, for each probe trial. The total number of platform crossings, average distance from the platform, and total distance traveled during the probe trials was compared between genotypes using t-tests. All data were analyzed using Statview (SAS Institute, USA).
Fig. 5. Df(16)A+/− mice do not display deficits in hippocampus-dependent reference memory in the MWM.
(A) Experimental design for the habituation, visible platform training, hidden platform training, and probe trials of the MWM task. Habituation and visible platform training were for 2 days each, followed by 5 days of hidden platform training. Probe trials were conducted 1 hr and 24 hrs after the last training session. n = 12 mice per genotype. (B) Percent time spent in each quadrant during the probe trials. For both the 1 hr (left) and 24 hr (right) probe trials, Df(16)A+/− and wild-type mice exhibited a preference for the target quadrant (main effect of quadrant: p < 0.0001), with the two genotypes showing equal spatial preferences for the target quadrant (effect of genotype: p = 0.9032, comparisons of all quadrants to target quadrant (all p < 0.0001). (C) The total number of platform crossings, average distance from the platform, and total distance traveled during the 1 hr (top) and 24 hr (bottom) probe trials did not differ between genotypes (all p > 0.05)
c-Fos mapping of hippocampal activation
Animals were 3 months of age at the time of the experiment and littermate controls were used. In total 28 male mice from 8 litters, separated in 3 batches, were used. The novel environment was a 20 × 42 cm rectangular, opaque plastic box containing 5 novel objects from the laboratory (a polystyrene block, a purple pipette tip rack, a ball of aluminum foil and a blue and an orange 50 ml tube cap containing water, food pellets were also present). For each animal, the enclosure had been washed and fresh bedding and novel objects were used. Control animals were taken immediately from the home cage. Mice were euthanized by CO2 inhalation prior to transcardial perfusion with PBS, followed by fixation with 4% paraformaldehyde in PBS. Brains were post-fixed at 4°C overnight and then transferred to PBS. 40 μm sections were cut on a VT1000S Leica vibrotome and kept at 4°C until further use. Sections were stained using rabbit anti-c-Fos (1:5000, Calbiochem) and mouse anti-NeuN (1:1000) at 4°C overnight. Secondary antibodies used were: goat anti-rabbit coupled to Alexa Fluor 546 and goat anti-mouse coupled to Alexa Fluor 488 (Both 1:700, Molecular Probes).
For each subject 3 brain sections at the level of the dorsal to dorsomedial hippocampus were stained and imaged and the average of 4–6 hippocampi was taken as the representative value for each mouse. Images were acquired on a Nikon Eclipse TE2000-E confocal microscope using a 20× objective lens; a single optical plane was taken at the level of the most intense NeuN signal for each section. Positive neurons were counted by eye or by automated protocols on Image J software.
Results
Intrinsic properties and firing behavior of Df(16)A+/− CA1 pyramidal neurons
As a first step in characterizing the properties of CA1 in Df(16)A+/− mice, we analyzed the intrinsic properties of pyramidal neurons in this region using whole-cell current clamp recordings. Firing patterns were assessed in response to incremental, 500 msec depolarizing current injections. When inter-cell membrane potential variation was normalized by adjusting the resting potential of all neurons to −65 mV (by injection of a small standing current), Df(16)A/+ neurons fired less in response to larger depolarizations (Figs. 1A and B). Both classes of neurons exhibited spike frequency accommodation in the action potential train (Madison and Nicoll, 1984), but Df(16)A+/− showed a steeper increase in interspike interval throughout, which resulted in the lower overall firing rate (Fig. 1D). Curiously, from the unadjusted resting potential there was no difference in the firing rates of the two populations of neurons (Fig. 1E). Resting potential did not significantly differ between genotypes, although overall the mean value was more depolarized in mutant neurons (Table 1). In addition, no significant genotypic differences were found for input resistance, capacitance, or the threshold for the first action potential (in response to a depolarizing current ramp) (Table 1). Consistent with these data, the rheobase (for a 100 msec square wave pulse of current required to elicit a single action potential) was also unchanged by the mutation (Table 1) and in addition, the voltage sag in response to hyperpolarizing current injections (caused by the activation of HCN channel-mediated Ih currents) was not different between genotypes (Fig. 1C).
Fig.1. Action potential firing by CA1 pyramidal neurons.
(A) Examples of action potential trains in WT (upper trace) and Df(16)A+/− neurons (lower trace) in response to a 500 msec depolarizing 0.4 nA current step from −65mV. (B) Df(16)A+/− neurons fired fewer action potentials in response to large current injections for 500 msec (p < 0.05 at ≥ 400 pA) when resting membrane potential was adjusted to −65 mV (2-way repeated measures ANOVA, genotype: p = 0.068, genotype x current: p < 0.001, WT: n = 8, Df(16)A+/−: n = 8). (C) Examples of voltage sags in WT and Df(16)A+/− neurons in response to a 75 pA hyperpolarizing current step. (D) Accommodation of spike rates was seen in both genotypes but it was greater in Df(16)A+/− neurons (2-way repeated measures ANOVA, genotype: p = 0.05, genotype x spike number: p = 0.002: n = 8, Df(16)A+/−: n = 7, 1 cell < 16 APs). (E) Action potential trains evoked from resting potential did not differ between genotypes (2-way repeated measures ANOVA, genotype: p = 0.49, genotype x current: p = 0.73, WT: n = 15, Df(16)A+/−: n = 13).
Table 1.
Intrinsic properties of CA1 pyramidal neurons.
| Rest Pot (mV) | Input Res. (MΩ) | Capacitance (pF) | APThreshold (mV) | Rheobase (pA) | Voltage Sag | |
|---|---|---|---|---|---|---|
| WT | 64.1 ± 1.1 (16) | 147.8 ± 11.8 (16) | 107.1 ± 4.9 (16) | −45.4 ± 0.7 (13) | 88.13 ± 8.27 (7) | 0.86 ± 0.01 (16) |
| Df(16)A+/− | 62.7 ± 0.9 (16) | 156.1 ± 9.8 (16) | 110.4 ± 5.5 (16) | −46.2 ± 0.4 (12) | 91.25 ± 14.41 (6) | .85 ± 0.01 (16) |
| P | 0.30 | 0.59 | 0.65 | 0.34 | 0.85 | 0.89 |
Functional Excitatory Input to Df(16)A+/− CA1 pyramidal neurons is normal
We also sought to characterize excitatory synaptic input to CA1 pyramidal neurons in Df(16)A+/− mice using a combination of extracellular field and whole-cell voltage-clamp recordings. Responses to stimulation of Schaffer collateral (SC, from CA3) and the temporoammonic (TA, from entorhinal cortex) pathways were measured as well as spontaneous and miniature synaptic currents. Field excitatory postsynaptic potentials (fEPSPs) (Figs. 2A and B) and whole-cell excitatory postsynaptic currents (EPSCs) (Figs. 2D and E) evoked by SC stimulation, across a range of stimulus intensities, were of similar magnitude in the two genotypes. The amplitude of the fiber volley in field recordings was similar between genotypes, indicating equal numbers of axons were activated (Fig. 2C) and the degree of paired-pulse facilitation (PPF) was equivalent suggesting comparable presynaptic release probability (Creager et al, 1980; Debanne et al, 1996) (Figs. 2F and S1A). Looking at postsynaptic properties neither EPSC kinetics (Figs. S1B and C) nor the balance of AMPA and NMDA receptor-mediated currents at SC-CA1 synapses (Figs. S1D and E, Béïque et al, 2006) significantly differed between genotypes. Finally, the deletion did not significantly change the size or PPF of fEPSPs recorded in stratum laconosum moleculare (SLM) when stimulating the TA pathway (Figs. S1F-H). Consistent with the evoked data, the frequency and amplitude of both spontaneous (sEPSCs) and miniature (mEPSCs, in the presence of TTX) EPSCs, recorded in the whole-cell configuration from CA1 pyramidal neurons, were comparable in the two genotypes (Figs. 2G–I).
Fig. 2. Excitatory synaptic input to CA1 pyramidal neurons.
(A) Example traces showing fEPSPs and fiber volleys recorded in CA1 SR in response to SC stimulation at incremental intensities (3–18 V). Upper, WT; lower, Df(16)A+/−. (B) fEPSPs recorded in SR of CA1 and evoked by extracellular stimulation of SC inputs were not different between genotypes (2-way repeated measures ANOVA, genotype: p = 0.45, genotype × stimulus intensity: p = 0.58, WT: n = 75, Df(16)A+/−: n = 66). (C) The average fiber volley recorded in the same conditions was similar in both genotypes (2-way repeated measures ANOVA, genotype: p = 0.98, genotype × stimulus intensity: p = 0.99, WT: n = 29, Df(16)A+/−: n = 37). (D) Example traces showing eEPSCs recorded in response to 3, 6 and 9 V stimuli. Upper; WT, lower; Df(16)A+/−. (E) EPSCs recorded in CA1 pyramidal neurons, evoked by extracellular stimulation of SC inputs were not different between genotypes (2-way repeated measures ANOVA, genotype: p = 0.71, genotype x stimulus intensity: p = 0.73, WT: n = 19, Df(16)A+/−: n = 19). (F) Paired-pulse facilitation (interstimulus interval, 50 msec) was not significantly affected by the mutation. Left, PPF for fEPSPs: WT: 1.51 ± 0.02, n = 73; Df(16)A+/−: 1.53 ± 0.02, n = 64; p = 0.36B. Right, for EPSCs: WT: 1.40 ± 0.05, n = 26; Df(16)A+/−: 1.46 ± 0.05, n = 29; p = 0.36. (G) Example traces showing sEPSCs in a WT neuron, upper, and a Df(16)A+/− neuron, lower. (H) The frequency of spontaneous EPSCs (Left) in WT and Df(16)A+/− neurons was similar (WT: 0.94 ± 0.12 Hz, n = 17; Df(16)A+/−: 0.98 ± 0.15 Hz, n = 17; p = 0.84), as was the frequency of miniature EPSCs (Right) (WT: 0.54 ± 0.06 Hz, n = 18; Df(16)A+/−: 0.46 ± 0.06, n = 15; p = 0.33). (I) The average amplitudes of sEPSCs and mEPSCs in WT and Df(16)A+/− neurons were similar. Left, sEPSCs (WT: 15.75 ± 0.82 pA, n = 17; Df(16)A+/−: 15.27 ± 0.73 pA, n = 17; p = 0.66), right, mEPSCs. (WT: 13.58 ± 0.62 pA, n = 18; Df(16)A+/−: 14.86 ± 0.70, n = 15; p = 0.18).
Overall, these data suggest that the number and properties of functional excitatory synapses on CA1 pyramidal neurons in Df(16)A+/− mice does not differ significantly from WT values.
The activity of inhibitory interneurons in Df(16)A+/− mice is reduced
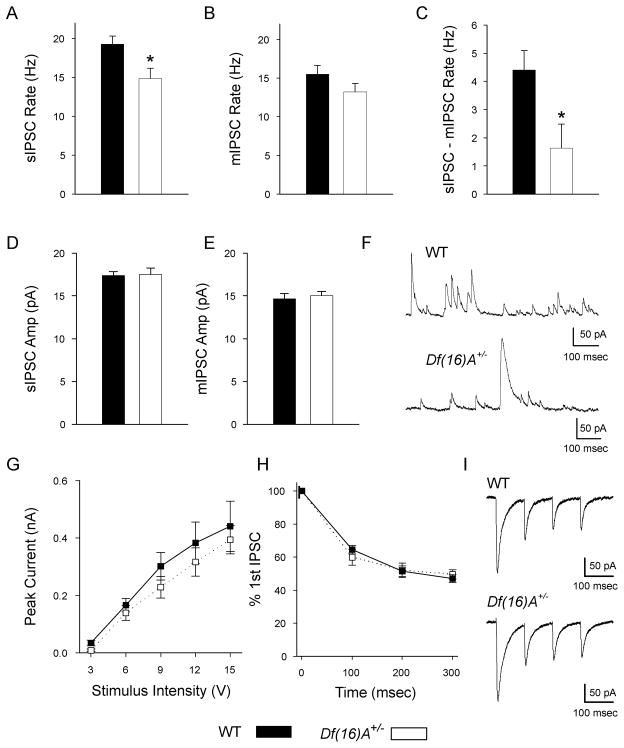
In recent years there has been considerable attention paid to putative dysfunction of parvalbumin-expressing interneurons in SCZ in neocortex and hippocampus (Lewis et al, 2006; Lisman et al, 2008; Belforte et al, 2010). Therefore, we also analyzed inhibitory, GABAergic innervation of CA1 pyramidal neurons. First, we used intracellular recordings to investigate spontaneous (sIPSCs) and miniature (mIPSCs) inhibitory synaptic currents (Fig. 3F). The rate of mIPSCs was not significantly different between genotypes suggesting that the number of functional GABAergic synapses on each neuron was similar between genotypes (Fig. 3B). However, there was a 22.9% (P = 0.01) decrease in the frequency of sIPSCs in Df(16)A+/− pyramidal neurons (Fig. 3A). As the frequency of sIPSCs is the sum of random quantal events (mIPSCs) and events caused by action potential firing in the presynaptic neuron, to estimate action potential firing rate in connected presynaptic neurons, the frequency of mIPSCs was subtracted from the sIPSC rate on a cell-by-cell basis. This subtraction indicated that input attributable to the firing of action potentials in presynaptic interneurons in the mutant mice was lower by 62.9% (P = 0.02, Fig 3C). The amplitude of events in both conditions was comparable (Figs. 3D and E). As GABAergic currents were recorded at 0 mV, the possibility existed that greater depolarization-induced suppression of inhibition (DSI), a phenomenon caused by depolarization-induced release of endocannabinoids presynaptically inhibit GABA release (Wilson et al., 2001), mediated this effect. However, when DSI was tested in a series of CA1 pyramidal neurons no difference was found between genotypes (Fig. S2). Together these results suggest that the number of functional inhibitory synapses on each pyramidal neuron is unchanged by the mutation but that the spontaneous firing of GABAergic interneurons in hippocampal slices from Df(16)A+/− mice is decreased.
Fig. 3. Inhibitory synaptic input to CA1 pyramidal neurons.
(A) The frequency of sIPSCs was 22.9% significantly lower in Df(16)A+/− neurons (14.9 ± 1.3 Hz, n = 15) than in WT neurons (19.3 ± 1.1 Hz, n = 17, p = 0.01). (B) WT and Df(16)A+/− neurons had comparable rates of mIPSCs (WT: 15.5 ± 1.2 Hz, n = 14; Df(16)A+/−: 13.2 ± 1.1 Hz, n = 15; p = 0.16). (C) The number of events attributable to firing of INs was reduced by 62.9% in Df(16)A+/− neurons (WT: 4.4 ± 0.7 Hz, n = 14; Df(16)A+/−: 1.6 ± 0.9 Hz, n = 15; p = 0.02). (D) The average amplitude of sIPSCs were similar in both genotypes (WT: 17.4 ± 0.4 pA, n = 17; Df(16)A+/−: 17.5 ± 0.7 pA, n = 15; p = 0.88). (E) The average amplitude of mIPSCs were also similar in both genotypes (WT: 14.6 ± 0.7 pA, n = 14; Df(16)A+/−: 15.0 ± 0.5 pA, n = 15; p = 0.61). (F) Example traces from a WT neuron, top, and a Df(16)A+/− neuron, bottom, showing sIPSCs recorded at 0 mV. (G) eIPSCs from stimulation of the SR region above the pyramidal neuron were not significantly different over a range of stimulus intensities (2-way, repeated measures ANOVA, genotype: p = 0.47, genotype × stimulus intensity: p = 0.94, WT, n = 10; Df(16)A+/−, n = 6). (H) Stimulation of GABAergic axons 4 times at 10 Hz showed no difference between short-term dynamics in WT and Df(16)A+/− neurons (2-way, repeated measures ANOVA, genotype: p = 0.90, genotype × stimulus number: p = 0.32, WT, n = 10; Df(16)A+/−, n = 6). (I) Example traces from a WT neuron, top, and a Df(16)A+/− neuron, bottom, of eIPSCs recorded at −90 mV in AP-V and NBQX.
Consistent with the inference that the number of GABAergic synapses is equivalent on each pyramidal neuron, when GABAergic inputs were stimulated directly in SR (with AMPA and NMDA receptors blocked) no difference was seen in the amplitude of evoked IPSCs over a range of stimulus intensities (Fig. 3G). In addition, the short-term dynamics of GABA release (in response to 4 stimuli at 100msec intervals) was unchanged by the mutation, suggesting normal release probability at these inputs (Fig. 3H).
Given this decrease in interneuron activity, we tested for functional correlates of reduced inhibition. Firstly, disynaptic GABAergic currents were measured by stimulating SCs and alternately recording glutamatergic (at −60 mV) and GABAergic currents (by stepping the membrane potential to 0 mV) (Fig. S3A). In doing this, excitatory input could be normalized (to ≈275 pA) and the corresponding GABAergic currents measured. Over a population of CA1 pyramidal neurons, for an eEPSC of ≈275 pA, the corresponding IPSC was not different between genotypes (Figs. S3B and C) and neurons stimulated at a range of stimulus intensities displayed comparable excitation to inhibition ratios (Fig. S3D).
Secondly, we assayed the spiking output of CA1 in relation to excitatory input as another test of feedforward inhibition. In each slice, the population spike in SP and the fEPSP in SR were recorded extracellularly as SC inputs were stimulated. The relationship of the population spike to the fEPSP magnitude is well described by a sigmoidal function (Fig. S4A) and decreased feedforward inhibition causes both a leftward shift in the population spike-EPSP curve and increases the slope of the linear part of the curve (Carvalho and Buonomano, 2009; Pouille et al, 2009). However, normalized population spikes and fEPSPs for WT and Df(16)A+/− slices showed that the relationships between these variables were not significantly different (Fig. S4B). Additionally, the average sigmoid slope and fEPSP size that evoked a 50% population spike for each slice was not different between genotypes (Figs. 4C and D).
Fig. 4. Synaptic plasticity in CA1.
(A) SC-CA1 LTP induced by two trains at 100 Hz was significantly reduced in Df(16)A+/− mice After 1 hr WT fEPSPs were potentiated by 63.12% (n = 9) and Df(16)A+/− responses by 29.77% (n = 8, p = 0.001). (B) SC-CA1 LTP induced by a theta burst protocol did not differ between genotypes. After 1 hr potentiation was 62.5 ± 10.5% in WT (n = 7) and 55.0 ± 5.3% in Df(16)A+/− (n = 9, p = 0.50). (C) SC-CA1 L-LTP induced by three applications of a 200 Hz based protocol did not differ between genotypes. After 2.5 hrs potentiation was 61.7 ± 7.3% in WT (n = 7) and 64.4 ± 13.6% in Df(16)A+/− (n = 7, p = 0.86). (D) TA-CA1 LTP induced by two trains at 100 Hz stimulation was similar in WT and Df(16)A+/− mice. After 1 hr potentiation was 61.7 ± 7.3% in WT (n = 7) and 64.4 ± 13.6% in Df(16)A+/− (n = 7, p = 0.86). (E) SC-CA1 LTD induced by 1 Hz stimulation for 15 min was comparable in the two genotypes. After 1 hr depression was 27.2 ± 2.8% in WT (n = 13) and 31.3.0 ± 3.0% in Df(16)A+/− (n = 18, p = 0.34). (F) SC-CA1 depotentiation induced by 1 Hz stimulation for 15 min after LTP induction by 2 × 100 Hz trains was not different between genotypes. After 1 hr depotentiation was 23.5 ± 5.6% in WT (n = 5) and 23.1 ± 3.6% in Df(16)A+/− (n = 8, p = 0.96).
Overall, these data suggest that in the hippocampal CA1 region of Df(16)A+/− mice feedforward inhibition is of similar strength to WT mice and similarly controls the spiking of CA1 pyramidal neurons.
To further assess interneuron function, whole-cell recordings were made from samples of CA1 interneurons in stratum oriens (SO)/alveus. The rate of spontaneous interneuron firing in the slice will depend largely on their intrinsic properties and the amount of spontaneous synaptic input, we therefore assessed those parameters. Neurons were classified by their firing properties as fast spiking, regular spiking or “slow” spiking (Fig. S5a). Each class was present in similar proportions in the two genotypes (Fig. S5b, Table S1). When compared overall or between subclasses no significant differences were seen in intrinsic properties or the frequency or amplitude of sEPSCs (Table S1).
Induction of LTP in Df(16)A+/− mice is altered under some conditions
Our final physiological experiments were concerned with synaptic plasticity at excitatory inputs to CA1 pyramidal neurons. To investigate this, we recorded fEPSPs at SC and TA inputs and used a number of protocols to induce either long-term potentiation (LTP) or long-term depression (LTD) of these inputs. At SC inputs one of three LTP induction protocols revealed a difference between genotypes. When LTP was induced by two 1 sec trains of 100 Hz stimulation significant LTP occurred in both genotypes but to a significantly lesser degree in Df(16)A+/−; after 1 hr the increase in fEPSP size was 52.8% (P = 0.001) less than in WT controls (Fig. 4A). The difference in potentiation was fairly constant throughout the 1 hr monitoring period, suggestive of a deficit in the establishment of increased excitatory transmission rather than a decreased maintenance of the potentiated state. In contrast, when a theta-burst protocol (12 bursts at 5 Hz of 5 stimuli at 100 Hz, Larson et al, 1986) was used to induce LTP no significant difference was observed between genotypes (Fig. 4B). We also investigated protein synthesis-dependent late-LTP (L-LTP) in Df(16)A+/− mice using a strong induction protocol of 200 Hz stimulation (40 stimuli at 200 Hz, repeated 10 times at 5 sec intervals, Paterlini et al., 2005) applied three times at 5 minute intervals. Responses were recorded for 2.5 hrs post-induction. At the end of this period, the induction protocol was applied again to check slice viability. Over the entire recording period the two genotypes showed comparable levels of potentiation, including the initial responses and the potentiation induced at the end (Fig. 4C). These data together suggest that the mechanisms that mediate long-term synaptic potentiation in CA1 pyramidal neurons are likely intact in Df(16)A+/− mice but that under certain conditions the induction of this plasticity is disrupted.
To test if induction of LTP by two 100 Hz trains of 1 sec was similarly affected at the other major input pathway to CA1 pyramidal neurons we repeated this paradigm at the TA pathway. The magnitude of potentiation at this pathway was less than at SC inputs but both genotypes showed similar increases in synaptic strength (Fig. 4D), arguing that diminished synaptic potentiation in response to this induction protocol is not ubiquitous.
LTD at SC inputs was tested by stimulation of these afferents at 1 Hz for 15 min (i.e. 900 pulses) in slices from younger (4 week old) animals, an age at which this form of LTD is NMDA receptor-dependent (Dudek and Bear, 1992). In WT and Df(16)A+/− mice, synaptic strength was decreased by approximately 30% 1 hr after the induction of LTD, indicating that this form of plasticity is functionally normal in Df(16)A+/− mice (Fig. 4E). Finally, depotentiation of enhanced fEPSPs was tested in a subsample of slices from adult mice that had undergone LTP induced by two trains at 100 Hz; 1 Hz stimulation was given for 15 min and the resultant decrease in fEPSP monitored for 30 min. Normalizing to the last 10 samples 1 hr after potentiation, fEPSPs were depotentiated to a similar degree in both genotypes (Fig. 4F).
Df(16)A+/− mice perform the hippocampus-dependent Morris water maze task at control levels
Having undertaken these physiological assays of hippocampal function we sought to determine if Df(16)A/+ mice showed differences in the Morris water maze (MWM, Morris, 1984; Fig. 5A), a behavioral task that assesses spatial reference memory and is dependent on hippocampal function generally (Morris et al. 1982) and CA1 plasticity in particular (Tsien et al, 1996).
During the initial training phase of the MWM, when animals swim to a visibly marked platform, there was no significant difference between Df(16)A/+ and WT mice in the distance traveled or time taken to reach the platform (data not shown). The distance the mice swam to reach the platform decreased over successive trials but there was no significant interaction of trial number with genotype (data not shown). These results suggest that Df(16)A/+ mice have normal motivational levels and visuomotor skills.
During the hidden platform training phase, again no significant differences were found between Df(16)A/+ mice and their WT littermates in either the distance travelled or time to reach the platform (data not shown). Finally, during the probe trials, where the platform was removed and the time spent searching for it in its previous location was measured, mice spent significantly more time in the target quadrant than in all others. However, there was no significant effect of genotype on the percent time spent within the quadrants for either the 1 hr probe or the 24 hr probe trial (Fig. 5B). Additionally, measures of the total number of platform crossings, the average distance from the platform, and the total distance traveled during the 1 hr and 24 hr probe trials (Fig. 5C) did not differ between genotypes. Overall, these data indicate that there were no deficits in the retention or decay of short- or long-term reference memory in the Df(16)A/+ mice.
Novelty-induced c-Fos expression is selectively decreased in the CA3 hippocampal subfield of Df(16)A+/− mice
Finally, we looked at neuronal activation in the hippocampal circuit following exploration of a novel environment, as indicated by the induction of c-Fos expression. Control Df(16)A/+ and WT subjects taken from their home cages showed no differences in the number of c-Fos positive principal neurons located in either CA1, CA3 or dentate gyrus (DG) (Figs. 6a–C, E). In CA3 and CA1 of control mice, expression of c-Fos was extremely low, with numerous sections containing no positive neurons (Figs. 6A–C) whereas in DG, control animals had low numbers of positive cells distributed throughout the supra- and infrapyramidal blades (Fig. 6E).
Fig. 6. c-Fos activation in principal neurons of the hippocampus in response to exploration of a novel environment.
(A) Representative images of c-Fos staining in the CA3 subfield of the hippocampus, left, are home-cage controls and right are images from animals exposed to novel environments. Upper is WT and lower in Df(16)A/+. (B) Quantification of the c-Fos expression in CA3. In the home cage mutant and WT animals displayed similar levels of positive cells (7.9 ± 2.6 pyramidal cells per section in WT, n = 5 and 6.0 ± 2.0 in Df(16)A+/−, n = 5, p = 0.59), whereas activation of CA3 by exposure to the novel environment was weaker in Df(16) A+/− mice than WT controls (52.1 ± 4.9 pyramidal cells per section in WT, n = 9, and 38.7 ± 2.6 in Df(16)A+/−, n = 10, p = 0.023). (C) Quantification of the c-Fos expression in CA1. In both the home cage condition and after novel environment exploration the number of c-Fos positive pyramidal neurons showed no significant differences between genotypes (Home cage: 3.8 ± 1.8 pyramidal cells per section in WT, n = 5, and 2.6 ± 1.3 in Df(16)A+/−, n = 5, p = 0.60. Novel environment: 156.1 ± 16.1 pyramidal cells per section in WT, n = 9, and 133.6 ± 15.7 in Df(16)A+/−, n = 10, p = 0.33). (D) Automated cell counts of c-Fos positive pyramidal neurons in CA1 (left) and CA3 (right) gave similar results to those gained using visual inspection. (CA1: (117.7 ± 23.1 pyramidal cells per section in WT, n = 9, and 91.7 ± 21.5 in Df(16)A+/−, n = 10, p = 0.42. CA3: 42.7 ± 5.7 pyramidal cells per section in WT, n = 9, and 28.6 ± 3.8 in Df(16)A+/−, n = 10, p = 0.049). (E) Quantification of the c-Fos expression in the dentate gyrus. Novel environment exposure induced robust c-Fos expression in the suprapyramidal blade of the dentate. This increase was comparable between genotypes. Likewise no effect of genotype was observed in the home cage controls or in the infrapyramidal blade following exploration (Home cage: Infrapyramidal blade: 12.2 ± 2.5 granule cells per section in WT, n = 5, and 14.1 ± 3.1 in Df(16)A+/−, n = 5, p = 0.65. Suprapyramidal blade: 12.5 ± 1.7 in WT, n = 5, and 13.2 ± 1.6 in Df(16)A+/−, n = 5, p = 0.77. Novel environment: Infrapyramidal blade: 9.5 ± 0.9 granule cells per section in WT, n = 9, and 8.6 ± 1.1 in Df(16)A+/−, n = 10, p = 0.54. Suprapyramidal blade: 27.2 ± 1.4 in WT, n = 9, and 26.2 ± 2.0 in Df(16)A+/−, n = 10, p = 0.70).
In all mice, exploration of the novel environment induced extensive expression of c-Fos throughout the hippocampus (Papa et al, 1993). In both CA subfields large numbers of principal neurons were activated by novel environment exposure. CA1 contained the highest (and most variable) density of pyramidal neurons expressing c-Fos but despite a slight trend to lower numbers in Df(16)A/+ mice no significant differences were observed here using either manual counts (Fig 6B) or totals given by automated cell counting software (Fig. 6D). In contrast, in CA3 the number of pyramidal neurons that expressed c-Fos after exploration was 25.7% lower (p = 0.023) in Df(16)A/+ mice than in WT controls (Figs. 6A and C). The distribution of activated cells was similar in both genotypes with a pronounced absence in staining around the CA3c region. This difference was confirmed using automated counts that, despite slightly lower overall cell counts, showed 33% (p = 0.049) fewer positive cells in the mutant mice (Fig. 6D).
Finally, consistent with previous studies, the activation of the DG was sparse (approximately a twofold increase over controls) and increases in c-fos expression were limited to the suprapyramidal blade (Chawla et al, 2005). This pattern of activation was similar in mice of both genotypes with no significant differences observed (Fig. 6E).
Discussion
In this study we have investigated hippocampal function in a mouse model of 22q11.2 deletion syndrome, a human condition associated with a 25–30% chance of developing SCZ (Pulver et al., 1994; Murphy et al., 1999). The results presented show a number of modest and specific disruptions of hippocampal physiology in the CA1 region. Recordings of spontaneous inhibitory input showed a decrease in Df(16)A+/− mice indicative of a decrease in the activity of interneurons. In addition, pyramidal neurons (when held at −65 mV) fired less in response to sustained strong depolarization and LTP at SC inputs to CA1 was reduced in response to trains of 100 Hz stimulation, although not to theta burst or 200 Hz bursts stimuli. To investigate the impact of these physiological changes we undertook a behavioral study and investigated activation of the hippocampus by novelty. In the MWM, preservation of short- and long-term spatial reference memory was observed in Df(16)A+/− mice. However, exploration of a novel environment revealed reduced recruitment of CA3 pyramidal neurons in mutant mice. These results suggest a pattern of changes in hippocampal function that may play a role in the altered cognitive function and/or susceptibility to psychosis in 22q11.2 deletion carriers.
The intrinsic properties of CA1 pyramidal neurons were largely unchanged, except that when resting potential was normalized Df(16)A+/− neurons fired less in response to sustained strong depolarizations due to increased spike frequency accommodation. It should be noted that although the resting potential did not significantly differ between genotypes, mutant neurons sat on average slightly more depolarized than WT controls. Accommodation is caused by accumulative activation of IM and IAHP potassium currents (Lancaster and Adams, 1986; Lancaster and Nicoll, 1987); expression and regulation of these channels needs to be assessed further in this model as dysregulation could affect cognition (Matthews et al, 2009).
Assessing the neuronal connectivity in any psychiatric disease model is essential and so we assayed in a number of ways excitatory and inhibitory input to CA1. Interestingly, our investigation of inhibitory input to CA1 pyramidal neurons yielded evidence of a deficit in interneuron function. Properties of miniature IPSCs and the amplitude of evoked IPSCs were all unchanged in Df(16)A+/− mice, indicative of a normal density and strength of inhibitory synapses onto pyramidal cells. By contrast, the rate of spontaneous IPSCs was robustly reduced in mutant neurons suggesting that interneuron function is compromised, i.e. in the context of the unstimulated slice preparation, interneurons were less active in Df(16)A+/− mice. Attempts to find a cause for decreased interneuron activity (in the physiology of interneurons) or a straightforward consequence of it (in measurements of feedforward inhibition and its effects on CA1 output) did not show clear differences and, overall, argue against a frank disinhibition of CA1 in Df(16)A+/− mice. Nevertheless, aberrant interneuron physiology would affect not only the degree of inhibition but also its timing and this could have profound effects on neural activity (Klausberger and Somogyi, 2008). In the future, more detailed studies of interneuron physiology are required, including measurement of their firing in awake, behaving mice (Nitz and McNaughton, 2004). It is of interest to note, the recent demonstration that genetic silencing of parvalbumin expressing interneurons specifically in CA1 impaired spatial working memory without impacting spatial reference memory (Murray et al, 2011), a pattern of deficits similar to those seen in Df(16)A+/− mice.
Multiple assays of excitatory input to CA1 showed that in Df(16)A+/− mice these parameters did not significantly deviate from WT levels. These results suggest that excitatory drive to CA1 is functionally preserved in the mutant mice, a conclusion consistent with the ability of these mice to successfully complete the MWM task and the recording of normal theta oscillations in CA1 in vivo (Sigurdsson et al, 2010). Previous data showed decreased spine density on cultured hippocampal neurons as well as CA1 pyramidal neurons in Df(16)A+/− mice (Mukai et al, 2008). Whereas in cultured hippocampal neurons there was good agreement between decreased spine density and reduced mEPSC frequency (Mukai et al, 2008), in slice preparations there is an apparent disconnect between these parameters. One caveat is that spine density was assessed using basal dendrites whereas evoked inputs assayed here were to apical dendrites. However, the rate of mEPSCs recorded also did not differ. One possibility that we tested was that Df(16)A+/− mice had fewer silent synapses (i.e. a lower proportion of AMPAR-lacking synapses would normalize the number of functional synapses, Isaacs et al, 1995), however, taking ratios of NMDAR to AMPAR-mediated currents showed no difference between genotypes. Subtle compensatory changes in presynaptic release probability (that do not impact paired-pulse ratios) or changes in the distribution of excitatory synaptic inputs could account for these differences.
In contrast to basal synaptic transmission, our studies of synaptic plasticity at excitatory inputs showed a significant deficit in LTP evoked by 100 Hz stimulation of SC inputs. However, two other stimulation protocols at SC inputs (theta burst (Larson et al, 1986) and 200 Hz bursts (Paterlini et al, 2005)) and 100 Hz stimulation of TA inputs induced equivalent levels of potentiation in the two genotypes. In the case of the 200 Hz induction protocol, LTP was maintained at similar levels for 2.5 hrs, i.e. through the period where it becomes dependent on protein synthesis (Frey et al, 1988). LTD and depotentiation were also similar in both genotypes. Together these data suggest that the mechanisms mediating synaptic plasticity in CA1 pyramidal neurons are likely intact in Df(16)A+/− mice but that their engagement by strong synaptic drive varies according to input pattern. Hence, it needs to be determined if specific (particularly long) stimulation trains of certain frequencies or patterns depolarize target neurons to a lesser extent in mutant mice, causing less NMDAR-mediated calcium influx and recruitment of downstream signaling. In this context, the decreased spiking of pyramidal neurons in response to sustained strong-depolarization may also affect the induction of plasticity.
Hippocampal synaptic plasticity is implicated in spatial learning. We therefore tested Df(16)A+/− mice in the MWM, an assay of short and long-term spatial reference memory that is sensitive to hippocampal lesions (Morris et al., 1982) and NMDA receptor blockade (Morris et al., 1986). Mutant and WT mice completed this task with similar efficacies. This is consistent with the unchanged theta burst-induced LTP, where the induction protocol is considered more related to physiological patterns of inputs received by CA1 than long 100 Hz trains (Larson et al, 1986).
Further evidence for relatively spared CA1 function came from our investigation of hippocampal activation during the exploration of a novel environment (Papa et al, 1993; Kubik et al, 2007). Interestingly, however, this experiment provided strong evidence for a decrease in the number of CA3 pyramidal neurons recruited. Immunoreactivity for the IEG c-Fos, a marker of neuronal activity (Dragunow and Faull, 1989), was reduced by 25–33% in CA3 but expressed at around WT levels in dentate granule cells and CA1 pyramidal neurons. Therefore, determining if CA3 hypoactivity is a key feature of these mice will be an important future direction of this work.
CA3 pyramidal neurons’ primary inputs are from dentate granule cells, the entorhinal cortex and each other (via extensive collateral projections); deficits in any of these pathways could underlie the reduced activation and future work will need to address if any of them are impaired or if the intrinsic properties of CA3 pyramidal neurons themselves are altered. Evidence for disinhibition in CA1 appears to stand in contrast to this finding of reduced pyramidal neuron activation in CA3 and suggests that changes in inhibitory interneuron activity may be subregion specific. However, it may be the case that altered interneuron function influences microcircuit activity in more complex ways. Future work will characterize interneuron physiology in CA3 and other hippocampal regions. That comparable numbers of granule cells were c-Fos positive suggests that the number of active mossy fibers in CA3 was similar but whether granule cell firing and/or presynaptic physiology are altered remains to be determined. Gene expression changes in granule cells have been observed in another mouse model of 22q11.2 deletion syndrome (Jurata et al. 2006) and alterations in dentate structure and function have been observed in a DISC1 mouse model of SCZ (Kvajo et al, 2008). Given that the more sparsely active CA3 region gives rise to the majority of excitatory input to CA1, the relatively similar activation of the latter region between genotypes may reflect an ability of entorhinal cortical inputs to drive these cells (Brun et al, 2002; Nakashiba et al, 2008). Alternatively, it is worth consideration that if interneuron activity is reduced in CA1, this disinhibition may allow SC inputs to more efficiently recruit CA1 pyramidal neurons under physiological conditions. Overall, a detailed analysis of hippocampal neuronal firing rates in vivo will be informative.
These results suggest a focus on CA3 and dentate function in Df(16)A+/− mice is required. Selective ablation of the obligatory NMDA receptor subunit NR1 (and so most associative synaptic plasticity) in the principal cells of CA3 did not change animals ability to complete the standard form of the MWM task, but did impair performance on a version of the task dependent on “pattern completion” in which most spatial cues were removed (Nakazawa et al, 2002). Hence, to further probe hippocampal dysfunction evaluation of Df(16)A+/− mice on tests of pattern completion, pattern separation (McHugh et al, 2007) and rapid learning (Nakazawa et al, 2003), which are more dependent on the dentate-CA3 network, will be needed. Interestingly, in his review of the SCZ neuropathology literature Harrison (2004) noted a relative sparing of CA1 compared to other subfields. Additionally, others have argued for changes in CA3 (Tamminga et al, 2010) and dentate (Kobayashi, 2009) function as possible substrates for pathophysiological changes in SCZ.
During the preparation of this manuscript, Earls et al. (2010) reported on another 22q11.2 deletion mouse model; Df(16)l/− (Paylor et al., 2001) that carries a smaller size deletion. Specifically, this model has 5 fewer genes (Dgcr2, Stk22a, Stk22b, Mrpl40 and Hira) deleted than the mice used in our study, all five of which are included in the typical human 1.5 Mb deletions (“SCZ critical region”; Karayiorgou et al., 1995; 2010). Age dependent (i.e. absent at 6–8 weeks, present at 16–20 weeks) enhancement of glutamate release from SC terminals and increased LTP (rather than reduced, as in the present study) at these synapses were observed; changes due, at least in part, to dysregulation of SERCA2 expression. Earls et al (2010) also reported deficits in the MWM assay, a result also in contrast with our data, despite us using animals 20 weeks or older for our MWM assay. This discrepancy in behavioral phenotypes is one of a number of such differences between the two strains [compare Paylor et al. (2001) to Stark et al. (2008); see also Drew et al. (2010)]. The reasons for the discrepancies between these studies are unclear; they may be related to procedural differences in the housing, training and testing protocols employed or they may reflect an important role of one or more of the differentially targeted genes and such discrepancies appear to also extend to physiological alterations.
Cataloguing the physiological properties of different brain regions, implicated in the pathophysiology of SCZ, in mouse models of bona fide genetic risk factors for this disease and comparison of these properties to observed behavioral and systems-level activity changes will allow us to build models of key pathogenic processes (Arguello and Gogos, 2006; Karayiorgou et al, 2010; Nestler and Hyman, 2010). As such the present work will serve as a framework for assessing the role of the hippocampus in expanded phenotyping of this and related models of SCZ.
Supplementary Material
(A) fEPSP PPRs at 20, 50 and 100 msec interstimulus intervals did not differ between genotypes. 20 msec: WT: 1.30 ± 0.07, n = 14; Df(16)A+/−: 1.34 ± 0.08, n = 12 (p = 0.71), 50 msec: WT: 1.38 ± 0.03, n = 14; Df(16)A+/−: 1.33 ± 0.03, n = 12 (p = 0.30), 100 msec: WT: 1.26 ± 0.03, n = 14; Df(16)A+/−: 1.19 ± 0.03, n = 12 (p = 0.26). (B) EPSCs decays were fitted with monoexponential curves; taus were not different between genotypes (WT: 7.74 ± 0.53 msec, n = 13; Df(16)A+/−: 7.61 ± 0.53, n = 16; p = 0.864). (C) Average 10–90% rise times for WT and Df(16)A+/− neurons were not significantly different (WT: 2.48 ± 0.13 msec, n = 17; Df(16)A+/: 2.30 ± 0.07, n = 28; p = 0.22). (D) Example traces showing eEPSCs recorded at −60 mV (inward current) and +60 mV (outward current). Upper; WT, lower; Df(16)A+/−. (E) The ratio of NMDA receptor-mediated current (measured 3× tau after the AMPA peak) to the AMPA receptor-mediated current was not significantly different between genotypes (WT: 0.49 ± 0.04, n = 4; Df(16)A+/−: 0.56 ± 0.11, n = 5; p = 0.313). (F) Example traces showing fEPSPs recorded in CA1 SLM response to TA stimulation at incremental intensities (3–24 V). Upper, WT; lower, Df(16)A+/−. (G) fEPSPs recorded in SLM of CA1 and evoked by extracellular stimulation of TA inputs were not different between genotypes (2-way repeated measures ANOVA, genotype: p = 0.46, genotype × stimulus intensity: p = 0.92, WT: n = 11, Df(16)A+/−: n = 11). (H) Paired-pulse facilitation (inter-stimulus interval, 50 msec) was not significantly affected by the mutation. Left, SC (WT: 1.51 ± 0.02, n = 73; Df(16)A+/−: 1.53 ± 0.02, n = 64; p = 0.36), right, TA (WT: 1.81 ± 0.14, n = 7; Df(16)A+/−: 2.03 ± 0.13, n = 10; p = 0.26)
Disynaptic IPSCs were evoked by SC stimulation and recorded at −40 mV, between the two stimuli the neuron was depolarized to 0 mV for 5 sec. (A) Suppression of the eIPSC was seen in both genotypes to a comparable degree. WT: 71.96 ± 8.94% of control, n = 7; Df(16)A+/−: 75.72 ± 4.26%, n = 13 (p = 0.67). (B) Example traces of control and suppressed GABAergic (outward) currents. Left, WT; right, Df(16)A+/−.
(A) Example traces from a WT neuron, top, and a Df(16)A+/− neuron, bottom, of glutamatergic currents, inwards, and GABAergic currents outwards (holding currents subtracted for clarity). (B) Left, the average glutamatergic current evoked at −60 mV following normalization to around 275 pA by adjusting the stimulation intensity (WT: 275.5 ± 25.8 pA, n = 14; Df(16)A+/−: 277.4 ± 24.4 pA, n = 19; p = 0.96), right, the average corresponding GABAergic current recorded at 0 mV did not differ between genotypes (WT: 829.0 ± 108.7 pA, n = 14; Df(16)A+/−: 917.4 ± 148.1 pA, n = 19; p = 0.66). (C) The ratio of the GABAergic current to the glutamatergic current was calculated for each neuron and the values were similar between genotypes (WT: 3.42 ± 0.54, n = 14; Df(16)A+/−: 3.77 ± 0.68, n = 19; p = 0.71). (D) Paired-pulse (interval: 100 msec) depression of second IPSC was similar in each genotype (WT: 0.93 ± 0.07, n = 7; Df(16)A+/−: 0.94 ± 0.07, n = 8; p = 0.97). (E) Shown are the corresponding glutamatergic and GABAergic currents in individual neurons at incremental stimulus intensities (typically 3, 6, 9 V). Each line is a neuron, WT are black boxes with solid lines, Df(16)A+/− are white circles with dotted lines. The populations are overlapping.
(A) Example data from a WT slice, top, and a Df(16)A+/− slice, bottom, showing the sigmoidal increase in spiking output in response to a linear increase in the input as assayed by the fEPSP. (B) The input and output were normalized for each slice and pooled for each genotype, the average input-output curves were similar. To compare genotypes, for each slice, from the fitted sigmoid curve, the normalized, (C), threshold (the fEPSP that gave a 50% spiking output, WT: 0.46 ± 0.04, n = 10; Df(16)A+/−: 0.46 ± 0.03, n = 13; p = 0.92) and, (D), slope (WT: 0.12 ± 0.01, n = 10; Df(16)A+/−: 0.10 ± 0.01, n = 13; p = 0.17) were taken and compared across genotypes – neither differed significantly.
(A) Example traces of the typical firing pattern associated with each class of interneuron in response to 500 msec rectangular injections of depolarizing currents. Left, Fast-spiking interneurons, shown are the three types of firing observed, large somatic current injections were required to evoke firing (see C) although small synaptic inputs to the dendrites could evoked firing (data not shown). Center, Regular-spiking interneurons fired in a graded fashion with low thresholds. Top trace 25 pA injection, bottom, 200 pA. Right, example of a slow spiking interneuron to 200 pA injection. Other properties of the neurons co-varied with firing pattern (see Table S1). In SO/alveus, many regular spiking cells are likely to be OLM, bistratifed and trilaminar neurons. Fast spiking interneurons normally express parvalbumin and are typically basket or axo-axonic neurons that target the cell body or axon hillock of pyramidal neurons. Four neurons were classified as “slow” spiking, the identity of these cells is unclear but their firing pattern was very distinctive and characterized by small afterhyperpolarizations. (B) Pie charts showing the proportions of each subtype of neuron found in Df(16)A+/− and WT slices. Proportions did not differ significantly between genotypes. 4/17 (22.2%) WT and 5/20 (25%, Chi-square, P = 0.795) Df(16)A+/− neurons showed spontaneous firing. 4/10 (25%) WT and 4/12 (33.3%, P = 0.746) Df(16)A+/− of regular spiking neurons displayed rebound firing after injection of 100 pA hyperpolarizing current. (C) Left, average AP thresholds for fast-spiking interneurons (WT: −35.43 ± 2.54, Df(16)A+/− −37.24 ± 1.91, P = 0.581). Average rheobase values for evoking a single spike, a partial train or a full train (as in A) from resting potential (center) and −60 mV (right). For all comparisons, P > 0.05.
Supp Table 1. Intrinsic properties of and spontaneous excitatory synaptic input to inhibitory interneurons.
Acknowledgments
This work was supported by US National Institute of Mental Health grants MH67068 (to MK and JAG) and MH077235 (to JAG) and a Simons Foundation Grant (to JAG). LJD and KLS have been supported in part by NARSAD Young Investigator Awards. We thank Heather McKellar and Greg Scherrer for assistance with the c-Fos experiment, Chi-Kun Tong for technical assistance, Gregg Crabtree for discussion and Megan Sribour, Allie Abrams-Downey and Yan Sun for maintenance of the mouse colony.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arguello PA, Gogos JA. Modeling madness in mice: one piece at a time. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Bassett AS, Marshall CR, Lionel AC, Chow EW, Scherer SW. Copy number variations and risk for schizophrenia in 22q11.2 deletion syndrome. Hum Mol Genet. 2008;17:4045–4053. doi: 10.1093/hmg/ddn307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béïque JC, Lin DT, Kang MG, Aizawa H, Takamiya K, Huganir RL. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl Acad Sci USA. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- Brunet A, Armengol L, Pelaez T, Guillamat R, Vallès V, Gabau E, Estivill X, Guitart M. Failure to detect the 22q11.2 duplication syndrome rearrangement among patients with schizophrenia. Behav Brain Funct. 2008;4:10. doi: 10.1186/1744-9081-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho TP, Buonomano DV. Differential effects of excitatory and inhibitory plasticity on synaptically driven neuronal input-output functions. Neuron. 2009;61:774–785. doi: 10.1016/j.neuron.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK, Worley PF, McNaughton BL, Barnes CA. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15:579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- Creager R, Dunwiddie T, Lynch G. Paired-pulse and frequency facilitation in the CA1 region of the in vitro rat hippocampus. J Physiol. 1980;299:409–424. doi: 10.1113/jphysiol.1980.sp013133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Guérineau NC, Gähwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol. 1996;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debbané M, Schaer M, Farhoumand R, Glaser B, Eliez S. Hippocampal volume reduction in 22q11.2 deletion syndrome. Neuropsychologia. 2006;44:2360–2365. doi: 10.1016/j.neuropsychologia.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Drew LJ, Crabtree GW, Markx S, Stark KL, Chaverneff F, Xu B, Mukai J, Fenelon K, Hsu PK, Gogos JA, Karayiorgou M. The 22q11.2 microdeletion: Fifteen years of insights into the genetic and neural complexity of psychiatric disorders. Int J Dev Neurosci. 2010 doi: 10.1016/j.ijdevneu.2010.09.007. Epub. Oct 8. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls LR, Bayazitov IT, Fricke RG, Berry RB, Illingworth E, Mittleman G, Zakharenko SS. Dysregulation of presynaptic calcium and synaptic plasticity in a mouse model of 22q11 deletion syndrome. J Neurosci. 2010;30:15843–15855. doi: 10.1523/JNEUROSCI.1425-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann L, Pandita RK, Morrow BE. Low-copy repeats mediate the common 3-Mb deletion in patients with velo-cardio-facial syndrome. Am J Hum Genet. 1999;64:1076–1086. doi: 10.1086/302343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J, Shifman S. Animal models of psychiatric disease. Curr Opin Genet Dev. 2008;18:235–240. doi: 10.1016/j.gde.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Liddle PF, Frith CD, Hirsch SR, Frackowiak RS. The left medial temporal region and schizophrenia: a PET study. Brain. 1992;115:367–382. doi: 10.1093/brain/115.2.367. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Perez J, Broome M, Borgwardt S, Placentino A, Caverzasi E, Cortesi M, Veggiotti P, Politi P, Barale F, McGuire P. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2007;31:465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Isaac JTR, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Jurata LW, Gallagher P, Lemire AL, Charles V, Brockman JA, Illingworth EL, Altar CA. Altered expression of hippocampal dentate granule neuron genes in a mouse model of human 22q11 deletion syndrome. Schizophr Res. 2006;88:251–259. doi: 10.1016/j.schres.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, Gos A, Nestadt G, Wolyniec PS, Lasseter VK, Eisen H, Childs B, Kazazian HH, Kucherlapati R, Antonarakis SE, Pulver AE, Housman DE. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci USA. 1995;92:7612–7616. doi: 10.1073/pnas.92.17.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M, Gogos JA. The molecular genetics of the 22q11-associated schizophrenia. Brain Res Mol Brain Res. 2004;132:95–104. doi: 10.1016/j.molbrainres.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11:402–416. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Suzuki M, Maeda Y, Urata K, Yamaguchi N, Matsuda H, Hisada K, Suzuki M, Takashima T. Regional cerebral blood flow in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1992;241:195–200. doi: 10.1007/BF02190252. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K. Targeting the hippocampal mossy fiber synapse for the treatment of psychiatric disorders. Mol Neurobiol. 2009;39:24–36. doi: 10.1007/s12035-008-8049-5. [DOI] [PubMed] [Google Scholar]
- Kubik S, Miyashita T, Guzowski JF. Using immediate-early genes to map hippocampal subregional functions. Learn Mem. 2007;14:758–770. doi: 10.1101/lm.698107. [DOI] [PubMed] [Google Scholar]
- Kvajo M, McKellar H, Arguello PA, Drew LJ, Moore H, MacDermott AB, Karayiorgou M, Gogos JA. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci USA. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986;55:1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2006;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews EA, Linardakis JM, Disterhoft JF. The fast and slow afterhyperpolarizations are differentially modulated in hippocampal neurons by aging and learning. J Neurosci. 2009;29:4750–4755. doi: 10.1523/JNEUROSCI.0384-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11:543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Mukai J, Dhilla A, Drew LJ, Stark KL, Cao L, MacDermott AB, Karayiorgou M, Gogos JA. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat Neurosci. 2008;11:1302–1310. doi: 10.1038/nn.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Murray AJ, Sauer J-F, Riedel G, MaClure C, Ansel L, Cheyne L, Bartos M, Wisden W, Wulff P. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci. 2011 doi: 10.1038/nn.2751. Epub. Jan 30. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319:1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitz D, McNaughton B. Differential modulation of CA1 and dentate gyrus interneurons during exploration of novel environments. J Neurophysiol. 2004;91:863–872. doi: 10.1152/jn.00614.2003. [DOI] [PubMed] [Google Scholar]
- Paylor R, McIlwain KL, McAninch R, Nellis A, Yuva-Paylor LA, Baldini A, Lindsay EA. Mice deleted for the DiGeorge/velocraniofacial syndrome region show abnormal sensorimotor gating and learning and memory impairments. Hum Mol Genet. 2001;10:2645–2650. doi: 10.1093/hmg/10.23.2645. [DOI] [PubMed] [Google Scholar]
- Papa M, Pellicano MP, Welzl H, Sadile AG. Distributed changes in c-Fos and c-Jun immunoreactivity in the rat brain associated with arousal and habituation to novelty. Brain Res Bull. 1993;32:509–515. doi: 10.1016/0361-9230(93)90299-q. [DOI] [PubMed] [Google Scholar]
- Paterlini M, Zakharenko SS, Lai WS, Qin J, Zhang H, Mukai J, Westphal KG, Olivier B, Sulzer D, Pavlidis P, Siegelbaum SA, Karayiorgou M, Gogos JA. Transcriptional and behavioral interaction between 22q11.2 orthologs modulates schizophrenia-related phenotypes in mice. Nat Neurosci. 2005;8:1586–1594. doi: 10.1038/nn1562. [DOI] [PubMed] [Google Scholar]
- Pouille F, Marin-Burgin A, Adesnik H, Atallah BV, Scanziani M. Input normalization by global feedforward inhibition expands cortical dynamic range. Nat Neurosci. 2009;12:1577–15785. doi: 10.1038/nn.2441. [DOI] [PubMed] [Google Scholar]
- Pulver AE, Nestadt G, Goldberg R, Shprintzen RJ, Lamacz M, Wolyniec PS, Morrow B, Karayiorgou M, Antonarakis SE, Housman D, Kucheriapati R. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J Nerv Ment Dis. 1994;182:476–478. doi: 10.1097/00005053-199408000-00010. [DOI] [PubMed] [Google Scholar]
- Roberts GW. Schizophrenia: the cellular biology of a functional psychosis. Trends Neurosci. 1990;13:207–211. doi: 10.1016/0166-2236(90)90161-3. [DOI] [PubMed] [Google Scholar]
- Scambler PJ. The 22q11 deletion syndromes. Hum Mol Genet. 2000;9:2421–2426. doi: 10.1093/hmg/9.16.2421. [DOI] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh TH, Kurahashi H, Saitta SC, O’Hare AM, Hu P, Roe BA, Driscoll DA, McDonald-McGinn DM, Zackai EH, Budarf ML, Emanuel BS. Chromosome 22-specific low copy repeats and the 22q11.2 deletion syndrome: genomic organization and deletion endpoint analysis. Hum Mol Genet. 2000;9:489–501. doi: 10.1093/hmg/9.4.489. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, Wan X, Pavlidis P, Mills AA, Karayiorgou M, Gogos JA. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40:751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Tan GM, Arnone D, McIntosh AM, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies in chromosome 22q11.2 deletion syndrome (velocardiofacial syndrome) Schizophr Res. 2009;115:173–181. doi: 10.1016/j.schres.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Xu B, Roos JL, Levy S, van Rensburg EJ, Gogos JA, Karayiorgou M. Strong association of de novo copy number mutations with sporadic schizophrenia. Nat Genet. 2008;40:880–885. doi: 10.1038/ng.162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) fEPSP PPRs at 20, 50 and 100 msec interstimulus intervals did not differ between genotypes. 20 msec: WT: 1.30 ± 0.07, n = 14; Df(16)A+/−: 1.34 ± 0.08, n = 12 (p = 0.71), 50 msec: WT: 1.38 ± 0.03, n = 14; Df(16)A+/−: 1.33 ± 0.03, n = 12 (p = 0.30), 100 msec: WT: 1.26 ± 0.03, n = 14; Df(16)A+/−: 1.19 ± 0.03, n = 12 (p = 0.26). (B) EPSCs decays were fitted with monoexponential curves; taus were not different between genotypes (WT: 7.74 ± 0.53 msec, n = 13; Df(16)A+/−: 7.61 ± 0.53, n = 16; p = 0.864). (C) Average 10–90% rise times for WT and Df(16)A+/− neurons were not significantly different (WT: 2.48 ± 0.13 msec, n = 17; Df(16)A+/: 2.30 ± 0.07, n = 28; p = 0.22). (D) Example traces showing eEPSCs recorded at −60 mV (inward current) and +60 mV (outward current). Upper; WT, lower; Df(16)A+/−. (E) The ratio of NMDA receptor-mediated current (measured 3× tau after the AMPA peak) to the AMPA receptor-mediated current was not significantly different between genotypes (WT: 0.49 ± 0.04, n = 4; Df(16)A+/−: 0.56 ± 0.11, n = 5; p = 0.313). (F) Example traces showing fEPSPs recorded in CA1 SLM response to TA stimulation at incremental intensities (3–24 V). Upper, WT; lower, Df(16)A+/−. (G) fEPSPs recorded in SLM of CA1 and evoked by extracellular stimulation of TA inputs were not different between genotypes (2-way repeated measures ANOVA, genotype: p = 0.46, genotype × stimulus intensity: p = 0.92, WT: n = 11, Df(16)A+/−: n = 11). (H) Paired-pulse facilitation (inter-stimulus interval, 50 msec) was not significantly affected by the mutation. Left, SC (WT: 1.51 ± 0.02, n = 73; Df(16)A+/−: 1.53 ± 0.02, n = 64; p = 0.36), right, TA (WT: 1.81 ± 0.14, n = 7; Df(16)A+/−: 2.03 ± 0.13, n = 10; p = 0.26)
Disynaptic IPSCs were evoked by SC stimulation and recorded at −40 mV, between the two stimuli the neuron was depolarized to 0 mV for 5 sec. (A) Suppression of the eIPSC was seen in both genotypes to a comparable degree. WT: 71.96 ± 8.94% of control, n = 7; Df(16)A+/−: 75.72 ± 4.26%, n = 13 (p = 0.67). (B) Example traces of control and suppressed GABAergic (outward) currents. Left, WT; right, Df(16)A+/−.
(A) Example traces from a WT neuron, top, and a Df(16)A+/− neuron, bottom, of glutamatergic currents, inwards, and GABAergic currents outwards (holding currents subtracted for clarity). (B) Left, the average glutamatergic current evoked at −60 mV following normalization to around 275 pA by adjusting the stimulation intensity (WT: 275.5 ± 25.8 pA, n = 14; Df(16)A+/−: 277.4 ± 24.4 pA, n = 19; p = 0.96), right, the average corresponding GABAergic current recorded at 0 mV did not differ between genotypes (WT: 829.0 ± 108.7 pA, n = 14; Df(16)A+/−: 917.4 ± 148.1 pA, n = 19; p = 0.66). (C) The ratio of the GABAergic current to the glutamatergic current was calculated for each neuron and the values were similar between genotypes (WT: 3.42 ± 0.54, n = 14; Df(16)A+/−: 3.77 ± 0.68, n = 19; p = 0.71). (D) Paired-pulse (interval: 100 msec) depression of second IPSC was similar in each genotype (WT: 0.93 ± 0.07, n = 7; Df(16)A+/−: 0.94 ± 0.07, n = 8; p = 0.97). (E) Shown are the corresponding glutamatergic and GABAergic currents in individual neurons at incremental stimulus intensities (typically 3, 6, 9 V). Each line is a neuron, WT are black boxes with solid lines, Df(16)A+/− are white circles with dotted lines. The populations are overlapping.
(A) Example data from a WT slice, top, and a Df(16)A+/− slice, bottom, showing the sigmoidal increase in spiking output in response to a linear increase in the input as assayed by the fEPSP. (B) The input and output were normalized for each slice and pooled for each genotype, the average input-output curves were similar. To compare genotypes, for each slice, from the fitted sigmoid curve, the normalized, (C), threshold (the fEPSP that gave a 50% spiking output, WT: 0.46 ± 0.04, n = 10; Df(16)A+/−: 0.46 ± 0.03, n = 13; p = 0.92) and, (D), slope (WT: 0.12 ± 0.01, n = 10; Df(16)A+/−: 0.10 ± 0.01, n = 13; p = 0.17) were taken and compared across genotypes – neither differed significantly.
(A) Example traces of the typical firing pattern associated with each class of interneuron in response to 500 msec rectangular injections of depolarizing currents. Left, Fast-spiking interneurons, shown are the three types of firing observed, large somatic current injections were required to evoke firing (see C) although small synaptic inputs to the dendrites could evoked firing (data not shown). Center, Regular-spiking interneurons fired in a graded fashion with low thresholds. Top trace 25 pA injection, bottom, 200 pA. Right, example of a slow spiking interneuron to 200 pA injection. Other properties of the neurons co-varied with firing pattern (see Table S1). In SO/alveus, many regular spiking cells are likely to be OLM, bistratifed and trilaminar neurons. Fast spiking interneurons normally express parvalbumin and are typically basket or axo-axonic neurons that target the cell body or axon hillock of pyramidal neurons. Four neurons were classified as “slow” spiking, the identity of these cells is unclear but their firing pattern was very distinctive and characterized by small afterhyperpolarizations. (B) Pie charts showing the proportions of each subtype of neuron found in Df(16)A+/− and WT slices. Proportions did not differ significantly between genotypes. 4/17 (22.2%) WT and 5/20 (25%, Chi-square, P = 0.795) Df(16)A+/− neurons showed spontaneous firing. 4/10 (25%) WT and 4/12 (33.3%, P = 0.746) Df(16)A+/− of regular spiking neurons displayed rebound firing after injection of 100 pA hyperpolarizing current. (C) Left, average AP thresholds for fast-spiking interneurons (WT: −35.43 ± 2.54, Df(16)A+/− −37.24 ± 1.91, P = 0.581). Average rheobase values for evoking a single spike, a partial train or a full train (as in A) from resting potential (center) and −60 mV (right). For all comparisons, P > 0.05.
Supp Table 1. Intrinsic properties of and spontaneous excitatory synaptic input to inhibitory interneurons.