Abstract
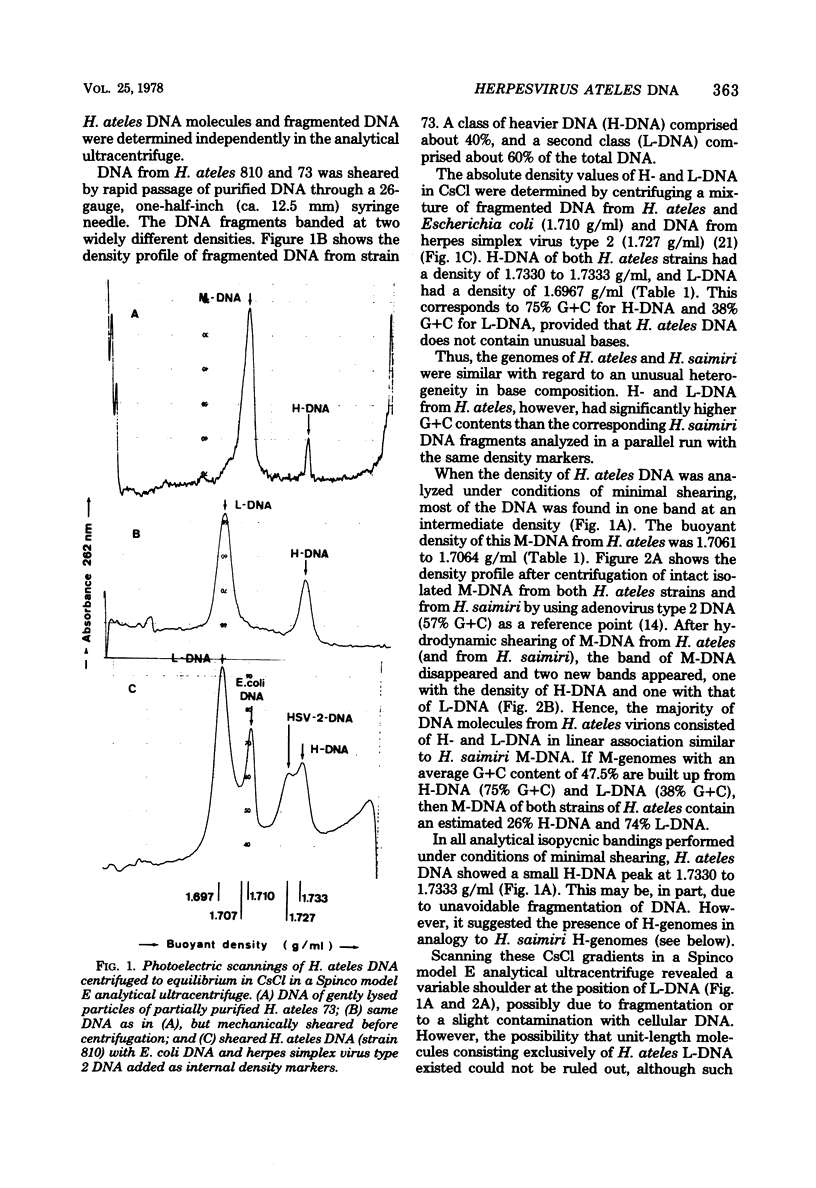
Analysis of the structural organization of Herpesvirus ateles DNA shows that two types of viral DNA molecules are encapsidated in virions: (i) M-genomes, which contain 74% light sequences (L-DNA, 38% guanine plus cytosine) and 26% highly repetitive heavy sequences (H-DNA, 75% guanine plus cytosine), and (ii) defective H-genomes, which consist exclusively of repetitive H-DNA. The structure of M-genomes from H. ateles consists of an L-DNA region of about 70 × 106 daltons inserted between H-DNA termini of variable length. M-genomes with a shorter H-DNA region at one end of the molecule have a long stretch of H-DNA at the other end, resulting in a total molecular weight of 89.8 ± 8.5 × 106. Thus it resembles the structure of M-genomes of H. saimiri. H-DNA of the two independent H. ateles isolates, strains 810 and 73, reveals different patterns after cleavage with restriction endonuclease Sma I. H-DNA of H. ateles 810 appears to consist of identical tandem repeat units with a molecular weight of 1,035,000; the H-DNA repeat unit of strain 73 is shorter (930,000 molecular weight). Corresponding DNA sequences of the two H. ateles strains (810 and 73) are completely homologous in cross-hybridizations. However, a discrete nucleotide sequence divergence between these virus strains is detected by measuring melting temperatures (Tm) of DNA hybrid molecules. Some homology exists between H. ateles and H. saimiri DNA. Hybridization of L-DNA from H. ateles with L-DNA from H. saimiri shows about a 35% homology between the respective L-DNA sequences; the resulting heteroduplex molecules show a decrease of Tm by 13.5°C, corresponding to about a 9% mismatching in cross-hybridizing parts of L-regions. Very little homology is found between H-DNA of H. ateles and H. saimiri.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornkamm G. W., Delius H., Fleckenstein B., Werner F. J., Mulder C. Structure of Herpesvirus saimiri genomes: arrangement of heavy and light sequences in the M genome. J Virol. 1976 Jul;19(1):154–161. doi: 10.1128/jvi.19.1.154-161.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J., Sack G. H., Jr, Nathans D. Studies of simian virus 40 DNA. VII. A cleavage map of the SV40 genome. J Mol Biol. 1973 Aug 5;78(2):363–376. doi: 10.1016/0022-2836(73)90122-8. [DOI] [PubMed] [Google Scholar]
- Falk L. A., Nigida S. M., Deinhardt F., Wolfe L. G., Cooper R. W., Hernandez-Camacho J. I. Herpesvirus ateles: properties of an oncogenic herpesvirus isolated from circulating lymphocytes of spider monkeys (Ateles sp.). Int J Cancer. 1974 Oct 15;14(4):473–482. doi: 10.1002/ijc.2910140407. [DOI] [PubMed] [Google Scholar]
- Falk L. A., Wolfe L. G., Deinhardt F. Herpesvirus saimiri: experimental infection of squirrel monkeys (Saimir sciureus). J Natl Cancer Inst. 1973 Jul;51(1):165–170. doi: 10.1093/jnci/51.1.165. [DOI] [PubMed] [Google Scholar]
- Falk L. A., Wolfe L. G., Deinhardt F. Isolation of Herpesvirus saimiri from blood of squirrel monkeys (Saimiri sciureus). J Natl Cancer Inst. 1972 May;48(5):1499–1505. [PubMed] [Google Scholar]
- Falk L., Wright J., Wolfe L., Deinhardt F. Herpesvirus ateles: transformation in vitro of marmoset splenic lymphocytes. Int J Cancer. 1974 Aug 15;14(2):244–251. doi: 10.1002/ijc.2910140213. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W., Ludwig H. Repetitive sequences in complete and defective genomes of Herpesvirus saimiri. J Virol. 1975 Feb;15(2):398–406. doi: 10.1128/jvi.15.2.398-406.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W. Structure and function of herpesvirus saimira DNA. IARC Sci Publ. 1975;(11 Pt 1):145–149. [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W., Werner F. J. The role of Herpesvirus saimiri genomes in oncogenic transformation of primate cells. Bibl Haematol. 1975 Oct;(43):308–312. doi: 10.1159/000399154. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B., Müller I., Werner J. The presence of Herpesvirus Saimiri genomes in virus-transformed cells. Int J Cancer. 1977 Apr 15;19(4):546–554. doi: 10.1002/ijc.2910190416. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B., Wolf H. Purification and properties of Herpesvirus saimiri DNA. Virology. 1974 Mar;58(1):55–64. doi: 10.1016/0042-6822(74)90140-8. [DOI] [PubMed] [Google Scholar]
- GREEN M., PINA M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology. 1963 May;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- Hayward G. S., Jacob R. J., Wadsworth S. C., Roizman B. Anatomy of herpes simplex virus DNA: evidence for four populations of molecules that differ in the relative orientations of their long and short components. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4243–4247. doi: 10.1073/pnas.72.11.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R. D., Meléndez L. V., García F. G., Trum B. F. Pathologic features of Herpesvirus ateles lymphoma in cotton-topped marmosets (Saguinus oedipus). J Natl Cancer Inst. 1972 Dec;49(6):1631–1639. doi: 10.1093/jnci/49.6.1631. [DOI] [PubMed] [Google Scholar]
- Hunt R. D., Meléndez L. V., King N. W., Gilmore C. E., Daniel M. D., Williamson M. E., Jones T. C. Morphology of a disease with features of malignant lymphoma in marmosets and owl monkeys inoculated with Herpesvirus saimiri. J Natl Cancer Inst. 1970 Feb;44(2):447–465. [PubMed] [Google Scholar]
- Laufs R., Fleckenstein B. Malignant lymphoma induced by partially purified Herpesvirus saimiri and recovery of infectious virus from tumorous lymph nodes. Med Microbiol Immunol. 1972;158(2):135–146. doi: 10.1007/BF02120479. [DOI] [PubMed] [Google Scholar]
- Laufs R., Fleckenstein B. Susceptibility to Herpesvirus saimiri and antibody development in old and new world monkeys. Med Microbiol Immunol. 1973 Mar 8;158(3):227–236. doi: 10.1007/BF02120558. [DOI] [PubMed] [Google Scholar]
- Laufs R., Meléndez L. V. Oncogenicity of Herpesvirus ateles in monkeys. J Natl Cancer Inst. 1973 Aug;51(2):599–608. [PubMed] [Google Scholar]
- Ludwig H. Untersuchungen am genetischen Material von Herpesviren. I. Biophysikalisch-chemische Charakterisierung von Herpesvirus=Desoxyribonucleinsäuren. Med Microbiol Immunol. 1972;157(3):186–211. doi: 10.1007/BF02121161. [DOI] [PubMed] [Google Scholar]
- Melendez L. V., Daniel M. D., Hunt R. D., Garcia F. G. An apparently new herpesvirus from primary kidney cultures of the squirrel monkey (Saimiri sciureus). Lab Anim Care. 1968 Jun;18(3):374–381. [PubMed] [Google Scholar]
- Melendez L. V., Hunt R. D., King N. W., Barahona H. H., Daniel M. D., Fraser C. E., Garcia F. G. Herpesvirus ateles, a new lymphoma virus of monkeys. Nat New Biol. 1972 Feb 9;235(58):182–184. doi: 10.1038/newbio235182b0. [DOI] [PubMed] [Google Scholar]
- Meléndez L. V., Hunt R. D., Daniel M. D., García F. G., Fraser C. E. Herpesvirus saimiri. II. Experimentally induced malignant lymphoma in primates. Lab Anim Care. 1969 Jun;19(3):378–386. [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett R. F., Hayward S. D., Kieff E. D. DNA of Epstein-Barr virus. I. Comparative studies of the DNA of Epstein-Barr virus from HR-1 and B95-8 cells: size, structure, and relatedness. J Virol. 1975 Mar;15(3):556–559. doi: 10.1128/jvi.15.3.556-559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Schulte-Holthausen H., Schneweis K. E. Differentiation of Herpes simplex virus serotypes 1 and 2 by DNA-DNA-Hybridization. Med Microbiol Immunol. 1975 Sep 19;161(4):279–285. doi: 10.1007/BF02122716. [DOI] [PubMed] [Google Scholar]
- Sheldrick P., Berthelot N. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):667–678. doi: 10.1101/sqb.1974.039.01.080. [DOI] [PubMed] [Google Scholar]
- Sugden B., Summers W. C., Klein G. Nucleic acid renaturation and restriction endonuclease cleavage analyses show that the DNAs of a transforming and a nontransforming strain of Epstein-Barr virus share approximately 90% of their nucleotide sequences. J Virol. 1976 May;18(2):765–775. doi: 10.1128/jvi.18.2.765-775.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman J. S., McCarthy B. J. The relationship between mismatched base pairs and the thermal stability of DNA duplexes. II. Effects of deamination of cytosine. Biochim Biophys Acta. 1973 Feb 4;294(1):416–424. doi: 10.1016/0005-2787(73)90096-8. [DOI] [PubMed] [Google Scholar]
- Werner F. J., Bornkamm G. W., Fleckenstein B. Episomal viral DNA in a Herpesvirus saimiri-transformed lymphoid cell line. J Virol. 1977 Jun;22(3):794–803. doi: 10.1128/jvi.22.3.794-803.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Wolf H. A procedure for simultaneous preparation of large amounts of DNA and RNA by the use of potassium iodide gradients. Anal Biochem. 1975 Oct;68(2):505–511. doi: 10.1016/0003-2697(75)90645-4. [DOI] [PubMed] [Google Scholar]
- Wolfe L. G., Falk L. A., Deinhardt F. Oncogenicity of herpesvirus saimiri in marmoset monkeys. J Natl Cancer Inst. 1971 Nov;47(5):1145–1162. [PubMed] [Google Scholar]






