Abstract
Objective
To identify correlates of perceived pain-related restrictions in a community sample of women with fibromyalgia.
Method
The fibromyalgia group was composed of white women with a self-reported, physician-given fibromyalgia diagnosis (N = 238) from the Biopsychosocial Religion and Health Study (BRHS). BRHS respondents had participated in the larger Adventist Health Study-2. To identify associations with pain-related restrictions, we used hierarchical linear regression. The outcome measure was subjects' pain-related restrictions (one SF-12 version 2 item). Predictors included age, education, body mass index (BMI), sleep apnea, and fibromyalgia treatment in the last year, as well as standardized measures for trauma, major life stress, depression, and hostility. To better interpret the findings, pain-related restrictions also were predicted in women with osteoarthritis and no fibromyalgia.
Results
Women with fibromyalgia reporting the more severe pain-related restrictions were those who had experienced trauma accompanied by physical pain, were older, less educated, more depressed, more hostile, had high BMI scores, and had been treated for fibromyalgia in the last 12 months (adjusted R2 = 0.308). Predictors in women with osteoarthritis were age, BMI, treatment in the last 12 months, experience of a major life stressor, and greater depression symptom severity (adjusted R2 = 0.192).
Conclusions
In both groups, age, BMI, treatment in the last 12 months, and depression predicted pain-related restrictions. Experience of a traumatic event with physical pain was the strongest predictor in the fibromyalgia group. These findings may be useful in constructing novel treatments and prevention strategies for pain-related morbidity in fibromyalgia patients.
Keywords: Fibromyalgia, Osteoarthritis, Pain, Central Nervous System, Trauma, Stress
Introduction
Fibromyalgia and Chronic Pain
Pain that persists for more than 3 months is considered chronic [1]. Perhaps the most perplexing, controversial, disabling, and difficult-to-treat of all chronic pain conditions is fibromyalgia. Fibromyalgia patients account for more medical cost, medication use, physician visits, and disability compared with other chronic pain patients [2,3]; therefore, identifying correlates of pain-related functional status may improve care in this patient group.
Fibromyalgia Pain
Patients with fibromyalgia report constant, widespread, amplified body pain (hyperalgesia) and pain to superficial and deep palpation that is out of proportion to the intensity of applied pressure (allodynia). Recent evidence suggests that fibromyalgia pain may be modulated at the level of the central nervous system (CNS) [4,5]. Functional imaging studies have shown alterations in brain networks in both fibromyalgia and other chronic pain patients [6,7]. Despite these similarities, fibromyalgia patients report more pain-related problems relative to other pain patients with comparable pain ratings [8,9]. This may be due, in part, to differences in genetic predisposition, life experiences, coping strategies, cognitive styles, and co-morbidities [10,11].
Correlates of Fibromyalgia, Pain, and Pain-Related Restrictions
Previous work suggests that traumatic events can influence the onset and progression of various diseases such as depression, anxiety disorders, and chronic pain (including fibromyalgia), and often there are associated CNS changes [7,12–16]. Many factors have been associated with pain and restrictions in fibromyalgia patients including traumatic events. In a recent study, fibromyalgia prevalence was associated with having experienced both sexual and physical assault/abuse [17]. What remains unclear, however, is whether negative life events (i.e., traumatic and major life stress experiences) can predict the extent of perceived pain-related restrictions in fibromyalgia patients.
In fibromyalgia patients, depression increases somatic complaints and functional disability [18] and can predict the extent of pain and functional restrictions [19]. Coping styles such as catastrophizing, the inability to identify emotions, and hostility (which entails isolation and mistrust of others) can lead to increased pain and disability in fibromyalgia patients [20,21].
Age, education, weight, and sleep disorders also can influence pain-related restrictions. Fibromyalgia patients who are younger and better educated maintain higher functional status [22]. Moreover, fibromyalgia patients who are overweight report increased disability, decreased quality of life, and lower pain thresholds [23]. The relationship between sleep problems and musculoskeletal pain is not completely understood, but nonrestorative sleep is associated with increased pain sensitivity [24].
Research Aim
The present study was designed to identify factors associated with perceived pain-related restrictions in a national (United States), community sample of women with fibromyalgia. Our primary aim was to determine whether various factors—shown to be related to pain severity and functional restrictions in previous studies—would be positively related/make independent contributions to pain restriction prediction in women with fibromyalgia. In designing this study, we sought to both identify and confirm potentially treatable factors not yet evaluated together in a single study in this difficult-to-treat patient population. To better interpret the results, we predicted pain-related restrictions in another pain group, women with osteoarthritis (and without fibromyalgia).
Method
Data Source
Data were from the Biopsychosocial Religion and Health Study (BRHS [25]). BRHS investigators randomly sampled 20,000 of the 96,000 respondents to the Adventist Health Study (AHS-2 [26]); of the 20,000 receiving a mailed questionnaire, 10,988 provided usable BRHS surveys. The AHS-2 is a cohort study investigating the interrelationships among cancer, diet, and lifestyle among Seventh-day Adventists, whereas the BRHS is a study of the interrelationships among traumatic and major life stressors, religion, and health/mental health. Seventh-day Adventists endorse many healthy lifestyle commitments, which are anchored in their faith; for example, only 1% smoke, 7% consume alcohol, and fewer than half eat meat.
Subjects
Fibromyalgia subjects in the present study were the 238 White women answering “yes” to a yes-no question, “Has a physician ever diagnosed you with fibromyalgia,” and providing responses to both the gender and race/ethnicity questions. The number of respondents answering “no” to the fibromyalgia question and answering the gender and race/ethnicity questions was 3,624. The numbers of men and Black women meeting these criteria were too small and precluded meaningful analyses.
Measures
Dependent Variable
Pain ratings were based on subjects' answers to the SF-12 version 2 [27] item, “During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?” Ratings were on a 5-point scale, ranging from 1 (not at all) to 5 (extremely). This criterion represents a “snapshot,” that is, how the women perceived the extent to which pain had interfered with their lives in the month preceding the survey.
Independent Variables
Age (in years) is a continuous variable. Education is a three-level categorical variable, high school or less, some college, and college graduate or higher. Body mass index (BMI, weight scaled by height) is a self-reported continuous variable. Labels have been given to three score ranges (obesity ≥ 30, overweight = 25–29.9, and normal = 18.5–24.9) [28]. Our sleep disorder measure (sleep apnea) was coded yes = 1 and no = 0, based on subjects' answers to the question, “Has a physician ever diagnosed you with sleep apnea?” Fibromyalgia status (treated the last 12 months) was coded yes = 1 and no = 0.
Trauma and major life stress items were from a modified version of the Trauma Assessment in Adults and Ryff and colleagues' child abuse scales [29–31]. In this section of the questionnaire, subjects were asked about “different types of stressful or difficult life events” (both in child and adulthood, with no event timeline). We classified the items as either a traumatic experience or a major life stressor. The traumatic experiences are those that likely involved actual or threatened death (actual death of another person) or serious injury (or threat to one's physical integrity), and elicited intense reactions, such as fear, helplessness, or horror [32–34]. We then grouped the items by trauma type: 1) life-threatening accident (one question, “Have you ever been in a really bad accident—car, at work, or somewhere else—and thought you might be killed or injured?), 2) emotional abuse/neglect (two questions about mother or father insulting, swearing at, or ignoring between ages 5 and 15 years), 3) physical assault/abuse (two assault questions, actual and threatened in subject's lifetime; four abuse questions between age 5 and 15 years, mother or father pushing, slapping, throwing objects, kicking, biting, striking with an object), and 4) sexual assault/abuse (three questions, actual and threatened in one's lifetime).
The major life stressors are serious illness (e.g., cancer, leukemia, AIDS, and multiple sclerosis), abortion (self/intimate partner), miscarriage (self/intimate partner), divorce/separation, homelessness, and death of a child. For each of these trauma/stressor variables, subjects were given a score of 0 in the category if their answers to all of the questions in that category were no/never (and given a score of 1, if otherwise).
With one exception, traumatic experience and major life stressor classification/scoring is consistent with Haviland et al. [17]. They grouped bad accident with war, natural disaster, and witnessing someone being killed. Only “bad accident” was related to the pain criterion in the present study, so it was isolated as a single trauma variable.
For depression severity, we used the 11-item version of the Center for Epidemiological Studies-Depression Scale (CES-D) (each item rated on a three-point scale, ranging from 0 or rarely/none to 2 or much/most of the time), which we converted to full 20-item CES-D equivalent scores [35]. CES-D equivalent scores of ≥16 are considered high; and this cut-point often is used to screen for clinical depression. For hostility, we used a modified version of the 9-item Cook–Medley hostility scale [36]. Items were rated on a 1–4 scale (originally a true-false format): 1 = definitely false, 2 = tends to be false, 3 = tends to be true, and 4 = definitely true. Total scores are expressed as average item scores. Missing item values for these two scales were replaced with the average of available items if subjects had answered at least eight of the depression and seven of the hostility items.
Data Analyses
To evaluate the bivariate relationships between independent variables and the dependent variable (pain ratings), we used correlation (age, BMI, depression, and hostility), one-way analysis of variance (education), and t-tests for independent samples (sleep apnea, fibromyalgia status, traumatic experiences, and major life stressors).
To evaluate multivariate relationships, we used hierarchical linear regression (presented are standardized betas, P-values, and adjusted R2s for each stage). Entered at the first stage was a set of personal/health variables (age, education, BMI, sleep apnea, and fibromyalgia status). At stage two, the traumatic/major life stress set was entered, and at stage three, the psychological set (depression and hostility). For all tests of statistical significance, alpha was set at 0.05.
Based on the results of the multivariate analysis, we created two “extreme” subgroups and compared their average pain ratings; in one group were those women possessing all of the associated factors, and, in the other group, those possessing none.
Finally, we ran a parallel analysis (hierarchical regression) with the women in the larger sample answering “yes” to the osteoarthritis question (“Has a physician ever diagnosed you with osteoarthritis?”), “no” to the fibromyalgia question, and with complete information on all of the independent variables. The independent variables were the same as those in the fibromyalgia analysis, with one exception: fibromyalgia status was replaced with osteoarthritis status (again, treated in the last 12 months).
Results
Descriptive statistics for the 238 women with fibromyalgia are shown in Tables 1 and 2. Subjects' average age was 62.6 years (SD = 10.8), and approximately three-quarters had either attended or graduated from college. Traumatic experiences and major life stressors were strikingly common. Moreover, subjects' average depression scores (15.7) were just under an established screening criterion for clinical depression (≥16), and their average BMIs (29.2) were just under the obesity criterion (≥30) (39.1% met the screening criterion for clinical depression and 27.3% for obesity). Slightly less than a fifth (18.1%) reported sleep apnea, and 45% reported having been treated for fibromyalgia in the last 12 months.
Table 1.
Women with a physician-given fibromyalgia diagnosis: descriptive statistics (continuous variables) and their relationships to pain ratings
| N | Range | Mean | SD | Correlation with pain | |
|---|---|---|---|---|---|
| Age | 233 | 38–88 | 62.6 | 10.8 | 0.12 |
| BMI | 236 | 16.8–75.9 | 29.2 | 8.0 | 0.24 |
| Depression | 237 | 0.7–48.6 | 15.7 | 10.4 | 0.35 |
| Hostility | 234 | 1–3.3 | 2.1 | 0.49 | 0.18 |
P < 0.05 shown in boldface type.
BMI = body mass index.
Table 2.
Women with a physician-given fibromyalgia diagnosis: descriptive statistics (categorical variables) and their relationships to pain ratings
| Pain ratings |
|||||
|---|---|---|---|---|---|
| n | % | Mean | SD | P | |
| Education† | 0.002 | ||||
| High school or less | 69 | 29.1 | 3.5 | 1.2 | |
| Some college | 105 | 44.3 | 3.3 | 1.3 | |
| College graduate or higher | 63 | 26.6 | 2.7 | 1.2 | |
| Sleep apnea | |||||
| Yes | 43 | 18.1 | 3.8 | 1.1 | 0.000 |
| No | 190 | 79.8 | 3.1 | 1.3 | |
| Fibromyalgia Tx last 12 months‡ | 0.000 | ||||
| Yes | 98 | 45.0 | 3.6 | 1.2 | |
| No | 120 | 55.0 | 2.8 | 1.2 | |
| Bad accident‡ | 0.014 | ||||
| Yes | 102 | 42.9 | 3.4 | 1.2 | |
| No | 136 | 57.1 | 3.0 | 1.3 | |
| Emotional abuse/neglect‡ | 0.217 | ||||
| Yes | 104 | 45.2 | 3.3 | 1.2 | |
| No | 126 | 54.8 | 3.1 | 1.3 | |
| Physical assault/abuse‡ | 0.001 | ||||
| Yes | 123 | 53.7 | 3.5 | 1.1 | |
| No | 106 | 46.3 | 2.9 | 1.3 | |
| Sexual assault/abuse‡ | 0.067 | ||||
| Yes | 109 | 46.6 | 3.4 | 1.2 | |
| No | 125 | 53.4 | 3.1 | 1.3 | |
| Major life stressor‡ | 0.072 | ||||
| Yes | 168 | 71.2 | 3.3 | 1.2 | |
| No | 68 | 28.8 | 3.0 | 1.3 | |
One-way analysis of variance.
t-test for independent samples.
P < 0.05 shown in boldface type.
Subjects' perceived pain ratings are shown in Figure 1. In response to the question, “During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?” slightly less than half responded quite a bit (n = 75, 31.8%) or extremely (n = 38, 16.1%), and, a fifth, moderately (n = 50, 21.2%). The mean score (±SD) was 3.21 ± 1.26 on the 5-point scale.
Figure 1.
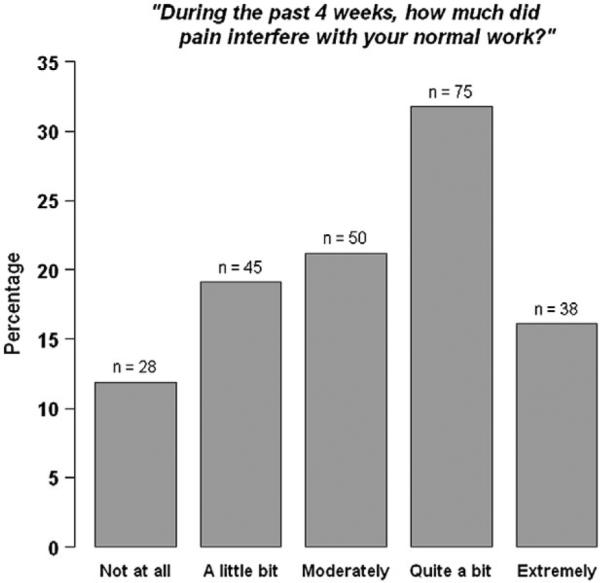
Pain responses of women with a physician-given fibromyalgia diagnosis (n = 236).
The bivariate relationships between predictor variables and the pain criterion are shown in Tables 1 and 2. Greater pain interference was associated with lower education, higher BMI, sleep apnea, treated for fibromyalgia in the last 12 months, having been in a bad accident, the experience of physical assault/abuse, and higher depression and hostility scores.
The multivariate (hierarchical regression) results are shown in Table 3. Personal/health characteristics were entered at stage 1. Lower education, higher BMI, sleep apnea, and fibromyalgia status (treated in the last 12 months) were significant predictors of greater perceived pain interference. Entered at stage 2 were the trauma and major life stress variables; significant (positive association) were having been in a bad accident and physical abuse/assault experience/s. At stage 3, the psychological variables—depression and hostility—were entered, and both were significant; higher scores were associated with greater pain interference. Age was significant (positive) only at stages 2 and 3 (narrowly missing statistical significance at stage 1, P = 0.05). Adjusted R2 for the final model was 0.308. In the final model, physical assault/abuse experience/s was the strongest pain restriction predictor. The next strongest, respectively, were age, lower education, and BMI. Not significant at any stage were emotional abuse/neglect, sexual assault/abuse, and major life stressor.
Table 3.
Hierarchical regression, pain predictors (fibromyalgia subjects, n = 195)
| Stage 1 |
Stage 2 |
Stage 3 |
||||
|---|---|---|---|---|---|---|
| St. beta | P | St. beta | P | St. beta | P | |
| Age | 0.128 | 0.050 | 0.176 | 0.007 | 0.191 | 0.003 |
| Education—high school or less† | 0.196 | 0.014 | 0.225 | 0.004 | 0.186 | 0.016 |
| Education—some college† | 0.189 | 0.018 | 0.218 | 0.006 | 0.171 | 0.027 |
| Body mass index | 0.242 | 0.000 | 0.212 | 0.002 | 0.185 | 0.006 |
| Sleep apnea | 0.141 | 0.042 | 0.138 | 0.043 | 0.114 | 0.088 |
| Fibromyalgia Tx last 12 months | 0.232 | 0.001 | 0.204 | 0.002 | 0.171 | 0.008 |
| Bad accident | 0.150 | 0.022 | 0.161 | 0.012 | ||
| Emotional abuse/neglect | −0.066 | 0.356 | −0.117 | 0.101 | ||
| Physical assault/abuse | 0.237 | 0.001 | 0.254 | 0.001 | ||
| Sexual assault/abuse | −0.025 | 0.711 | −0.038 | 0.564 | ||
| Major life stressor | 0.034 | 0.607 | 0.010 | 0.870 | ||
| Depression | 0.155 | 0.024 | ||||
| Hostility | 0.146 | 0.036 | ||||
Reference = education—college graduate or higher.
P < 0.05 at Stage 3 shown in boldface type. Stage 3 adjusted R2 = 0.308; Stage 2 adjusted R2 = 0.262; Stage 1 adjusted R2 = 0.237.
Finally, average perceived pain ratings for the five women with all of the significant characteristics were 4.40 ± 0.55. Average scores for the seven without them were 1.43 ± 0.54. Women in the first group met all of the following criteria: age ≥ 62.6 (sample mean), education = high school or less, BMI ≥ 30 (obese criterion), fibromyalgia status = yes, experienced physical assault/abuse or bad accident = yes, depression ≥ 16 (clinical depression criterion), and hostility ≥ 2.1 (sample mean). Women in the second group met none of these criteria.
Osteoarthritis Regression
The osteoarthritis and fibromyalgia groups differed in important ways. For the continuous variables, subjects in the osteoarthritis group (means and SDs for the osteoarthritis and the fibromyalgia groups are given in parentheses): had lower pain-related restriction scores (2.67 + 1.18 vs 3.21 ± 1.26), were older (70.4 ± 11.0 vs 62.6 ± 10.8), had slightly lower BMIs (27.9 ± 7.0 vs 29.2 ± 8.0), and had lower depression scores (10.7 ± 8.6 vs 15.7 ± 10.4). Hostility scores were similar (2.05 ± 0.5 vs 2.1 ± 0.5). For the categorical variables (percentages by group are given in parentheses), individuals in the osteoarthritis group: had fewer subjects in the lowest education category (20.4% vs 29.1%) and more in the highest education category (34.2% vs 26.6%), had fewer with sleep apnea (7.0% vs 18.1%), had fewer reporting bad accidents (34.6% vs 42.9%), had less emotional abuse/neglect (32.9% vs 45.2%), had less physical assault/abuse (38.2%. vs 53.7%), less sexual assault/abuse (28.8% vs 46.6%), and fewer major life stressors (63.6% vs 71.2%). With the exception of education and hostility, all differences were statistically significant (and all but BMI differences were substantial).
A total of 744 women answered “yes” to the osteoarthritis question (and “no” to the fibromyalgia question) and had complete information on all of the independent variables (and, thus, were included in the regression analysis). Five variables were significant in the final model (staged as in the fibromyalgia model; standardized beta coefficients in the final model are shown in parentheses): age (0.196), BMI (0.243), treated in the last 12 months (0.172), major life stressors (0.078), and depression (0.213). Adjusted R2 for the final model was 0.192.
Discussion
The primary purpose of the present study was to identify factors—including traumatic life events—that were associated with the extent of perceived pain-related restrictions in women self-reporting a physician-given fibromyalgia diagnosis. In the final regression model, older age, lower education, higher BMI, treatment for fibromyalgia in the last 12 months, experiencing a bad accident or physical assault/abuse, and higher depression, and hostility scores, all were associated with greater pain-related restrictions in the fibromyalgia group. In the osteoarthritis group, only older age, higher BMI, treatment for osteoarthritis in the last 12 months, major life stressors, and depression were predictors of pain-related restrictions.
In contrast to the fibromyalgia group, traumatic events (having been in a bad accident or experiencing physical assault/abuse), hostility, and education were not associated with pain-related restrictions in the osteoarthritis group. Particularly noteworthy is that traumatic experiences accompanied by physical pain (bad accident or physical abuse/assault) were the strongest predictors of pain-related restrictions in our cohort of women with fibromyalgia (and not in women with osteoarthritis). On the other hand, having experienced at least one major life stressor (e.g., serious illness, abortion, miscarriage, divorce/separation, homelessness, or death of a child) predicted pain restrictions for women with osteoarthritis but not for women with fibromyalgia. Future research may be designed to elucidate the process by which these contrasting stressors lead to pain-related restrictions in the two disease groups.
Life Experience and Pain-Related Restriction
In the fibromyalgia group, the strongest predictor of pain-related interference was the traumatic life experience of physical assault/abuse. In previous studies, traumatic life experiences have been shown to influence subsequent behavior, health trajectories, as well as CNS and hypothalamic-pituitary-adrenal (HPA) axis function and structure [37]. In our fibromyalgia subjects, 72.5% had experienced physical trauma (physical assault/abuse or a bad accident) and concomitant acute pain. This supports the possibility that traumatic events predisposed some women to later develop fibromyalgia, hypersensitivity to pain [17,38], and mood disorders.
Traumatic events can influence brain function [39]; when a traumatic event occurs, brain neural networks assess the event for controllability. Events perceived to be uncontrollable affect the degree of stress mediator release and subsequent neuroplastic changes that, in turn, alter neuronal function and presumably lessen one's ability to handle further stress [40]. Although unproven, it is possible that similar changes may be relevant to participants in the present study.
There is growing evidence that adverse life experiences can lead to alterations in HPA axis function, which in turn can affect normal brain function, especially frontal lobe/executive function, and have lasting effects [41]. This occurs, in part, from altered levels of biogenic amines [41]. Similar alterations in neurotransmitter levels and synaptic function have been reported in fibromyalgia patients [42–44] and have been hypothesized to contribute to cognitive alterations, such as catastrophizing, depression, hostility, “fibro fog” (cognitive impairment), and the inability to modulate pain and pain's effects. In the present study, greater perceived pain-related restrictions in the fibromyalgia group were associated with having experienced “physical” traumatic events (i.e., physical assault/abuse and bad accident). Future studies should uncover 1) whether these events can lead to changes in CNS functions and 2) the consequences of the timing of such events.
Other Correlates of Pain-Related Restriction
Depression was associated with worse pain-related restrictions in both the fibromyalgia and the osteoarthritis groups, despite depression scores being considerably lower on average in the osteoarthritis group. Among the fibromyalgia cohort, 39.1% had CES-D equivalent scores ≥ 16 (typically used as a screen for clinical depression), and the group's mean depression scores fell just short of this criterion. In previous studies, fibromyalgia patients have reported poorer quality of life, lower functional status, and more psychiatric symptoms than patients with other pain conditions [9,45–47]. In fact, some suggest that depression exerts a greater effect on fibromyalgia patients than on patients with other pain syndromes [9]. Moreover, fibromyalgia patients with concomitant mood disorders report more intense somatic complaints, pain levels, and functional restrictions than patients with other pain syndromes [18,19]. Our data, however, show that depressive symptoms—regardless of severity—predict pain-related restrictions in both individuals with fibromyalgia and osteoarthritis, although the fibromyalgia group reported both worse depression and higher pain-related restrictions.
Hostility also predicted pain-related restrictions in the fibromyalgia cohort. Hostility often has been defined as general cynicism, interpersonal mistrust, and lack of social support [48]. Hostility relates to a cognitive style that is maladaptive and has been associated with the progression of various diseases, including cardiovascular disease [49]. A recent large community-based study reported that hostility was significantly associated with depression [50]. The mechanism by which hostility relates to pain-related restrictions in the fibromyalgia group is unknown but may relate to a cognitive style [51] that may lead to perceived pain-related restrictions.
In both groups, older age and higher BMI were associated with an increase in pain-related restrictions, and, in the fibromyalgia group, low education was a significant predictor. Advancing age has been associated with a decline in functional ability in fibromyalgia patients, with an estimated 4% decline in functional capacity for each year of advancing age [22]. In agreement with our findings, previous studies have shown that low education levels generally are associated with poor health outcomes [52] and increased depression rates [53]. Obesity has been shown to have a negative effect on the musculoskeletal system [54]. In our fibromyalgia sample, 27.3% of the subjects met the National Heart, Lung, and Blood Institute criterion for obesity [28]. Obesity in fibromyalgia patients leads to greater disability, poorer quality of life, and reduced pain thresholds [23]. It is likely that increased age and being overweight/obese increase stress to a system that already is unable to handle the ever present burden of pain.
Thus, older age, high BMI, having treatment within the last 12 months, and depression emerge as common factors that predict perceived pain-related restrictions in women with fibromyalgia and osteoarthritis, whereas traumatic experiences, hostility, and low education are specific to fibromyalgia.
Study Strengths
The present sample was a relatively large, community group. In many fibromyalgia studies, researchers have studied smaller groups in clinical (treatment) settings. An additional strength is that the BRHS survey contained detailed lifetime trauma/stressor questions, thus, allowing us to clarify the relationship between specific traumas and stressors, as well as other correlates that lead to perceived pain-related restrictions in women with fibromyalgia and osteoarthritis. Our sample size and comprehensive questionnaire afforded us the ability to evaluate a large number of variables in a single study. Moreover, our relatively large cohort of women with osteoarthritis allowed for similar meaningful comparisons to be made in this group.
Study Limits
First, our fibromyalgia criterion was based on yes-no answers to a single question, “Has a physician ever diagnosed you with fibromyalgia?” Although clearly an imperfect criterion, Haviland et al. [17] have provided some support for its validity: 1) their reported rates were reasonable given sample age and composition, and the rate for women was higher than that of men, and 2) individuals reporting a fibromyalgia diagnosis had more pain, less energy, worse sleep, higher depressive symptom scores, and diagnoses of depression and irritable bowel syndrome (life-to-date). Second, although our pain criterion is interpretable, it does not necessarily represent pain intensity/severity or disability. Third, our trauma/stress data are uncorroborated, self-reported, “point events,” and we had no measures/indices of chronic stress/present anxiety, which both have long been associated with chronic pain. Fourth, this is a unique sample, although how this may limit generalizability is not obvious. Fifth, only 50% of those sent questionnaires responded (although the response rate for white women—the group from which the present samples were drawn—was 62%), and finally, this is a cross-sectional, not longitudinal, study and a secondary data analysis.
Conclusion
We have identified a number of associations with perceived pain-related restrictions in women with fibromyalgia and contrasted these with pain predictors for women with osteoarthritis. The factors that provide the most striking contrast are traumatic events accompanied by physical pain, which predicted greater perceived pain-related interference in the fibromyalgia group only. Thus, individuals with fibromyalgia who have experienced traumatic life events may be predisposed to greater pain-related restrictions than those who have not experienced trauma [55]. The fibromyalgia subgroup with the most extreme pain restrictions in the present study may have been genetically predisposed to cope poorly with past and present stressors. Future studies could be designed to explore the intriguing relationships between genetic predisposition, traumatic life events, and present pain-related restrictions. Successful treatment likely will occur only after the development of treatment paradigms that consider the full spectrum of the fibromyalgia disease phenotype we have reported. Strategies that consider, for example, cortical alterations and past and present cognitive, physical, and emotional elements of disease, have been successful in treating chronic pain patients [56]. Our results underscore the importance of investigating traumatic experiences when approaching fibromyalgia patients or those susceptible for the development of the disease. In the ideal scenario, traumatic events would be prevented, but when present, they should be addressed in the predisease or early disease stage. Finally, the reported data may help researchers design novel studies that explore genetic and epigenetic factors underlying the development of risk factors for perceived pain-related restrictions in fibromyalgia patients.
Acknowledgments
Funding for this study was from the National Institute on Aging (Biopsychosocial Religion and Health Study, 5R01AG026348-05) and from the National Cancer Institute for the parent study (Adventist Health Study 2, 5R01 CA094594).
References
- 1.Merskey H, Bogduk N. Classification of Chronic Pain. IASP Press; Seattle, WA: 1994. [Google Scholar]
- 2.White LA, Birnbaum AG, Kaltenboeck A, et al. Employees with fibromyalgia: Medical comorbidity, healthcare costs, and work loss. J Occup Environ Med. 2008;50:13–24. doi: 10.1097/JOM.0b013e31815cff4b. [DOI] [PubMed] [Google Scholar]
- 3.Silverman S, Dukes EM, Johnston SS, et al. The economic burden of fibromyalgia: Comparative analysis with rheumatoid arthritis. Curr Med Res Opin. 2009;25:829–40. doi: 10.1185/03007990902728456. [DOI] [PubMed] [Google Scholar]
- 4.Shweinhardt P, Sauro KM, Bushnell MC. Fibromyalgia: A disorder of the brain? Neuroscientist. 2008;14:415–21. doi: 10.1177/1073858407312521. [DOI] [PubMed] [Google Scholar]
- 5.Williams DA, Clauw DJ. Understanding fibromyalgia: Lessons from the broader pain research community. J Pain. 2009;10:777–91. doi: 10.1016/j.jpain.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Tracey I. Imaging pain. Br J Anaesth. 2008;101:32–9. doi: 10.1093/bja/aen102. [DOI] [PubMed] [Google Scholar]
- 8.DeWalt DA, Reed GW, Pincus T. Further clues to recognition of patients with fibromyalgia from a simple 2-page multidimensional health assessment questionnaire (MDHAQ) Clinical Experiments Rheumatology. 2004;22:453–61. [PubMed] [Google Scholar]
- 9.Gormsen L, Rosenberg R, Bach FW, Jensen TS. Depression, anxiety, health related quality of life and pain in patients with chronic fibromyalgia and neuropathic pain. Eur J Pain. 2010;14:127, e1–8. doi: 10.1016/j.ejpain.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Turk DC, Flor H. Primary fibromyalgia is greater than tender points: Towards a multiaxial taxonomy. J Rheumatol. 1989;16:80–6. [PubMed] [Google Scholar]
- 11.Thieme K, Turk DC, Flor H. Comorbid depression and anxiety in fibromyalgia syndrome: Relationship to somatic and psychosocial variables. Psychosom Med. 2004;66:827–44. doi: 10.1097/01.psy.0000146329.63158.40. [DOI] [PubMed] [Google Scholar]
- 12.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Pre-clinical and clinical studies. Biol Psychiatry. 2001;49:1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 13.Caspi A, McClay J, Moffitt TE, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–4. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 14.Leonardo ED, Hen R. Anxiety as a developmental disorder. Neuropsychopharmacology. 2008;33:134–40. doi: 10.1038/sj.npp.1301569. [DOI] [PubMed] [Google Scholar]
- 15.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: A convergent mechanism. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 16.Paras ML, Hassan-Murad M, Chen P, et al. Sexual abuse and lifetime diagnosis of somatic disorders: A systematic review and meta-analysis. JAMA. 2009;302:550–61. doi: 10.1001/jama.2009.1091. [DOI] [PubMed] [Google Scholar]
- 17.Haviland MG, Morton KR, Oda K, Fraser GE. Traumatic experiences, major life stressors, and self-reporting a physician-given fibromyalgia diagnosis. Psychiatry Res. 2010;177:335–41. doi: 10.1016/j.psychres.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White KP, Nielson WR, Harth M, Ostbye T, Speechley M. Chronic widespread musculo-skeletal pain with and without fibromyalgia: Psychological distress in a representative community adult sample. J Rheumatol. 2002;29:588–94. [PubMed] [Google Scholar]
- 19.Forseth KO, Husby G, Gran JT, Forre O. Prognostic factors for the development of fibromyalgia in women with self-reported musculoskeletal pain. A prospective study. J Rheumatol. 1999;26:2458–67. [PubMed] [Google Scholar]
- 20.Geisser ME, Casey KL, Brucksch CB, et al. Perception of noxious and innocuous heat stimulation among healthy women and women with fibromyalgia: Association with mood, somatic focus, and catastrophizing. Pain. 2003;102:243–50. doi: 10.1016/S0304-3959(02)00417-7. [DOI] [PubMed] [Google Scholar]
- 21.Sayar K, Gulec H, Topbas M. Alexithymia and anger in patients with fibromyalgia. Clin Rheumatol. 2004;23:441–8. doi: 10.1007/s10067-004-0918-3. [DOI] [PubMed] [Google Scholar]
- 22.Rutledge DN, Jones K, Jones CJ. Predicting high physical functioning in people with fibromyalgia. J Nurs Scholarsh. 2007;39:319–24. doi: 10.1111/j.1547-5069.2007.00187.x. [DOI] [PubMed] [Google Scholar]
- 23.Neumann L, Lerner E, Glazer Y, et al. A cross-sectional study of the relationship between body mass index and clinical characteristics, tenderness measures, quality of life, and physical functioning in fibromyalgia patients. Clin Rheumatol. 2008;12:1543–7. doi: 10.1007/s10067-008-0966-1. [DOI] [PubMed] [Google Scholar]
- 24.Moldofsky H. Rheumatic manifestations of sleep disorders. Curr Opin Rheumatol. 2010;22:59–63. doi: 10.1097/BOR.0b013e328333b9cc. [DOI] [PubMed] [Google Scholar]
- 25.Lee JW, Morton KR, Walters J, et al. Cohort profile: The Biopsychosocial Religion and Health Study (BRHS) Int J Epidemiol. 2009;38:1470–8. doi: 10.1093/ije/dyn244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler TL, Fraser GE, Beeson WL, et al. Cohort profile: The Adventist Health Study-2 (AHS-2) Int J Epidemiol. 2008;37:260–5. doi: 10.1093/ije/dym165. [DOI] [PubMed] [Google Scholar]
- 27.Ware JE, Jr, Kosinski M, Dewey JE. How to Score Version 2 of the SF-12® Health Survey. QualityMetric, Inc.; Lincoln, RI: 2002. [Google Scholar]
- 28.National Heart, Lung, and Blood Institute [(accessed August 2010)];Calculate your body mass index. 2009 Available at: http://www.nhlbisupport.com/bmi/
- 29.Cusack KJ, Frueh BC, Brady KT. Trauma history screening in a community mental health center. Psychiatr Serv. 2004;55:157–62. doi: 10.1176/appi.ps.55.2.157. [DOI] [PubMed] [Google Scholar]
- 30.Ryff CD, Singer BH, Palmersheim KA. Social inequalities in health and well-being: The role of relational and religious protective factors. In: Brim OG, Ryff CD, Kessler RC, editors. How Healthy Are We?: A National Study of Well-Being at Midlife. University of Chicago Press; Chicago: 2004. pp. 90–123. [Google Scholar]
- 31.McHugo GJ, Caspi Y, Kammerer N, et al. The assessment of trauma history in women with co-occurring substance abuse and mental disorders and a history of interpersonal violence. J Behav Health Serv Res. 2005;32:113–27. doi: 10.1007/BF02287261. [DOI] [PubMed] [Google Scholar]
- 32.Lazarus RS, Folkman S. Stress, Appraisal, and Coping. Springer; New York: 1984. [Google Scholar]
- 33.Shalev AY. Stress versus traumatic stress: From acute homeostatic reactions to chronic psychopathology. In: van der Kolk BA, McFarlane AC, Weisaeth L, editors. Traumatic Stress: The Effects of Overwhelming Experience on Mind, Body, and Society. Guilford; New York: 1996. pp. 77–101. [Google Scholar]
- 34.American Psychiatric Association . DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 35.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D Depression Symptoms Index. J Aging Health. 1993;5:179–93. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 36.Strong DR, Kahler CW, Greene RL, Schinka J. Isolating a primary dimension within the Cook–Medley Hostility Scale: A Rasch analysis. Pers Indiv Differ. 2005;39:21–33. [Google Scholar]
- 37.Hyman S. How adversity gets under the skin. Nat Neurosci. 2009;12:241–3. doi: 10.1038/nn0309-241. [DOI] [PubMed] [Google Scholar]
- 38.Raphael K, Marbach J, Klausner J. Myofascial face pain. Clinical characteristics of those with regional vs widespread pain. J Am Dent Assoc. 2000;131:161–71. doi: 10.14219/jada.archive.2000.0143. [DOI] [PubMed] [Google Scholar]
- 39.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behavior, and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 40.Maier SF, Watkins LR. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotrophin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–41. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 41.Arnsten AF. Stress signaling pathways that impair pre-frontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–22. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russell IJ, Vaeroy H, Javors M, Nyberg F. Cerebrospinal fluid biogenic amines metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550–6. doi: 10.1002/art.1780350509. [DOI] [PubMed] [Google Scholar]
- 43.Larson AA, Giovengo SL, Russell IJ, Michalek JE. Changes in the concentrations of amino acids in the cerebrospinal fluid that correlate with pain in patients with fibromyalgia: Implications for nitric oxide pathways. Pain. 2000;87:201–11. doi: 10.1016/S0304-3959(00)00284-0. [DOI] [PubMed] [Google Scholar]
- 44.Wood PB, Glabus MF, Simpson R, Patterson JC. Changes in gray matter in fibromyalgia: Correlation with dopamine metabolism. J Pain. 2009;10:609–18. doi: 10.1016/j.jpain.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Picavet HS, Hoeymans N. Health related quality of life in multiple musculoskeletal diseases: SF-36 and EQ-5D in the DMC3 study. Ann Rheum Dis. 2004;63:723–9. doi: 10.1136/ard.2003.010769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffman DL, Dukes EM. The health status burden of people with fibromyalgia: A review of studies that assessed health status with the SF-36 or the SF-12. Int J Clin Pract. 2008;62:115–26. doi: 10.1111/j.1742-1241.2007.01638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verbunt JA, Pernot DH, Smeets RJ. Disability and quality of life in patients with fibromyalgia. [(accessed August 2010)];Health Quality Life Outcomes. 2008 6:8. doi: 10.1186/1477-7525-6-8. doi:10.1186/1477-7525-6-8. Available at: http://www.hqlo.com/content/6/1/8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hardy JD, Smith TW. Cynical hostility and vulnerability to disease: Social support, life stress, and physiological response to conflict. Health Psychol. 1988;7:447–59. doi: 10.1037//0278-6133.7.5.447. [DOI] [PubMed] [Google Scholar]
- 49.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: The problems and implications of overlapped affective dispositions. Psychol Bull. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 50.Nabi H, Sing-Manoux A, Ferrie JE, et al. Hostility and depressive mood: Results form the Whitehall II Prospective Cohort Study. Psychol Med. 2010;40:405–13. doi: 10.1017/S0033291709990432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sullivan MJ, Rodgers WM, Kirsch I. Catastrophizing, depression, and expectancies for pain and emotional distress. Pain. 2001;91:147–54. doi: 10.1016/s0304-3959(00)00430-9. [DOI] [PubMed] [Google Scholar]
- 52.Pignone M, DeWalt DA, Sheridan S, Berkman N, Lohr KN. Interventions to improve health outcome for patients with low literacy: A systematic review. J Gen Intern Med. 2005;20:185–92. doi: 10.1111/j.1525-1497.2005.40208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopez-Leon S, Choy WC, Aulchenko YS, et al. Genetic factors influence the clustering of depression among individuals with lower socioeconomic status. Plos One. 2009;4:e5069. doi: 10.1371/journal.pone.0005069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anandacoomarasamy A, Fransen M, March L. Obesity and the musculoskeletal system. Curr Opin Rheumatol. 2009;21:71–7. doi: 10.1097/bor.0b013e32831bc0d7. [DOI] [PubMed] [Google Scholar]
- 55.Goldenberg DM. The interface of pain and mood disturbances in the rheumatic diseases. Semin Arthritis Rheum. 2010;40:15–31. doi: 10.1016/j.semarthrit.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Przekop P, Przekop A, Haviland MG, Riggs ML. Neurocognitive enhancement for the treatment of chronic pain. J Bodywork Mov Ther. 2010;14:334–5. [Google Scholar]


