Abstract
We recently investigated the roles of the phototropin 1 (PHOT1) LOV (light, oxygen or voltage) domains in mediating phototropic curvature in transgenic Arabidopsis seedlings expressing either wild-type PHOT1 or PHOT1 with one or both LOV domains inactivated by a single amino acid replacement. We have now investigated the role of the PHOT1 LOV domains in chloroplast movement and in leaf positioning in response to blue light. Low fluence rate blue light is known to mediate a chloroplast accumulation response and high fluence rate blue light an avoidance response in Arabidopsis leaves. As was the case for phototropism, LOV2 of PHOT1 is essential for chloroplast accumulation and LOV1 is dispensable. PHOT1 LOV2 is also essential to maintain developing primary leaves in a horizontal position under white light from above and LOV1 is again dispensable. A red light pulse given to dark-adapted light-grown plants followed by 2 h of darkness enhances both the chloroplast accumulation response under dim blue light and the chloroplast avoidance response under strong blue light. The effect is far-red reversible. This photoreversible response is normal in a phyB null mutant but does not appear in a phyA null mutant. These results suggest that phyA mediates the enhancement, induced by a red light pulse, of blue light-induced chloroplast movements.
Keywords: Arabidopsis, Chloroplast, movement, LOV, domain, Phototropin, Phytochrome
Introduction
Phototropins are blue light receptors that mediate a wide range of physiological and developmental responses, including phototropism, chloroplast movements, stomatal opening, leaf flattening, leaf positioning and the rapid inhibition of growth of etiolated hypocotyls (Christie 2007). Some of these responses serve to maximize photosynthetic potential in weak light, while others act to prevent damage to the photosynthetic apparatus in intense light. For example, light-induced chloroplast relocation is thought to provide an adaptive function. In response to low-intensity blue light, chloroplasts accumulate along the outer periclinal cell walls of mesophyll cells, in a plane perpendicular to the incident light, thus maximizing light harvesting. However, in high-intensity blue light, chloroplasts move to the anticlinal walls, parallel to the incident light (Wada et al. 2003). This avoidance response allows chloroplasts to minimize high-intensity light stress. Mutants impaired in phototropin activity show hypersensitivity to light stress (Kasahara et al. 2002). Appropriate young leaf positioning is also essential for photosynthesis in response to blue light. In low-intensity blue light, newly emerged leaves become oriented at right angles to incident light, a response that optimizes photosynthetic performance (Inoue et al. 2008).
There are two phototropins in Arabidopsis, PHOT1 and PHOT2, which share sequence homology. Although PHOT1 and PHOT2 overlap in function in phototropism, stomatal opening, leaf expansion and chloroplast accumulation, they exhibit certain distinct functions and photosensitivities. The chloroplast high-light avoidance response is mediated exclusively by PHOT2 (Jarillo et al. 2001, Kagawa et al. 2001) whereas the rapid inhibition of elongation of etiolated hypocotyls is mediated solely by PHOT1 (Folta and Spalding 2001).
The N-terminal halves of both phototropins contain two FMN-binding domains approximately 110 amino acids in length (Christie et al. 1999) designated LOV1 and LOV2, respectively, because they resemble similar domains in otherwise very dissimilar signaling proteins that respond exclusively either to light, oxygen or voltage (Huala et al. 1997). A recent C-terminal/N-terminal swapping experiment between PHOT1 and PHOT2 showed that the different sensitivities of PHOT1 and PHOT2 in phototropism depend entirely on the N-terminal (LOV1 plus LOV2) domain (Aihara et al. 2008). Curiously, both the PHOT1 N-terminal moiety (LOV1 and LOV 2) fused to the PHOT2 C-terminal moiety and the PHOT2 N-terminal moiety fused to the PHOT1 C-terminal moiety were capable of mediating the chloroplast avoidance response. Thus either half of PHOT2 is sufficient for an avoidance response. Kaiserli et al. (2009) performed a series of finer resolution domain-swapping experiments with PHOT1, concentrating on the roles of LOV1, LOV2 and the intervening linker sequence. Their results support a mechanism whereby PHOT1 LOV2 acts as a dark state repressor of PHOT1 kinase activity. Replacing LOV2 with LOV1 no longer represses the kinase activity and it becomes constitutive. Their results also suggest that PHOT1 LOV1 can suppress chloroplast accumulation at high-light intensities, evidence for a possible role of PHOT1 LOV1 in a physiological response to blue light.
Our previous studies have shown that the LOV2 domain of PHOT1 plays a major role in phototropism and leaf expansion, while the PHOT1 LOV1 domain is not functional for these responses (Cho et al. 2007). However, the LOV1 domain of PHOT2 was found to provide partial complementation for phototropism, the first evidence for a possible role for PHOT2 LOV1 in a physiological response to blue light. In the present study, we investigated the physiological functions of LOV domains of Arabidopsis PHOT1 and PHOT2 in chloroplast movement and leaf positioning. In order to characterize each LOV domain of PHOT1 and PHOT2, we used a phot1 phot2 double mutant transformed separately with constructs encoding each phototropin with both LOV domains functional, with only LOV2 functional, with only LOV1 functional or with neither LOV domain functional, as described by Cho et al. (2007). Unlike the above-mentioned domain-swapping experiments, involving major protein rearrangements, these experiments involved only the minimal perturbation caused by a single amino acid substitution. In all cases, LOV domains were inactivated by substituting an alanine for the reactive cysteine. In the course of studying aspects of the regulation of chloroplast movements by blue light, we also investigated the possible role of the phytochromes A and B (phyA and phyB) in modulating chloroplast movements mediated by the phototropins in response to blue light.
Results
Role of phototropin LOV domains in chloroplast movement
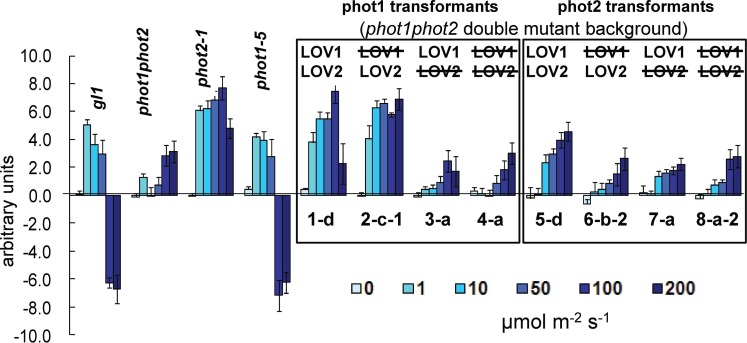
To investigate the function of the two phototropin LOV domains in PHOT1 and PHOT2 on chloroplast movement, we grew the transgenic lines transformed with the wild-type phototropin genes PHOT1 or PHOT2, or these genes with one or both of the two LOV domains inactivated. We measured chloroplast accumulation or avoidance in a central strip across Arabidopsis leaves on illumination with blue light of different fluence rates (Fig. 1). Blue light treatment of any of the four non-transformed lines (gl1, phot1-5/phot2-1, phot 1-5 or phot2-1) produced a response as predicted by the literature (Kagawa et al. 2001, Suetsugu and Wada 2003): whenever PHOT2 was present (gl1 and phot1-5), low fluence rates induced accumulation (above the 0.0 line in the figure) and high fluence rates induced avoidance (below the 0.0 line). The double mutant (phot1-5 phot2-1) was not entirely inactive, but showed a slight accumulation response, probably caused because the phot2-1 mutation is known to be leaky (Cho et al. 2007). With wild-type levels of PHOT1 present (the phot2-1 mutant), accumulation was the only response. The reduced chloroplast accumulation in this line at the highest fluence rate tested could have been caused by the small amount of PHOT2 produced by the leaky phot2-1 mutant which has a point mutation in the splice site juction, in antagonism to the PHOT1-mediated accumulation movement (see Discussion).
Fig. 1.
Chloroplast movement phenotypes in wild-type (gl1), phot single mutants (phot1-5 and phot2-1), the untransformed double mutant (phot1-5 phot2-1) and representative double mutant phot1-5 phot2-1 seedlings transformed with PHOT expression constructs. Leaves were treated for 30 min at the indicated fluence rates of blue light. Images of the chloroplast movement; a comparison of the darkness of the illuminated strip with that of the covered areas was analyzed by Image J software. Error bars are the SEM. n = 12. The experiment was carried out three times with similar results.
Although the various transformant lines selected for this study (1-d, 2-c-1. etc., in fig. 1 from Cho et al. 2007) all expressed mRNA levels equal to or greater than gl1; protein levels as determined by Western blotting were considerably lower than those of the wild type in all cases (Supplementary Fig. S1; fig. 1B in Cho et al. 2007). Nevertheless the results can be interpreted in terms of the levels of the phototropin proteins actually produced by the transformants. When the wild-type PHOT1 gene was transformed into the double mutant (line 1-d), the response was entirely in the direction of accumulation. However, at the highest fluence rate tested, the accumulation response was strongly inhibited. While the protein level for this transformant was below that of PHOT1 in wild-type plants (fig. 1 in Cho et al. 2007), the amount could have been sufficient to allow for at least a partial PHOT2-mediated avoidance response. Alternatively, the presence of functional LOV1 may have suppressed the accumulation response at the highest fluence rate, a mechanism supported by the results of Kaiserli et al. (2009). With the transgene expressing phot1 with only LOV2 functioning (line 2-c-1), the level of protein was sufficient for a strong positive accumulation response. Note that in the absence of LOV1, there is no suppression of the accumulation response in this line, again supporting the mechanism proposed by Kaiserli et al. (2009). Line 3-a (only LOV1 functional) and line 4-a (neither LOV domain functional) both failed to show more than the slight response attributable to a small amount of PHOT2 protein produced in the leaky phot2 mutant. The results above are consistent with other studies indicating the primary role of LOV2 in several physiological responses.
As was the case with the PHOT1 transformants, mRNA levels in all of the PHOT2 transformants were equal to or higher than those in wild-type plants (Cho et al. 2007). However, as with the PHOT1 transformants, protein levels were well below those of the gl1 line. Only those plants transformed with the PHOT2 wild-type gene showed some limited chloroplast accumulation (Fig. 1, line 5-d). No complementation was observed for the PHOT1 LOV1 LOV2 plant (line 6-b-2). Indeed, except for the PHOT2 transformant with both LOV domains intact, the weak accumulation response in all of the PHOT2 transformants did not differ from the responses of the phot1 phot2 double mutant. In no case did we observe an avoidance response in the phot2-complemented plants in this experiment. Thus, we hypothesized that in the absence of all PHOT1 and almost all PHOT2, there is likely to be insufficient expression of the transgenic PHOT2, plus a trace of the wild-type protein, to mediate an avoidance response. It could be that the avoidance response is not activated until the accumulation response is saturated—by either or both phototropins.
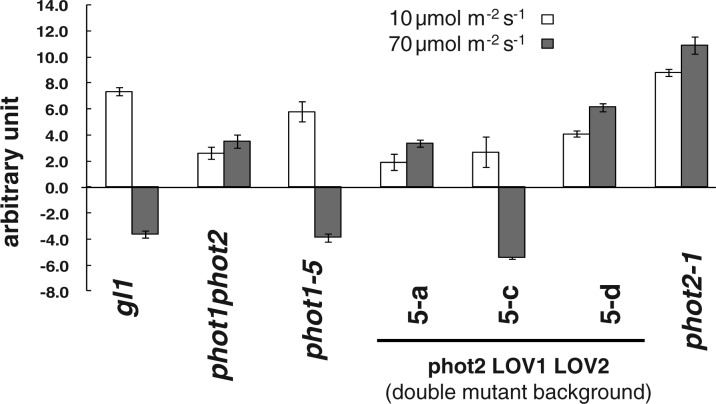
To test this hypothesis, we carried out Western blot analysis and chloroplast movement experiments for lines 5-a, 5-c and 5-d from Cho et al. (2007) that had all been transformed with the fully functional PHOT2 gene. These lines all showed mRNA levels above those for wild-type PHOT2 plants (Cho et al. 2007). Line 5-c showed the highest expression both of the phot2 transgene (fig. 1C in Cho et al. 2007) and of the PHOT2 protein (Fig. 2). Not surprisingly, line 5-a was no different from the phot1 phot2 double mutant and failed to show any chloroplast response (Fig. 3). Line 5-d showed slight accumulation at both light intensities, somewhat above that shown by the double mutant. However, line 5-c, with its higher expression level (Fig. 2), actually showed a significant avoidance response at the higher fluence rate (Fig. 3). Evidently the levels of PHOT2 protein present were sufficient for an avoidance response in this case. Note that line 5-c (Fig. 2) shows two bands. This is also the case for lines 7-a, 8-a-2 and 8-c-2 (Supplementary Fig. S1). All of these lines show expression of mRNA well above the basal level seen in the phot1 phot2 double mutant. We attribute the lower band to leaky expression of the wild-type gene. We suggest that the protein from the transgene is fully phosphorylated whereas the leaked protein from the mutant gene is not. The separation is what we would expect if the difference is the consequence of differential phosphorylation (see Cho et al. 2007, fig. 7, lanes 2 and 4 to compare unphosphorylated and phosphorylated phot2.
Fig. 2.
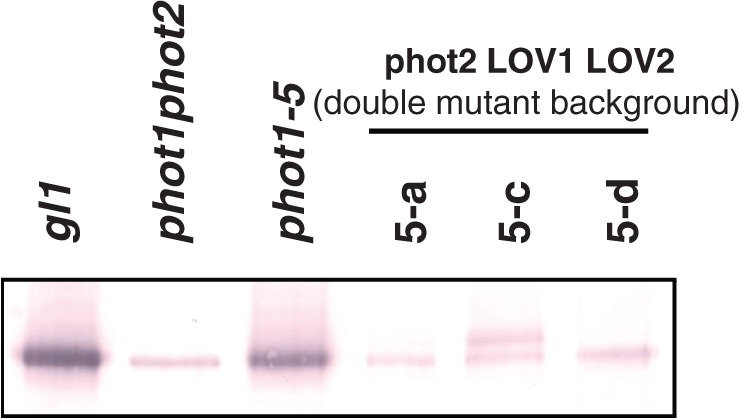
Western blots of membrane fractions prepared from seven different Arabidopsis lines and probed with anti-PHOT2 antibody. Note that the transformant lines 5-a, 5-c and 5-d express levels of PHOT2 far below those of the wild type (gl1) or phot1-5. Lines 5-a and 5-d do not appear to express more protein than the phot1 phot2 double mutant although line 5-d does show some limited complementation (see text).
Fig. 3.
Quantitative representation of chloroplast movements in the seven lines shown in Fig. 2 in response to blue light. The two fluence rates were chosen so that the lower one induced an accumulation response and the higher one induced an avoidance response in wild-type (gl1) leaves. Of the three transformant lines, only line 5-c shows an avoidance response. Error bars are the SEM. n = 12. The experiment was carried out three times with similar results.
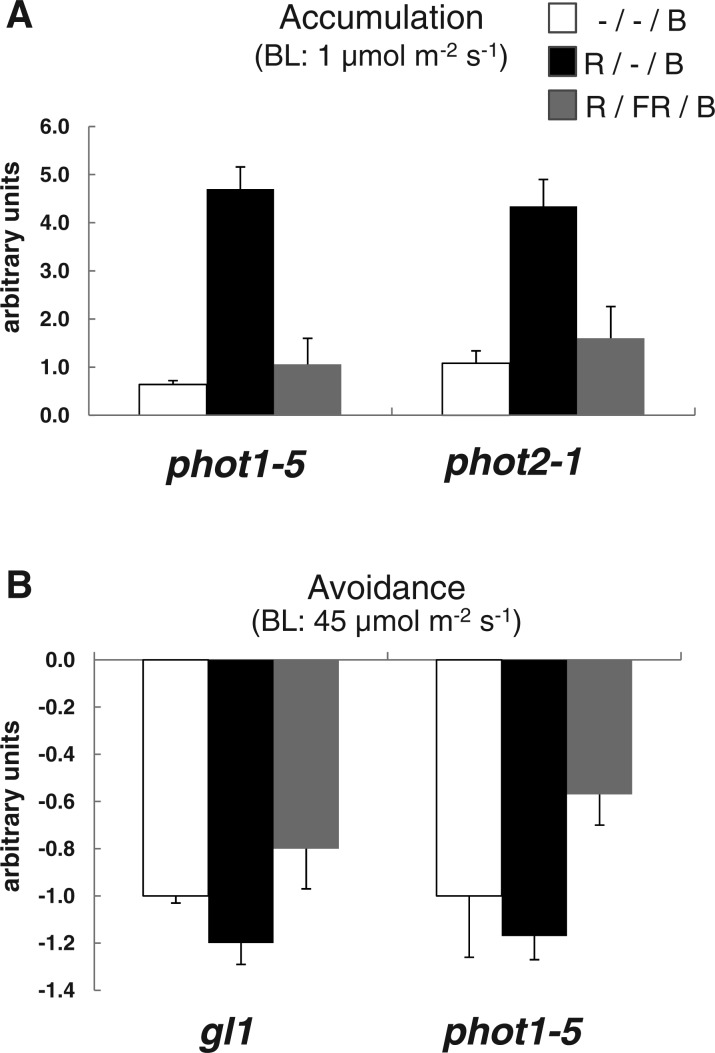
Fig. 7.
Red light enhances chloroplast movement and its effect is reversed by far-red light. Leaves were treated with the indicated fluence rate of blue light. Quantitative representation of the chloroplast movement. Light-grown Arabidopsis plants 3–4 weeks old were dark adapted for 1.5 h before red light treatment: either red light alone (R) (10 µmol m−2 for 10 s) or red light (10 µmol m−2 for 10 s) followed immediately by far-red (FR) (42 µmol m−2 for 30 s). A 2 h dark incubation period followed the red or red/far-red treatment prior to blue light (B) treatment (either 1 or 45 µmol m−2 s−1 for chloroplast accumulation or avoidance, respectively). (A) Chloroplast accumulation during 30 min low fluence rate blue light (1 µmol m−2 s−1). (B) Chloroplast avoidance during 30 min high fluence rate blue light (45 µmol m−2 s−1). Images of chloroplast movement were analyzed by Image J software as described for Fig. 1. Error bars are the SEM. n = 12 or more. The experiment was carried out three times with similar results.
Note that line 5-c (both LOV domains intact) also complemented the leaf flattening response (Fig. 4, Table 1), whereas lines 5-a and 5-d both failed to do so. Cho et al. (2007) did obtain partial complementation of flattening with line 5-d, possibly because their plants were slightly younger than ours. In addition, in their hands, line 5-d showed complete complementation for PHOT2-mediated phototropism of etiolated hypocotyls. Thus the level of PHOT2 protein expression in the etiolated hypocotyl of line 5-d is sufficient for phototropism whereas the level in the leaves is insufficient for complete leaf flattening.
Fig. 4.
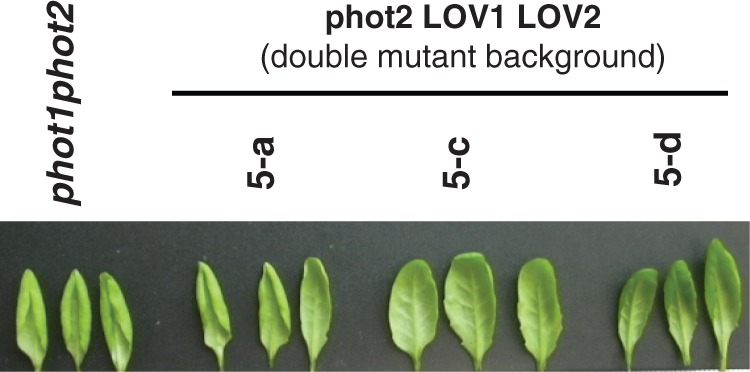
Leaf shapes of the transformed plants with the PHOT2 LOV1 LOV2 construct. Only line 5-c complements the leaf-flattening response.
Table 1.
Leaf unrolling indices for the wild type and for the lines shown in Fig. 4 (see text for details)
| Line | Average index | n | SEM |
|---|---|---|---|
| gl1 | 0.9 | 210 | ±0.006 |
| phot1 phot2 | 0.6 | 911 | ±0.013 |
| Line 5-a | 0.6 | 88 | ±0.011 |
| Line 5-c | 0.8 | 39 | ±0.010 |
| Line 5-d | 0.6 | 58 | ±0.011 |
Role of the LOV domain in leaf positioning
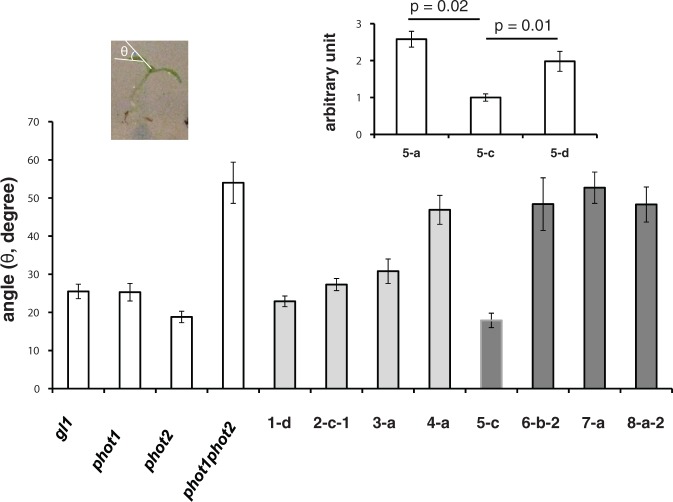
To optimize photosynthesis, plants are capable of responding to blue light by appropriate leaf positioning (Takemiya et al. 2005). We characterized the leaf-positioning phenotypes of the selected PHOT LOV transgenic lines as previously described (Inoue et al. 2008). These plants were irradiated with white light (100 µmol m−2 s−1) from above for 24 h. Not surprisingly leaf orientation was normal in the wild type (gl1) and in both phot2-1 and phot1-5. As illustrated in Fig. 5, the first true leaves were oriented horizontally, at right angles to the incident white light in these three lines. As expected, the double mutant phot1-5 phot 2-1 lacked normal leaf positioning. Fig. 6 shows the quantitative data for the controls and the various transformant lines. Among the transformants, the PHOT1 wild-type gene (line 1-d) and PHOT1 LOV1 LOV2 transgenic line (line 2-c-1) both showed complementation, and line 3-a showed partial complementation. However, neither the PHOT1 LOV1 LOV2 gene (line 3-a) nor the PHOT1 LOV1LOV2 gene (line 4-a) provided normal leaf positioning in response to white light. Although line 7-a showed partial complementation of hypocotyl phototropism (fig. 3B in Cho et al. 2007), it failed to complement the leaf positioning response. This observation is consistent with our results (Fig. 1) indicating that LOV1 in PHOT2 does not complement the chloroplast movement response. These different apparent sensitivities suggest that different phototropin levels may be required for the different responses. Note that lines 5-a and 5-d, both expressing detectable amounts of the transformant protein (Fig. 2), complement the leaf positioning phenotype less than line 5-c. Since none of the other transgenic lines showed complementation, probably because of low protein expression levels (Supplementary Fig. S1), little can be said about the role of LOV2 in PHOT2 for this response.
Fig. 5.

Leaf positioning of wild-type, phot1, phot2 and phot1 phot2 mutants of Arabidopsis grown under white light. Normal leaf positioning was induced by illumination with white light (∼35 µmol m−2) for 24 h. This experiment was carried out three times with 12 plants, each time with similar results.
Fig. 6.
Quantitation of the leaf positioning results. In the mutants and the transformants, the higher the angles between the leaf blades and the petioles, the smaller the positioning response and the weaker the complementation. This experiment was carried out twice with similar results. In all cases, n = 15 or above. Error bars are the SEM.
Red light effects on chloroplast movement
A recent study reported that brief pulses of red light given 2 h prior to phototropic induction by low fluence rates of blue light could prevent the movement of PHOT1–green fluorescent protein (GFP) from the plasma membrane into the cytoplasm in etiolated Arabidopsis seedling, and that this retention of PHOT1–GFP may contribute to the red light-induced enhancement in phototropic response to blue light (Han et al. 2008). This red light-induced increase in phototropic sensitivity was shown to be mediated by phyA and not by phyB. It was necessary to interpose a 2 h dark period between the red light pulse and the blue light treatment in order to obtain the full phytochrome effect. Thus we tested whether there might be a similar red light effect on the chloroplast movement responses induced by blue light.
Plants dark-adapted for at least 1.5 h as for the chloroplast experiments above were given a pulse of red light and then kept in the dark for 2 h prior to blue light treatment. Fig. 7A shows that a red light pulse (100 µmol m−2) prior to exposure to low-fluence blue light significantly increases chloroplast accumulation in both phot1-5 and phot2-1 plants. Chloroplast movement after blue light treatment was quantified as before. If the effect of red light is indeed phytochrome mediated, it should be at least partially far-red reversible. We treated leaves with red light as above followed immediately by 1,260 µmol m−2 of far-red light. The far-red light reversed the enhanced chloroplast accumulation by the red light (Fig. 7A). The enhancement and its reversal occurred whether the accumulation response was activated by PHOT1 or by PHOT2. To examine the effect of red light on the chloroplast avoidance response, we used the wild type (gl1) and also phot1-5 (because only PHOT2 contributes to the avoidance response). Although the effect of red light on chloroplast avoidance was marginal (Fig. 7B), subsequent far-red light clearly reduced the avoidance response. Thus, both the accumulation and avoidance results show far-red reversibility and hence phytochrome participation (Fig. 7).
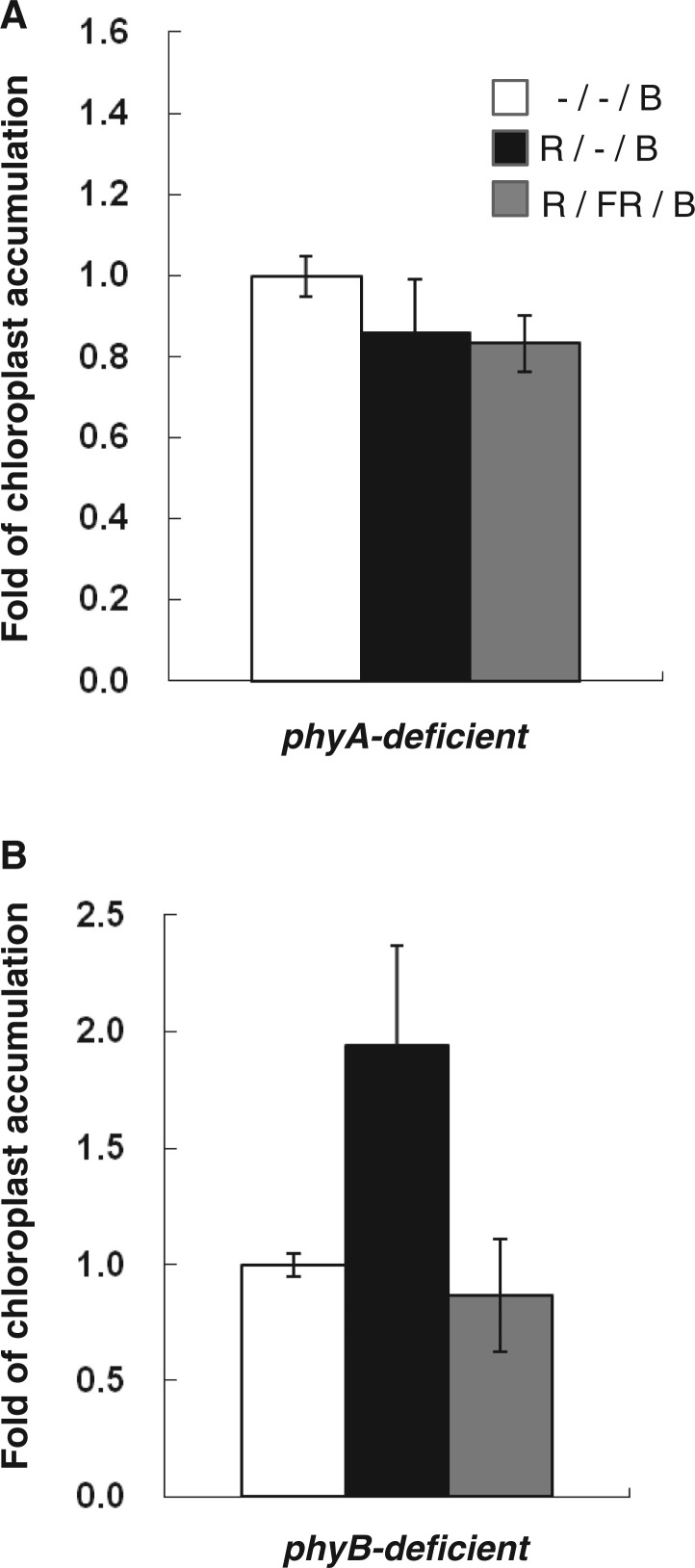
To test whether the red light-enhanced chloroplast movement induced by blue light was mediated by phyA or phyB, we tested the phytochrome-deficient mutants phyA-211 or phyB-9 (Reed et al. 1993, Reed et al. 1994) for the enhancement of blue light-induced chloroplast accumulation by red light. Fig. 8 shows that whereas red light was fully effective in enhancing chloroplast accumulation, this effect was fully reversed by immediate subsequent far-red light in phyB-deficient plants. However, any effect of red light alone or red light followed by far-red light was absent in phyA-deficient plants. Hence, chloroplast accumulation in response to blue light is enhanced by phyA-mediated red light pre-treatment, as is the case for hypocotyl phototropism (Han et al. 2008), but not by phyB.
Fig. 8.
Red light inhibition of blue light-induced chloroplast accumulation is mediated by phyA. (A) In the absence of phyA, red light fails to enhance blue light-induced chloroplast accumulation. (B) In the absence of phyB, red light is fully effective in enhancing blue light-induced chloroplast accumulation. Irradiation and dark incubation protocols as in Fig. 6. Blue light fluence rate (1 µmol m−2 s−1). Images of the chloroplast movement were analyzed by Image J software. Error bars are the SEM. n = 12 or more. The experiment was carried out three times with similar results.
Discussion
We have investigated the relative roles of LOV1 and LOV2 of phototropins, PHOT1 and PHOT2 in phototropin-mediated chloroplast movement and leaf positioning by using PHOT-expressing transgenic plants (Cho et al. 2007). Our previous study showed that the LOV2 domains of both PHOT1 and PHOT2 play a major role in phototropism and leaf flattening. To examine additionally the specific roles of the LOV domains of PHOT1 and PHOT2, we first examined the chloroplast movement in mesophyll cells in response to blue light as PHOT1 and PHOT2 have different photosensitivities for these movements (Sakai et al. 2001). PHOT1 functions in mediating chloroplast accumulation with a threshold of 0.4 µmol m−2 s−1, whereas PHOT2 has a higher fluence rate threshold for accumulation (2 µmol m−2 s−1). Its upper limit for maximum accumulation is near 16 µmol m−2 s−1. Only PHOT2 can mediate the avoidance response of chloroplasts to stronger blue light (>32 µmol m−2 s−1).
In our study (Fig. 1), PHOT1 wild-type (1-d) and PHOT1 LOV2 (2-c-1) both complemented the low light accumulation response, indicating that the LOV2 domain of PHOT1 alone is fully functional for choloroplast accumulation as in the phototropic response (Cho et al. 2007). Under high-light conditions (200 µmol m−2 s−1), the PHOT1 wild-type transformant (line 1-d) exhibited less accumulation than it showed at 100 µmol m−2 s−1, while PHOT1 LOV1 LOV2 (2-c-1) did not show any such loss of accumulation. The loss in the wild-type transformant (line 1-d) could be attributed to the PHOT2 protein because the phot2-1 mutant and phot1phot2 double mutant still produces a small level of full-length PHOT2 protein (Cho et al. 2007). Likewise, the reduction in accumulation in phot2-1 at the highest fluence rate could also be mediated by a small amount of functional PHOT2. This hypothesis requires that the accumulation response must be fully saturated by either or both phototropins before an avoidance response can be induced. The absence of such a loss of accumulation at the highest fluence rate in line 2-c-1 (only LOV2 functional) could be because of the lower protein expression for line 2-c-1 compared with that for line 1-d (Supplementary Fig. S1). Alternatively, LOV1 may be required to down-regulate the response mediated by LOV2. Indeed LOV1 can down-regulate LOV2 in an in vitro phosphorylation reaction (Matsuoka and Tokutomi 2005). A recent study also identified a photoactive role for LOV1 in PHOT1 in arresting chloroplast accumulation at high light intensities (Kaiserli et al. 2009).
Overall, the functional role(s) of LOV1 remains somewhat obscure. It was also proposed that the LOV1 domain may serve as a dimerization site, although it is unknown what effect dimerization might have on phototropin function (Salomon et al. 2004). Since PHOT2 LOV1 could mediate a small amount of blue light-activated autophosporylation, it might be expected to play a minor role in physiological functions of PHOT2 without its LOV2 domain (Cho et al. 2007). Finally, Kagawa et al. (2004) suggested that the function of LOV1 is to prolong the lifetime of phototropin receptor activation. Thus although the primary role of LOV1 is still not resolved, there are both physiological and biochemical hints that might eventually resolve its function(s).
We observed that high fluence rate blue light could not induce chloroplast avoidance in a PHOT2 wild-type transgenic plant of line 5-d (Fig. 3). Instead, chloroplast accumulation was only increased with the intensity of blue light. Note, however, that the expression level in line 5-d was sufficient to support a strong phototropism response (Cho et al. 2007). We hypothesize that the higher expression of the PHOT2 protein is required to trigger the chloroplast avoidance response because the phototropic response mediated by the PHOT2 N-terminal moiety is about 250 times less sensitive than that by PHOT1 (Aihara et al. 2008). We investigated the possibility by using the line 5-c plant, which has more heterologous PHOT2 wild type than either line 5-a or line 5-d. The avoidance response to high fluence blue light was indeed detected only in the line 5-c plant (PHOT2 wild type). The study suggests that a fairly high level of PHOT2 wild type is required to carry out the chloroplast avoidance response. Again, perhaps the accumulation response must be at saturation before a PHOT2-mediated avoidance response can be initiated. This suggestion is supported by another study showing that a phot2 heterozygous mutant (PHOT2/phot2-1) retains the avoidance response, but the velocity of its chloroplast movement during avoidance is reduced to half of that found in wild-type plants (Kagawa and Wada, 2004). Note that line 5c has sufficient phot2 to support normal leaf positioning (Fig. 6).
The nature of phototropin-mediated responses is diverse. Phototropic responses and leaf flattening are slower symmetric growth processes coordinated by cell expansion and division. On the other hand, chloroplast relocations, leaf positioning and stomatal opening are cell-autonomous and are reversible processes. The LOV2 domain in both phototropins appears to be fundamental to both types of responses. PHOT1 LOV1 may play some role in chloroplast movement (Kaiserli et al. 2009). When protein levels of PHOT2 are very low, LOV1 alone fails to support chloroplast movement, but is nevertheless sufficient to mediate at least a weak phototropic response in etiolated Arabidopsis hypocotyls (Cho et al. 2007). Thus LOV1 may play a minor role in both types of responses—growth processes and reversible processes.
Previous studies (DeBlasio et al. 2003, Luess et al. 2010) showed that red light plays a role in the blue light-induced chloroplast movement through phytochrome. Both phyA and phyB mutants show altered chloroplast movement responses to blue light. Our experiments probe a different aspect of phytochrome's role in the chloroplast movement response. In our hands, very small amounts of red light can enhance both chloroplast accumulation and chloroplast avoidance when given to dark-adapted leaves 2 h prior to the onset of blue light. The red light effect is fully far-red reversible. This result is parallel to the red light enhancement of phototropism where a similar red light treatment followed by a dark period enhances phototropism (Parks et al. 1996, Janoudi et al. 1997, Han et al. 2008), a response that is far-red reversible. In the present study, however, the red light-induced enhancement response reported here is occurring in light-grown plants that have had only a 1.5 h pre-treatment in darkness prior to red light treatment. Nevertheless, the response is mediated by phyA and not phyB, although phyB is the dominant phytochrome in light-grown plants. Clearly these light-grown plants have sufficient phyA to mediate an important physiological response, and the enhancement of a phototropin-mediated response by phyA activation is not restricted to etiolated seedlings.
Han et al. (2008) showed that red light prevented the blue light-induced migration of PHOT1–GFP away from the plasma membrane in etiolated hypocotyls and in some way enhanced its capacitiy to induce lateral auxin transport. Whether red light-induced retention of the phototropins at the plasma membrane of leaf mesophyll cells can account for the enhancement of chloroplast movements in response to red light remains to be investigated. At present, the retention phenomenon reported by Han et al. (2008) for PHOT1 has not been reported for PHOT2. Both the effect of red light on phototropism and that on chloroplast movement are mediated by phyA and not phyB, as is the requirement for the phytochrome-mediated retention of PHOT1–GFP at the plasma membrane. It will be interesting to determine whether other phototropin-mediated responses are also similarly affected by prior photoactivation of PHYA.
Materials and Methods
Plant materials and growth conditions
We used the transformant lines of Arabidopsis thaliana (Columbia ecotype, gl1 background) constructed by Cho et al. (2007). These transformants were phot1-5 phot2-1 double mutants transformed with a construct encoding either one or the other of the two phototropins. In different constructs, the various LOV domains were inactivated by replacing the reactive cysteines with alanines. Thus studies on the possible function of PHOT1 LOV domains were carried out in plants lacking functional PHOT2 and vice versa. For investigations of chloroplast movement, seeds were germinated and the seedlings grown in soil in pots for 3–4 weeks under a photoperiod of 16 h light and 8 h dark in a greenhouse after initial treatment at 4°C for 3 d. The phytochrome null mutants phyA-211 and phyB-9, expressing the PHOT1::PHOT1-GFP gene (Han et al. 2008), were used to test for any phytochrome-modulated blue light-induced chloroplast movement. For investigations on leaf positioning, seedlings were germinated and grown for 8 d under continuous white light from above.
Light sources
For the induction of chloroplast movement, a bank of light-emitting diodes (LEDs; λmax 470 nm) was used to obtain a range of fluence rates of blue light from 1 to 200 µmol m−2 s−1. Blue light treatments were always for 30 min. The red light source used to treat seedlings in experiments investigating phytochrome effects on chloroplast movement was a bank of red LEDs (λmax 630 nm, fluence rate 10 µmol m−2 s−1). The source of far-red light was a 12 V, 20 W tungsten halogen lamp filtered through a Rohm and Haas FRF Plexiglas filter (fluence rate 4 µmol m−2 s−1).
Analysis of chloroplast movement
Leaves from 3- or 4-week-old plants were cut at the petioles and placed adaxial side up on wet filter paper in a custom-made assembly essentially as described by Kagawa et al. (2001). The leaves were covered by a black cover glass with a slit window approximately 1.5 mm in width at right angles to the main vein and dark-adapted for at least 1.5 h before each experiment. They were then irradiated with blue light through the slits for 30 min. After removing the cover slips, the leaves were scanned with an Epson 3170 scanner. Chloroplast movements were monitored by following changes in leaf color in the irradiated region compared with the region kept shaded. Areas of chloroplast accumulation appear darker compared with the shaded areas, whereas areas of chloroplast avoidance appear lighter. Quantitative data were obtained using Image J software (http://rsb.info.nih.gov/ij/). Error bars indicate the SEM. Four to 12 leaves were tested for each experiment.
Determination of leaf positioning
Wild-type and transformant Arabidopsis plants were grown under constant white light (45 µmol m−2 s−1) at 22°C for 8 d and then immediately photographed. The angles between the petioles and the first leaf blades were measured from photographs.
Western blotting
Crude protein extracts were prepared from 2-week-old light-grown plants. Western blot analysis was performed using a polyclonal PHOT2 antibody (1 in 2,500 dilution) as described previously (Cho et al. 2007).
Supplementary data
Supplementary data are available at PCP online.
Funding
This work was supported by the Ministry of Education, Science and Technology [the General Researcher Supporting Fund through the National Research Foundation of Korea (NRF) (2009-0072217) to I.-S.H]; National Science Foundation [grant 843617 to W.R.B.]; the BK-21 Fund [to A.M. and A.L.].
Supplementary Material
Acknowledgment
The authors thank Dr. Tong-Seung Tseng for maintaining the many transformant lines and for his advice throughout the project and his careful review of the manuscript
Glossary
Abbreviations
- GFP
green fluorescent protein
- LED
light-emitting diode
- LOV domain
light, oxygen or voltage domain
- phy
phytochrome
- PHOT
phototropin.
References
- Aihara Y., Tabata R., Suzuki T., Shimazaki K., Nagatani A. Molecular basis of the functional specificities of phototropin 1 and 2. Plant J. 2008;56:364–375. doi: 10.1111/j.1365-313X.2008.03605.x. [DOI] [PubMed] [Google Scholar]
- Cho H.Y., Tseng T.S., Kaiserli E., Sullivan S., Christie J.M., Briggs W.R. Physiological roles of the light, oxygen, or voltage domains of phototropin 1 and phototropin 2 in Arabidopsis. Plant Physiol. 2007;143:517–529. doi: 10.1104/pp.106.089839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie J.M. Phototropin blue-light receptors. Annu. Rev. Plant Biol. 2007;58:21–45. doi: 10.1146/annurev.arplant.58.032806.103951. [DOI] [PubMed] [Google Scholar]
- Christie J.M., Reymond P., Powell G.K., Bernasconi P., Raibekas A.A., Liscum E., et al. Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science. 1998;282:1698–1701. doi: 10.1126/science.282.5394.1698. [DOI] [PubMed] [Google Scholar]
- Christie J.M., Salomon M., Nozue K., Wada M., Briggs W.R. LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proc. Natl Acad. Sci. USA. 1999;96:8779–8783. doi: 10.1073/pnas.96.15.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBlasio S.L., Mullen J.L., Luesse D.R., Hangarter R.P. Phytochrome modulation of blue light-induced chloroplast movements in Arabidopsis. Plant Physiol. 2003;133:1471–1479. doi: 10.1104/pp.103.029116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folta K.M., Spalding E.P. Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J. 2001;26:471–478. doi: 10.1046/j.1365-313x.2001.01038.x. [DOI] [PubMed] [Google Scholar]
- Han I.S., Tseng T.S., Eisinger W., Briggs W.R. Phytochrome A regulates the intracellular distribution of phototropin 1–green fluorescent protein in Arabidopsis thaliana. Plant Cell. 2008;20:2835–2847. doi: 10.1105/tpc.108.059915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huala E., Oeller P.W., Liscum E., Han I.S., Larsen E., Briggs W.R. Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science. 1997;278:2120–2123. doi: 10.1126/science.278.5346.2120. [DOI] [PubMed] [Google Scholar]
- Inoue S., Kinoshita T., Takemiya A., Doi M., Shimazaki K. Leaf positioning of Arabidopsis in response to blue light. Mol. Plant. 2008;1:15–20. doi: 10.1093/mp/ssm001. [DOI] [PubMed] [Google Scholar]
- Janoudi A.K., Gordon W.R., Wagner D., Quail P., Poff K.L. Multiple phytochromes are involved in red-light-induced enhancement of first-positive phototropism in Arabidopsis thaliana. Plant Physiol. 1997;113:975–979. doi: 10.1104/pp.113.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarillo J.A., Gabrys H., Capel J., Alonso J.M., Ecker J.R., Cashmore A.R. Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature. 2001;410:952–954. doi: 10.1038/35073622. [DOI] [PubMed] [Google Scholar]
- Kagawa T., Kasahara M., Abe T., Yoshida S., Wada M. Function analysis of phototropin2 using fern mutants deficient in blue light-induced chloroplast avoidance movement. Plant Cell Physiol. 2004;45:416–426. doi: 10.1093/pcp/pch045. [DOI] [PubMed] [Google Scholar]
- Kagawa T., Sakai T., Suetsugu N., Oikawa K., Ishiguro S., Kato T., et al. Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science. 2001;291:2138–2141. doi: 10.1126/science.291.5511.2138. [DOI] [PubMed] [Google Scholar]
- Kagawa T., Wada M. Velocity of chloroplast avoidance movement is fluence rate dependent. Photochem. Photobiol. Sci. 2004;3:592–595. doi: 10.1039/b316285k. [DOI] [PubMed] [Google Scholar]
- Kaiserli E., Sullivan S., Jones M.A., Feeney K.A., Christie J.M. Domain swapping to assess the mechanistic basis of Arabidopsis phototropin 1 receptor kinase activation and endocytosis by blue light. Plant Cell. 2009;21:3226–3244. doi: 10.1105/tpc.109.067876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M., Kagawa T., Oikawa K., Suetsugu N., Mihao M., Wada M. Chloroplast avoidance movement reduces photodamage in plants. Nature. 2002;420:829–832. doi: 10.1038/nature01213. [DOI] [PubMed] [Google Scholar]
- Luess D.R., DeBlaio S.L., Hangarter R.P. Integration of phot1, phot2, and PhyB signaling in light-induced chloroplast movements. J. Exp. Bot. 2010;61:4387–4397. doi: 10.1093/jxb/erq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka D., Tokutomi S. Blue light-regulated molecular switch of Ser/Thr kinase in phototropin. Proc. Natl Acad. Sci. USA. 2005;102:13337–13342. doi: 10.1073/pnas.0506402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks B.M., Quail P.H., Hangarter R.P. Phytochrome A regulates red-light induction of phototropic enhancement in Arabidopsis. Plant Physiol. 1996;110:155–162. doi: 10.1104/pp.110.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J.W., Nagatani A., Elich T.D., Fagan M., Chory J. Phytochrome A and Phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J.W., Nagpal P., Poole D.S., Furuya M., Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Kagawa T., Kasahara M., Swartz T.E., Christie J.M., Briggs W.R., et al. Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocations. Proc. Natl. Acad. Sci. USA. 2003;98:6969–6974. doi: 10.1073/pnas.101137598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon M., Lempert U., Rudiger W. Dimerization of the plant photoreceptor phototropin is probably mediated by the LOV1 domain. FEBS Lett. 2004;572:8–10. doi: 10.1016/j.febslet.2004.06.081. [DOI] [PubMed] [Google Scholar]
- Suetsugu N., Wada M. Chloroplast photorelocation movement mediated by phototropin family proteins in green plants. Biol. Chem. 2007;388:927–935. doi: 10.1515/BC.2007.118. [DOI] [PubMed] [Google Scholar]
- Takemiya A., Inoue S., Doi M., Kinoshita T., Shimazaki K. Phototropins promote plant growth in response to blue light in low light environments. Plant Cell. 2005;17:1120–1127. doi: 10.1105/tpc.104.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M., Kagawa T., Sato Y. Chloroplast movement. Annu. Rev. Plant Biol. 2003;54:455–468. doi: 10.1146/annurev.arplant.54.031902.135023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.