Abstract
The Cat-301 monoclonal antibody identifies aggrecan, a chondroitin sulfate proteoglycan in the cat visual cortex and dorsal lateral geniculate nucleus (dLGN). During development, aggrecan expression increases in the dLGN with a time course that matches the decline in plasticity. Moreover, examination of tissue from selectively visually deprived cats shows that expression is activity dependent, suggesting a role for aggrecan in the termination of the sensitive period. Here, we demonstrate for the first time that the onset of aggrecan expression in area 17 also correlates with the decline in experience-dependent plasticity in visual cortex and that this expression is experience dependent. Dark rearing until 15 weeks of age dramatically reduced the density of aggrecan-positive neurons in the extragranular layers, but not in layer IV. This effect was reversible as dark-reared animals that were subsequently exposed to light showed normal numbers of Cat-301-positive cells. The reduction in aggrecan following certain early deprivation regimens is the first biochemical correlate of the functional changes to the γ-aminobutyric acidergic system that have been reported following early deprivation in cats.
Keywords: amblyopia, area 17, dark rearing, monocular deprivation, sensitive period
Introduction
Four decades of research, summarized in a number of reviews (Movshon and Van Sluyters 1981; Mitchell and Timney 1984; Movshon and Kiorpes 1990; Berardi et al. 2000; Daw 2005; Morishita and Hensch 2008), has shown that the anatomical and functional development of the visual cortex of higher mammals continues long after birth and that the postnatal events can be modulated by visually driven activity. At one extreme, in the absence of any visual input, the visual cortex fails to develop normally. However, the clearest evidence for modulation of postnatal development by experiential factors has been provided by studies of the consequences of rearing animals with various types of abnormal early visual input, most notably monocular deprivation (MD) where one eye is deprived of a patterned visual image.
Research with this experiential paradigm has demonstrated that perturbations of normal visual experience can alter neuronal structure and function, provided they occur within restricted intervals, referred to as sensitive or critical periods (Wiesel 1982; Berardi et al. 2000; Daw 2005; Lewis and Maurer 2005; Hooks and Chen 2007). An early period of MD produces a shift in ocular dominance (OD) in visual cortical neurons such that the majority of cells respond solely to visual stimulation through the nondeprived eye. In the cat, shifts in OD in cortical area 17 in response to brief MD can be first induced at about 2 weeks, are most pronounced at 4–5 weeks of age, but thereafter susceptibility to changes in OD declines gradually to very low levels at 10–12 months of age (Cynader et al. 1980; Olson and Freeman 1980; Daw et al. 1992). Laminar analyses show that the sensitive period to MD lasts longer in the extragranular layers of cat area 17 than in the granular layer where it may persist to only 2 months of age (Daw 2005). The duration of sensitive periods, particularly in extragranular layers, is extended in complete darkness (Cynader and Mitchell 1980; Mower et al. 1981; Cynader 1983).
Following the phenomenological studies of developmental plasticity, attention gradually turned to examination of its molecular basis. The initial focus of these studies was on proteins expressed early in development that may instruct the developmental changes, followed more recently by attempts to identify candidate genes whose expression is regulated by experiential manipulations (e.g., Bear and Rittenhouse 1999; Prasad et al. 2002; Lachance and Chaudhuri 2004; Majdan and Shatz 2006). With respect to proteins, considerable attention has been focused on the potential roles of glutamate receptors (Bear 2003), neurotrophins (e.g., Bonhoeffer 1996; Huang et al. 1999; Berardi et al. 2003), and γ-aminobutyric acid (GABA) receptors (Hensch 2005). At a biochemical level, however, the principal receptors for all of these signaling pathways continue to be expressed in adults, suggesting that the expression of other proteins may limit plasticity. While several candidate molecules have been suggested (Hensch 2005; Morishita and Hensch 2008), those that exist in the extracellular matrix (ECM) deserve attention because of the transformation of this matrix from a largely soluble complex of proteins during prenatal and early postnatal development to a highly insoluble complex in the adult brain. So, while the ECM in the developing brain is conducive to the plastic processes of development, the gradual elaboration of an insoluble matrix in the maturing brain could play a role in the termination of sensitive periods (Hockfield 1990; Hockfield et al. 1990). In support of this hypothesis, Pizzorusso et al. (2002) showed that enzymatic degradation of key ECM components, called chondroitin sulfate proteoglycans (CSPGs), reinstated plasticity to MD in adult rats suggesting that these molecules play a key role in the decline in OD plasticity with age.
A candidate CSPG for regulating the age-dependent decrease in OD plasticity is aggrecan (Matthews et al. 2002). The Cat-301 monoclonal antibody recognizes a particular glycosylation state of aggrecan that is expressed in the perineuronal nets on a subset of neurons in the central visual pathway of the cat and monkey (Hockfield et al. 1983; Hendry et al. 1984; Sur et al. 1988; DeYoe et al. 1990; Matthews et al. 2002). In the cat dorsal lateral geniculate nucleus (dLGN), the perisynaptic cell surface expression of Cat-301 immunoreactivity is first detected at 4 weeks of age and then increases gradually to attain adult levels by about 6 months (Sur et al. 1988), a profile that is approximately the inverse of the time course of the sensitive period to MD in the visual cortex. Furthermore, Cat-301 immunoreactivity in the dLGN is reduced dramatically in animals deprived of visual experience from birth to 1 year, and MD imposed near birth but not in adulthood selectively reduces immunoreactivity in the A laminae innervated by the deprived eye (Sur et al. 1988; Kind et al. 1995). Cat-301 and other CSPGs also appear late in the development of the visual cortex, and their expression is dependent on visual activity (Lander et al. 1997) although the precise timing and laminar expression have not been examined. These findings raise the possibility that aggrecan is a key CSPG that mediates the gradual stabilization of cortical synapses during the sensitive period (Hockfield et al. 1983; Hockfield 1990; Pizzorusso et al. 2002).
Although the characteristic laminar pattern of Cat-301 in adult cat area 17 and its reduction following dark rearing have been described previously (Guimaraes et al. 1990; Lander et al. 1997), there have been no studies of the time course of Cat-301 expression during development. Hence, the initial aim of the present study was to document the profile of postnatal expression of Cat-301 immunoreactivity in cortical area 17 to determine the degree to which expression correlates with the decline in experience-dependent plasticity as demonstrated in earlier electrophysiological studies of the visual cortex. In order to probe the possibility of a link between expression of Cat-301 immunoreactivity with other documented developmental changes in the visual cortex, we examined cortical Cat-301 immunoreactivity following 2 early postnatal experiential manipulations. The first explored whether the decrease in Cat-301 expression previously reported (Guimaraes et al. 1990) in dark-reared animals could be rescued by visual activity. Previously, it has been shown that following restoration of normal visual input, dark-reared animals can recover normal visual acuity (Timney et al. 1978), and many of the functional deficits observed in cortical neurons also decline in severity (Cynader et al. 1976; Cynader and Mitchell 1980). The second manipulation we examined explored certain combinations of MD and reverse occlusion (also known as reverse lid-suture, RLS) that were previously shown to result in both bilateral amblyopia (Murphy and Mitchell 1987) and profound reductions of Cat-301-immunopositive cells in the dLGN (Kind et al. 1995), in order to determine whether Cat-301 expression in area 17 was reduced in a similar fashion in the visual cortex.
Materials and Methods
Subjects
Fifty-one cats from an outbred domestic cat lineage were raised from birth in a closed laboratory colony. The rearing conditions and surgical procedures that were employed followed protocols approved by the University Committee on Laboratory Animals at Dalhousie University and were in adherence with the standards of the Canadian Council on Animal Care. To study the developmental onset of Cat-301 immunoreactivity, 13 cats were reared with normal visual input and sacrificed at various postnatal ages (at 2 days or at 2 [N = 2], 3, 5, 8, 10, 12, 15 [N = 2], 26.9, 28.6, and 38.6 weeks of age). Thirty-eight animals were reared in order to study the activity dependence of Cat-301 expression. Of the latter, 6 animals were reared from birth in complete darkness until 100 days, while the remaining 32 were either deprived by eyelid suture in one or both eyes or else were reared with surgically induced strabismus. Two of the animals that were dark-reared to 100 days were perfused prior to any exposure to light. The remaining 4 were subsequently moved into normal colony lighting conditions (16:8 h light:dark illumination cycle) for an additional 100 days. Two of these were monocularly deprived by eyelid suture throughout the 100 days of visual exposure, while the other 2 received binocular visual experience during this period (Additional data on the effects of dark rearing were obtained from sections kindly provided by Dr Nigel Daw at Yale University from 2 animals that were dark reared from birth to either 200 or 341 days of age.).
Cat-301 expression was examined in 38 cats, including 27 from our earlier study of the dLGN (Kind et al. 1995) and one additional animal (C427) that was reared specifically for the present study. Cat-301 expression in area 17 was also examined in 4 strabismic animals, 2 esotropes (CS1 and CS2), and 2 exotropes (DS1 and DS2) that were reared for another study (Sengpiel et al. 1994). The pattern of Cat-301 expression in the visual cortex was assessed qualitatively in all of these animals, from which 23 were chosen for quantitative counts of Cat-301-positive cells. These included animals that were representative of the different rearing conditions as well as all of the animals that displayed a large reduction in the expression of Cat-301 in the dLGN. The animals excluded from the quantitative analysis all demonstrated quantitatively normal Cat-301 expression in the dLGN and qualitatively normal expression in area 17. The rearing history of the 17 monocularly deprived or reverse lid–sutured animals chosen for quantitative assessment are listed in Supplementary Table 1 together with a categorization (L, low; M, medium; N, normal) of the level of Cat-301 expression observed earlier in the dLGN (Kind et al. 1995). As kittens, 12 of these animals had the eyelids of the left eye sutured closed when they were 6–10 days old (at the approximate age of normal eyelid separation). The period of MD continued until the animals were either 4, 5, 6, 8, or 12 weeks of age at which time the eyelids of the left eye were opened. For the majority of the MD animals (all but C299, and C439), the eyelids of the nondeprived eye were sutured closed at the same time as vision was restored to the deprived eye for various periods (RLS). All of the animals received binocular visual exposure following the period of MD (C299, C439) or the subsequent period of RLS. The procedures for eyelid suture and reopening were identical to those described previously (Murphy and Mitchell 1987; Mitchell 1991; Kind et al. 1995).
In order to determine the effect of reduced cortical binocularity on Cat-301 expression in area 17, as well as to control for the possibility that any effects observed in MD kittens could be explained by a misalignment of the 2 eyes induced by lid suture, we investigated Cat-301 expression in 4 cats (2 esotropes, CS1 and CS2; 2 exotropes, DS1 and DS2) on which artificial strabismus had been induced surgically at 10–11 days of age by procedures described elsewhere (Sengpiel et al. 1994). Quantitative assessment of the density of Cat-301-positive cells was made on only 2 of the strabismic animals (CS1 and DS1) although data are available (Sengpiel et al. 1994) on the distribution of cortical OD based on physiological recording from all 4 animals.
Histological and Immunocytochemical Procedures
All animals were sacrificed with an overdose of sodium pentobarbitol (>250 mg/kg of body weight, intraperitoneally) and perfused through the heart with phosphate buffered saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer. The brains were removed from the skull, postfixed for at least 1 week in 4% paraformaldehyde, and then placed in 30% sucrose. The brains were dissected, and frozen blocks of tissue containing the dLGN and visual cortex were sectioned at 40 or 50 μm and incubated with the monoclonal Cat-301 antibody (diluted 1:5 to 1:50 in Dulbecco’s modified Eagle’s medium [Sigma or Gibco] with 10% fetal calf serum and 2% Triton X-100) overnight. The Cat-301 antibody was visualized in 1 of 2 ways, either using a Vectastain ABC mouse IgG kit (PK-4001, Dimension Laboratories) with an avidin–biotin amplification (final Cat-301 dilution 1:20 to 1:50), as described in Kind et al. (1995) or by use of an horseradish peroxidase (HRP)-conjugated secondary antibody (final Cat-301 dilution 1:5 or 1:10; IgGAM, Cappel Laboratories; Kind et al. 1994). No differences in the expression pattern or the overall numbers of immunoreactive cells were observed between the 2 reaction protocols. Furthermore, formal counts of the number of Cat-301-positive cells in sections from the dLGNs reacted with different antibody concentrations revealed negligible variation in counts of Cat-301-positive cells with antibody dilutions in the range we employed (Kind et al. 1995; Fig. 1). Some sections were also stained, by use of the non-avidin/biotin amplified protocol, with antibodies to parvalbumin (1:20 000, Sigma) and to the phosphorylated form of neurofilament (SMI-32, 1:100, Sternberger).
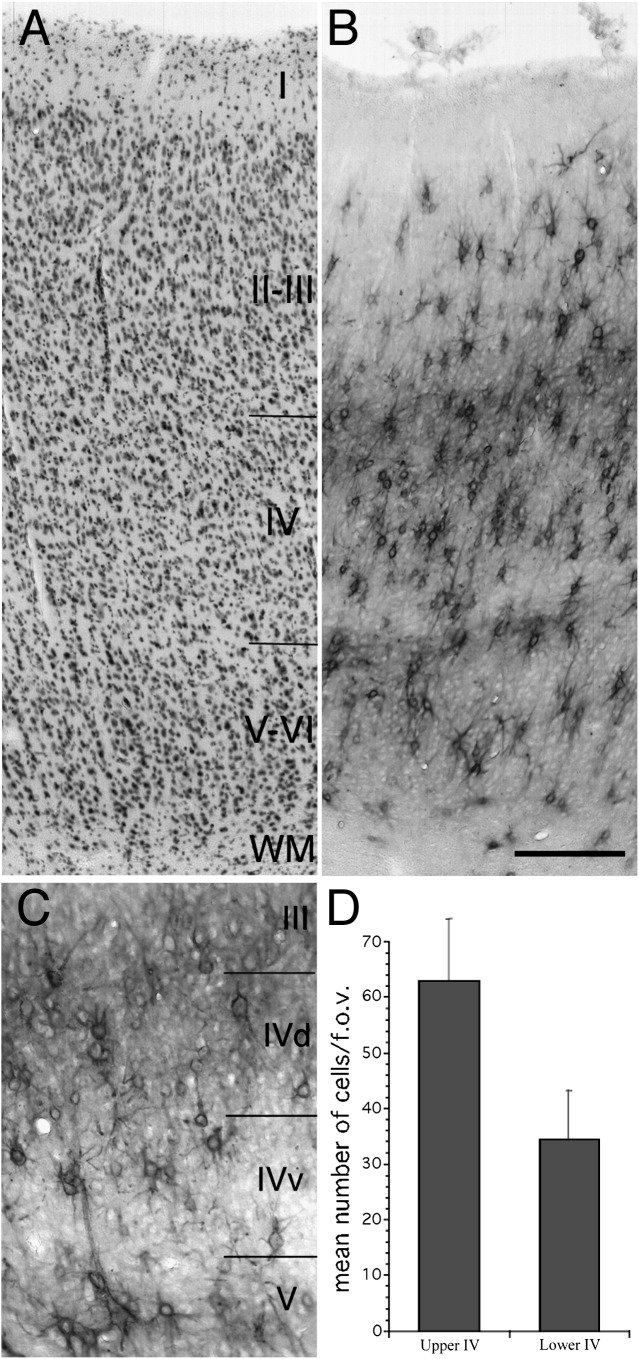
Figure 1.
Laminar distribution of Cat-301 immunoreactive cells in adult cat area 17. Photomicrographs of cresyl violet (A) and Cat-301 (B) stained adjacent sections through area 17 of a normal adult cat (C517). (C) Higher power photomicrograph of layer IV of a Cat-301 stained section of C517. (D) Histograms that show counts of Cat-301-positive neurons in dorsal versus ventral layer IV. Cat-301 immunoreactivity appears as 2 cell-dense bands. The lower band extends throughout layers V and VI with its ventral border with layer IV demarcated by the large layer V pyramidal cells. The upper band extends throughout layer IV and approximately 200 μm into lower layer III. The number of Cat-301-positive neurons is nearly twice as high in upper layer IV as compared with lower layer IV (D). The distribution of Cat-301 correlates well with the distribution of Y-cell geniculocortical terminations as reported by Humphrey et al. (1985). Scale bar (B) = 200 μm.
Data Analysis
In a previous study (Kind et al. 1995), we noted that when sections with low levels of Cat-301 were counterstained with cresyl violet, immunoreactivity for Cat-301 was masked. Therefore, to determine more accurately the number of Cat-301-positive neurons, all cell counts were performed on noncounterstained sections. To account for changes in cell density with age and dark rearing, Cat-301 counts in the developmental series as well as in dark-reared animals were expressed as a percentage of total neuronal number. Neuronal density was estimated by counting from Nissl-stained sections adjacent to those used for Cat-301-positive cell counts.
The cell counts were performed in accordance with the stereological optical dissector method of Coggeshall and Lekan (1996). All measurements are expressed relative to normal rather than as absolute values. Counts were made from 2 or 3 sections separated by at least 120 μm. At least 6 fields of view per cortical layer were counted at a magnification of either ×500 (field of view diameter = 0.35 mm for dark-reared and dark-reared recovery animals) or ×375 (field of view diameter = 0.55 mm for all monocularly deprived and RLS animals).
Counts of Cat-301-positive cells were made along the medial bank of area 17 of both hemispheres from AP +3 to AP +6. The counts from the 2 hemispheres were very similar, and therefore, the results were combined. For the purpose of this analysis, the cortex was divided into 3 major layers corresponding to the laminar borders demarcated by the Cat-301 antibody, namely the supragranular layers (layers II and III), the infragranular layers (layers V and VI), and the granular layer (layer IV). The criteria employed for laminar border assignments are provided in Results.
Results
Distribution of Cat-301 in the Adult Cat Visual Cortex
As previously reported, the Cat-301 monoclonal antibody labeled a subset of neurons in the cat visual cortex (Hockfield et al. 1983, 1990; Hendry et al. 1984; Guimaraes et al. 1990). Also consistent with earlier reports, Cat-301 immunoreactivity was distributed in 2 major bands in the adult cat visual cortex. However, while the dorsal border of the upper band was described previously as corresponding to the border between layers III and IV (Guimaraes et al. 1990), as identified from sections stained for Nissl (Fig. 1A) or immunoreacted for neurofilament (SMI-32), we show here that the upper band of Cat-301 staining extends several hundred microns into layer III, as confirmed by the band's overlap with layer III pyramidal cells. The large layer V pyramidal neurons present in the lower band of dense Cat-301 immunoreactivity demarcate the border between layer V and lower layer IV. As seen in Figure 1B, many of the Cat-301-positive neurons are not pyramidal in shape. Double labeling experiments with Cat-301 and antibodies to parvalbumin indicate that the majority of nonpyramidal neurons are GABAergic (Supplementary Fig. 1). Interestingly, the distribution of Cat-301 staining in layer IV was not uniform (Fig. 1C). Qualitatively, there appeared to be many more Cat-301-positive cells in dorsal (upper) than in ventral (lower) layer IV, and cell counts confirmed this impression, showing approximately twice as many Cat-301 immunoreactive neurons in upper layer IV (Fig. 1D).
Cat-301 Immunoreactivity Increases with Age in the Cat Visual Cortex
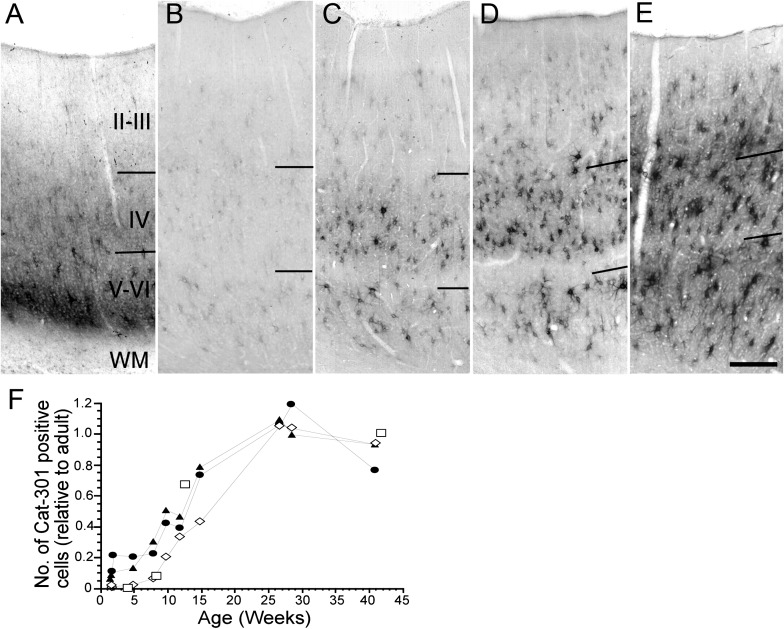
In the cat dLGN, development of Cat-301 expression is inversely correlated with the decline in visual cortical plasticity (Sur et al. 1988). We now show the developmental profile of Cat-301 expression in area 17 (Fig. 2). At 2 days, no Cat-301 immunoreactivity was detected in area 17 (data not shown), but by 15 days, a band of Cat-301 staining appeared in layer VI with a lighter band extending into layer 4 (Fig. 2A). The layer 6 band of immunoreactivity was transient and disappeared by 21 days. Furthermore, this transient Cat-301 immunoreactivity was located mainly in the neuropil; very few Cat-301-positive neuronal cell bodies were visible. Thereafter, the density of Cat-301-positive neurons in all layers increased gradually. By 12–15 weeks (Fig. 2D), the density of Cat-301 cells in layers V/VI had reached about 50 percent of the adult values that were not attained until 6 months of age (Fig. 2E). In layer IV, Cat-301-positive neuronal cell bodies were first detected in animals between 2 and 5 weeks of age (Fig. 2A,B, respectively), but their density did not increase substantially until 10 weeks (Fig. 2C). By 15 weeks, their density had increased to 70% of adult levels, but like layers V and VI, adult levels were not attained until 6 months. Interestingly, the development of Cat-301 immunoreactivity in layer II/III was delayed compared with that in the granular and infragranular layers. Cat-301-positive neurons were not seen in layer II/III until 5 weeks (Fig. 2B) after which their density increased at a slower rate than in the other layers, such that 50% of the adult density was not attained until after 15 weeks (Fig. 2D). Nevertheless, adult levels were reached by 6 months as in the other layers (Fig. 2E).
Figure 2.
Development of Cat-301 immunoreactivity in area 17. Photomicrographs showing the distribution of Cat-301 immunoreactivity in kittens at the following ages: 15 days (A), 5 weeks (B), 10 weeks (C), 15 weeks (D), and 6 months (E). Cat-301 immunoreactivity first appears by 15 days of age as a transient band of staining of neuropil in layer VI (A). Also, a few immunoreactive cell bodies can be seen scattered throughout the granular and infragranular layers at this age. The immunoreactive band of neuropil is only transitory and is undetectable by 5 weeks of age (B). The density of immunoreactive soma in layers IV and VI have increased by this time, but very few immunoreactive cells are present in the supragranular layers. By 10 weeks of age (C), the density of Cat-301-positive cells increases substantially in layer IV and reaches near adult levels by 6 months of age (D). The density of immunopositive cells in the infragranular layers also increases rapidly between 5 and 15 weeks of age and reaches adult levels between 4 and 6 months of age (E). The development of immunoreactive neurons in the supragranular layer is delayed in comparison with the other layers. Immunopositive neurons in these layers first appear by 5 weeks of age (B) but remain relatively sparse until 15 weeks (D). Adult levels of Cat-301 immunoreactive cells in the supragranular layers are attained by 6 months of age. Scale bar (E) = 250 μm. (F) Quantitative assessment of Cat-301 development in area 17. The density of Cat-301-positive cells (normalized to their respective adult values) in the granular (filled triangle symbols), supragranular (open diamond symbols), and infragranular (filled circle symbols) layers as a function of age. The curve for the supragranular layers is skewed to the right indicating delayed development compared with that in layer IV or the infragranular layers. For comparison purposes, the open square symbols show a replot of data of Sur et al. (1988) on the development of Cat-301 immunoreactivity in the dLGN.
The rate of maturation of Cat-301 expression in the granular and extragranular layers is shown quantitatively in Figure 2F. The cell densities have been normalized with respect to the adult levels for each curve. Because adult values had been attained in all layers by 6 months, we combined data from animals older than 6 months to arrive at an average adult value for the purpose of normalization. The data obtained by Sur et al. (1988) on the development of Cat-301 expression in the dLGN have been replotted in Figure 2F (open square symbols) for comparison. Interestingly, until about 8 weeks of age, Cat-301-positive neuronal density develops more slowly in the dLGN than in layer IV of area 17, with a time course that is closer to that seen in the supragranular layers. Thereafter, the limited data from the dLGN suggest that its development may more closely match that of layer IV. As can be seen in Figure 2F, the increase in the density of Cat-301-positive neurons in the supragranular layers is delayed by about a month relative to that in the granular or infragranular layers.
Cat-301 Recovers to Normal Levels Following an Initial Period of Dark Rearing
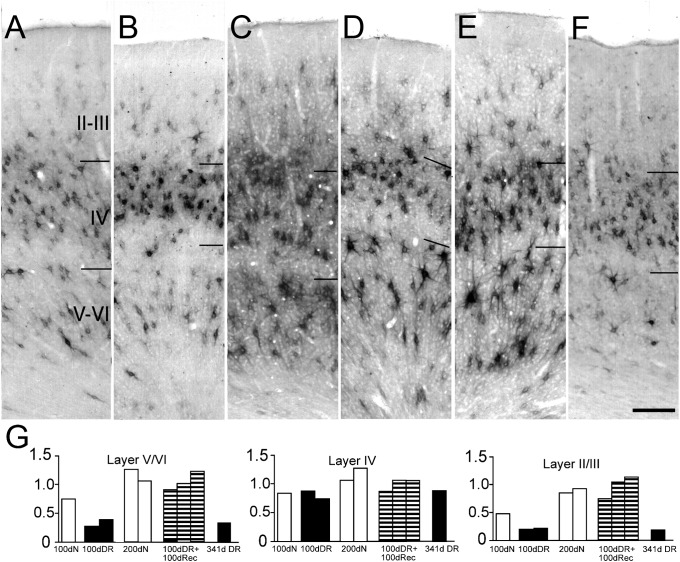
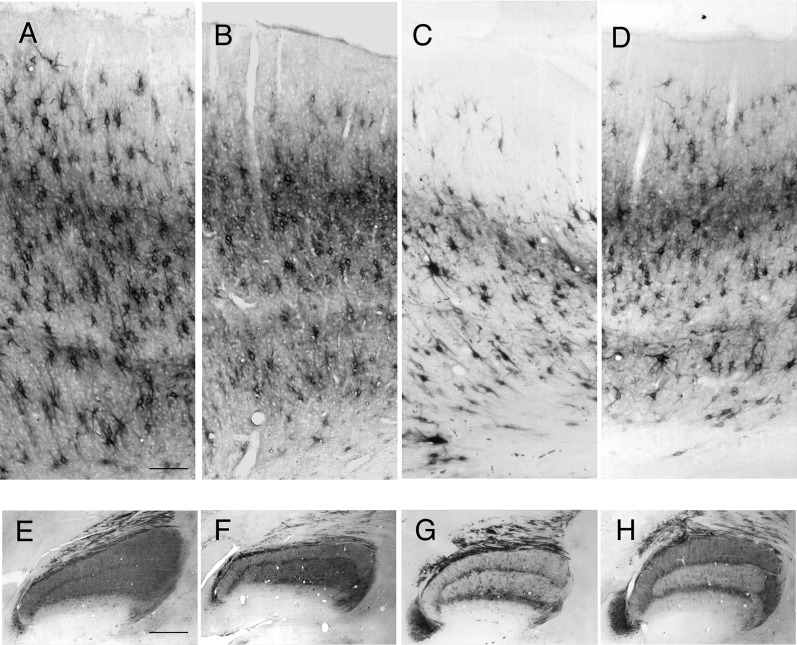
The behavioral and physiological effects of dark rearing to 3–4 months of age can be largely reversed by subsequent rearing under normal lighting conditions (Timney et al. 1978; Cynader et al. 1980; Kaye et al. 1982). Dark rearing reduces the expression of Cat-301 in both the dLGN and the extragranular layers of area 17 (Sur et al. 1988; Guimaraes et al. 1990; Lander et al. 1997; and Fig. 3B,F,G, this study). To determine whether the reduction in Cat-301 immunoreactivity induced by dark rearing was permanent, we examined the expression of Cat-301 in area 17 following a 100-day period of either binocular or monocular recovery imposed after 100 days of dark rearing from birth (Fig. 3). Following 100 days of recovery, with either binocular (Fig. 3D) or monocular (Fig. 3E) experience, Cat-301 immunoreactivity in the extragranular layers increased to levels similar to those observed in area 17 of normally reared age-matched animals. This increase following exposure to light was not simply due to the increase in age because an animal maintained in the dark for either 200 days or 1 year had markedly reduced levels of Cat-301 immunoreactivity (Fig. 3F). These results indicate that visual experience initiated after a prolonged period of dark rearing can induce Cat-301 expression to normal adult levels.
Figure 3.
Visual experience following dark rearing from birth restores normal levels of Cat-301 immunoreactivity in area 17. Photomicrographs showing comparisons of Cat-301 expression in area 17 of a 105 day normal kitten (A), a 100-day-old dark-reared animal (B), a 200-day-old normal kitten (C), a 100-day dark-reared animal that subsequently received 100 days of binocular visual experience (D), a 100-day dark-reared animal that subsequently received 100 days of monocular vision (E), and a 341-day-old dark-reared cat (F). Dark rearing to 100 days of age (B) dramatically reduced the number of Cat-301-positive cells in the extragranular layers compared with age-matched controls (A). Furthermore, in agreement with a previous study (Guimaraes et al. 1990), there appears to be a decrease in the soma size of the remaining Cat-301-positive neurons (B and F). However, 100 days of subsequent visual exposure, either binocular (D) or monocular (E), causes a rapid reinduction of Cat-301 expression to levels observed in age-matched normal controls, and soma size appears to increase toward that observed in normal animals. Levels of Cat-301 immunoreactivity in the extragranular layers remained low in animals reared in the dark to 1 year of age (F), indicating that the increase in Cat-301 expression observed in the visual recovery animals (D and E) was a direct result of their visual experience. Scale bar (F) = 200 μm. (G) Quantitative assessment of Cat-301-positive cell numbers in dark-reared animals as well as dark-reared animals that subsequently received a period of either binocular or monocular visual exposure. The data for all animals have been normalized to values observed in normally reared adult animals. The 3 panels display data in the form of histograms for layers V/VI, layer IV, and layers II/III. In each panel, data are shown for 3 normal (open columns), 3 dark-reared animals (filled columns) reared to either 100 or 341 days, and three 100-day-old dark-reared animals that subsequently received a 100-day period of either binocular or monocular exposure (hatched columns).
Quantitatively, the density of cells expressing Cat-301 in layer IV was not affected by dark rearing (see also Guimaraes et al. 1990) and remained unchanged following the period of recovery (Fig. 3G). Qualitative examination of Cat-301-positive cell size in layer IV of dark-reared cats revealed smaller cell body sizes compared with control animals, confirming the findings of Guimaraes et al. (1990). In layers V/VI, the density of Cat-301 cells was 50% lower in 100-day dark-reared animals relative to normal animals. The density of Cat-301-positive neurons decreased further with longer periods of dark rearing; by 341 days, the number of Cat-301-positive neurons in layers V/VI was only 20% of that in normal (200 days) or adult animals. A 100-day period of either monocular or binocular visual exposure following 100 days of dark rearing restored the levels of Cat-301 immunoreactivity in layers V/VI to normal. Similar results were observed in layers II/III. At 100 days of age, the density of Cat-301 immunoreactive neurons in the supragranular layers of normal animals was approximately 50% of the value observed in adults. In animals that were dark-reared to the same age, the density of Cat-301-positive neurons was reduced to 40% of the levels of age-matched controls. However, following 100 days of visual recovery, the density of Cat-301-positive neurons was restored to levels similar to those observed in 200-day-old normal animals. A prolonged period of dark rearing resulted in a drastic reduction in the density of Cat-301 immunoreactive neurons compared with 200-day-old normally-reared animals.
Dark Rearing Decreases Cat-301 Expression on Both GABAergic and Non-GABAergic Neurons
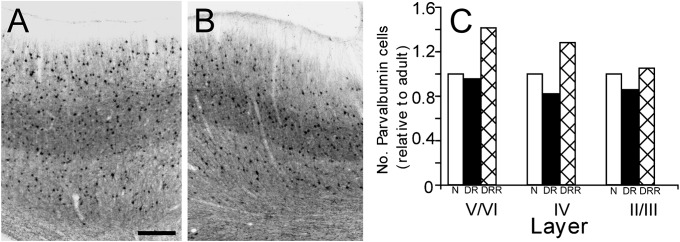
In a preliminary attempt to determine whether a particular neuronal subtype was affected by dark rearing, sections were double-labeled with antibodies to Cat-301 and to parvalbumin, which labels a subclass of fast-spiking GABAergic neurons. In area 17 of normal animals, most parvalbumin-positive cells were also Cat-301 positive. As illustrated qualitatively by the parvalbumin stained sections of Figure 4 A,B and by quantitative counts (Fig. 4C), the density of parvalbumin-containing cells in area 17 was similar in animals dark-reared to 100 days (DR), as in those that were subsequently exposed to light (DRR), and to values obtained from age-matched normal animals. When viewed in conjunction with the fact that the vast majority of Cat-301-positive cells are also parvalbumin positive (Supplementary Fig. 1) and the large decrease in Cat-301-positive cells in extragranular layers of dark-reared animals, these results indicate that dark rearing affects Cat-301 expression in GABAergic cells. In addition, in dark-reared animals, there was an almost complete loss of Cat-301-positive pyramidal cells (see Fig. 3), demonstrating that Cat-301 expression on both excitatory (pyramidal) and inhibitory (GABAergic) neurons is dependent on visual experience.
Figure 4.
Parvalbumin expression is unaffected by dark rearing. Photomicrogrpahs of sections through area 17 of a kitten dark-reared to 100 days of age (A) and a normal age-matched kitten (B) that have been stained with antibodies to parvalbumin (to identify a subclass of GABAergic neurons). The results of counts of parvalbumin-positive cells in normal (N; white bars), dark reared (DR; black bars), and dark-reared animals that subsequently received visual exposure (DRR; hatched bars) are shown in C. Scale bar (A) = 350 μm.
MD and Reverse Occlusion
We have previously shown that following certain regimens of combined MD and RLS that result in bilateral amblyopia (Murphy and Mitchell 1987), the density of Cat-301-positive cells in the dLGN was often dramatically reduced “in both A laminae” (Kind et al. 1995). Since aggrecan may have a role in regulating the decline of plasticity in the visual cortex, we examined the expression of Cat-301 in area 17 of the same animals reared for the earlier study (Kind et al. 1995). Included in the analysis were animals that were subjected to simple extended regimens of MD or else long periods of reverse occlusion at 4 or 5 weeks of age that resulted in low levels of Cat-301 expression in just one of the A laminae of the dLGN. The data from these animals were used as a benchmark for comparison of the effects on cortical Cat-301 expression of other rearing regimens that resulted in low levels of Cat-301 expression in both A laminae. A summary of the rearing conditions and cell counts in area 17 are presented in Supplementary Table 1 together with a categorization (identical to that used for the dLGN results) that summarizes the level of Cat-301 expression in the visual cortex.
Long-Term MD and Long-Term Reverse Occlusion
Animals in these conditions were either monocularly deprived from near birth to 12 weeks or else were monocularly deprived to 4 or 5 weeks of age at which time they were reverse occluded (RLS) for a period of 16 and 12 weeks, respectively. In all cases, qualitative examination of Cat-301 expression in the striate cortex revealed normal patterns of immunoreactivity. This qualitative similarity is apparent in the photomicrographs of Figure 5 that shows a comparison of representative sections through the visual cortex of a normal cat (Fig. 5A) and from 2 cats (C439 and C362) that had been either monocularly deprived to 12 weeks of age (Fig. 5B) or which had received a 12-week period of RLS following MD to 5 weeks of age (Fig. 5C). As with normal animals, 2 bands of immunoreactivity were visible in the neuropil corresponding to layers III/upper IV and layers V/VI. Furthermore, many large Cat-301-positive pyramidal cells were visible in layers V and lower layer III. Density measurements confirmed the qualitative assessment of normal levels of Cat-301 immunoreactivity in all layers of area 17 (Fig. 5D). In situ hybridization for aggrecan messenger RNA (mRNA) also showed high aggrecan levels in the primary visual cortex (Supplementary Fig. 2). In the dLGN, however, there was a dramatic reduction of aggrecan mRNA selectively in the deprived layers, indicating that the reduced protein levels in deprived dLGN is at least in part due to a reduction in either transcription or aggrecan mRNA stability.
Figure 5.
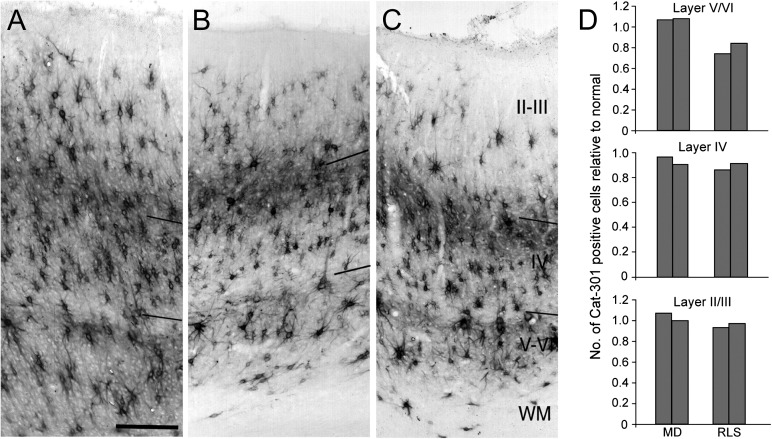
Normal expression of the Cat-301 CSPG in area 17 following MD and long-term RLS. Cat-301 immunoreacted sections through area 17 of a normal kitten (A; C518), and kittens subjected to either a 12-week MD (B, C439) or a 12-week period of RLS following 5 weeks of MD (C; C362). In all 3 animals, Cat-301-positive cells can be detected in all cortical layer but are most dense in layers V/VI and upper layer IV/lower layer III. Qualitatively, no differences could be detected in Cat-301 immunoreactivity between animals in these rearing conditions. Large pyramidal neurons were evident in both layer V and layer III, and the 2 bands of staining of neuropil in upper layer IV and layer V/VI appeared identical. Scale bar (A) = 250 μm. (D) Quantitative assessment of the number of Cat-301-positive cells in the granular and extragranular layers of area 17 of monocularly deprived (MD) and long-term animals. The cell numbers in each layer have been normalized to the mean cell density of 200-day normal animals. With the exception of a hint of lower numbers of Cat-301-positive cells in layers V/VI of the RLS animals, the cell counts were very similar in all animals.
Similar conclusions were reached concerning both the qualitative and the quantitative findings from 2 further animals that had received long periods of reverse occlusion (12 or 16 weeks) beginning at either 4 (C484 and C379) or 5 (C362) weeks of age. The normal pattern of Cat-301 expression in area 17 of these deprived animals is illustrated in Figure 5C. Quantitative measurements from 2 of the animals (C379 and C362; Table 1) confirmed the qualitative impressions. The only difference between the MD and the RLS animals was a slight decrease in the number of Cat-301-positive neurons in the infragranular layers of RLS cat C362 (Fig. 5D).
Table 1.
Cat-301 cell density measurements in Area 17 following monocular deprivation, reverse occlusion and strbismus.
| Animal | Condition | II/III | IV | V/VI | Area 17 | dLGN |
| C517 | Normal | 5628 ± 420 | 7291 ± 70 | 4049 ± 252 | N | N |
| C522 | Normal | 6283 ± 378 | 10 189 ± 529 | 4746 ± 437 | N | N |
| C200 | Normal | 6199 ± 193 | 7207 ± 150 | 3990 ± 202 | N | N |
| MD | ||||||
| C299 | 6-week MD + B | 6443 ± 244 (1.07) | 7896 ± 244 (0.96) | 4536 ± 227 (1.06) | N | N |
| C439 | 12-week MD + B | 6048 ± 109 (1.00) | 7367 ± 328 (0.90) | 4578 ± 269 (1.07) | N | N |
| MD + long-term RLS | ||||||
| C379 | 4-week MD + 16-week RLS + B | 5628 ± 185 (0.93) | 7031 ± 134 (0.85) | 3133 ± 143 (0.74) | N | N |
| C362 | 5-week MD + 12-week RLS + B | 5880 ± 294 (0.97) | 7375 ± 210 (0.90) | 3570 ± 260 (0.84) | N | N |
| MD + medium-term RLS | ||||||
| C398 | 6-week MD + 6-week RLS + B | 1369 ± 218 (0.23) | 4444 ± 378 (0.54) | 1789 ± 193 (0.42) | L | L |
| C418 | 5-week MD + 5-week RLS + B | 874 ± 126 (0.14) | 3268 ± 512 (0.40) | 1294 ± 50 (0.30) | L | L |
| MD short-term RLS | ||||||
| C422 | 4-week MD + 9-day RLS + B | 3536 ± 277 (0.59) | 5158 ± 269 (0.63) | 2360 ± 176 (0.55) | M | M |
| C425 | 4-week MD + 9-day RLS + B | 1470 ± 202 (0.24) | 3637 ± 151 (0.44) | 1327 ± 42 (0.31) | L | L |
| C450 | 4-week MD + 9-day RLS + B | 6048 ± 277 (1.00) | 8274 ± 109 (1.01) | 4494 ± 252 (1.05) | N | L |
| C426 | 5-week MD + 9-day RLS + B | 2780 ± 160 (0.46) | 6014 ± 286 (0.73) | 2150 ± 134 (0.50) | M | N |
| C427 | 5-week MD + 9-day RLS + B | 4603 ± 151 (0.76) | 7216 ± 218 (0.88) | 3058 ± 210 (0.72) | M | N* |
| C451 | 5-week MD + 9-day RLS + B | 6342 ± 269 (1.05) | 7644 ± 319 (0.93) | 3822 ± 403 (0.90) | N | M |
| Strabismus | ||||||
| CS1 | Esotrope | 5502 ± 210 (0.91) | 7182 ± 176 (0.87) | 3973 ± 277 (0.93) | N | N* |
| DS1 | Exotrope | 5863 ± 118 (0.97) | 7098 ± 344 (0.86) | 3318 ± 160 (0.78) | N | N* |
Note: L, low; M, medium; N, normal.
*animals in which level of Cat-301 immunoreactivity in the dLGN was assessed qualitatively.
Medium- and Short-Length Reverse Occlusion
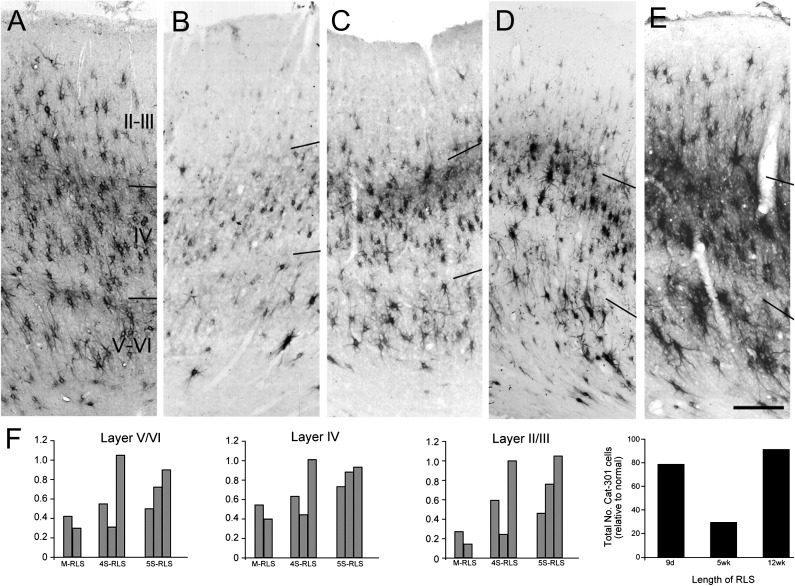
For the medium-length reverse-occluded animals, the initial period of MD extended to 5 or 6 weeks of age and the subsequent RLS lasted for the same period of time (C416, C398). Previously, it was shown that animals with this rearing history demonstrated a severe reduction of Cat-301 immunoreactivity throughout the dLGN (Kind et al. 1995; Figs 6 and 7). Qualitatively, in area 17 of C416 and C398, neuropil staining was very low in all layers, and few Cat-301-positive pyramidal cells were observed in layers III and V (Fig. 6B,C) compared with normal animals (Fig. 6A). Quantitatively (Fig. 6F, M-RLS animals), there was a massive reduction in Cat-301-positive cell density throughout area 17 with the most severe reduction evident in the supragranular layers. The density of Cat-301-positive neurons in the supragranular layers were reduced by 86% and 77% for C416 and C398, respectively (Supplementary Table 1). The decrease in the density of Cat-301-positive neurons in the infragranular layers of these 2 animals were, respectively, 70% and 58%, while in the granular layers, the densities were reduced by, respectively, 60% and 46%.
Figure 6.
Photomicrographs of coronal sections through area 17 that illustrate the expression of Cat-301 immunoreactivity in area 17 of a normal animal (A: C517) 4 monocularly deprived animals that received a medium-length (B, C416: 5-week RLS at 5 weeks; C, C398: 6-week RLS at 6 weeks) or short-length RLS (D, C425: 9-day RLS at 4 weeks; E, C427: 9-day RLS at 5 weeks). There was a substantial reduction in both the staining of neuropil as well as the density of Cat-301-positive cells in all layers for all medium-length RLS animals. There was more variability in the outcome of the short-length RLS animals (see Results). Quantitative analysis of Cat-301 cell density is shown in F. All density estimates are normalized to adult control animals with “normal” levels of Cat-301 expression (calculated based on an average of 8 normal, long-term MD, and long-term RLS animals). A comparison of length of RLS following 5 weeks of MD showing that the length of the RLS prior to binocular recovery is critical to the expression of Cat-301 in area 17 is also shown in F. Scale bar in A–C = 250 μm; in D and E = 225 μm.
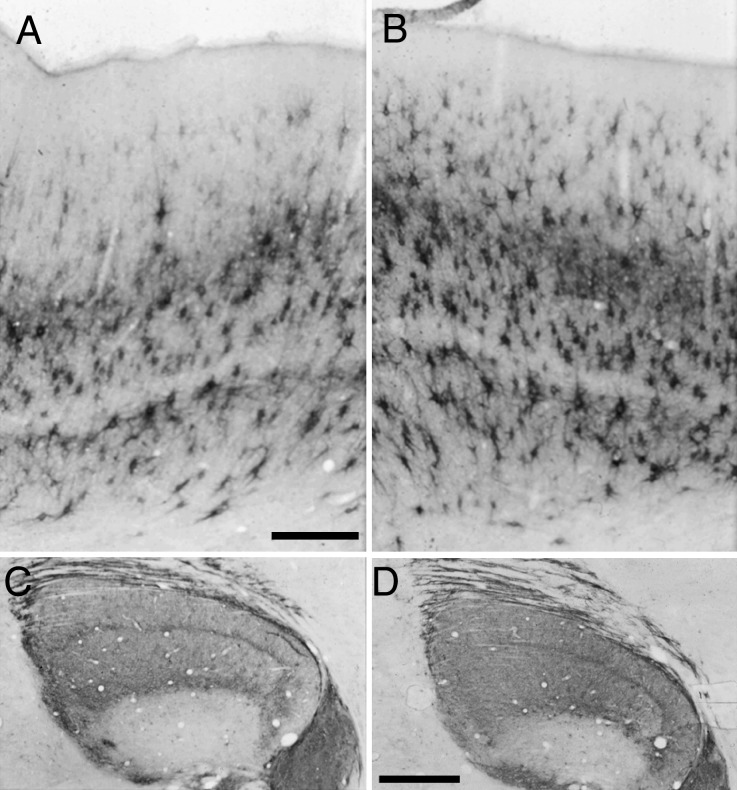
Figure 7.
Summary figure showing effects of MD and RLS on Cat-301 expression in area 17 and dLGN. Photomicrographs through area 17 (A–D) and dLGN (E,H) from a normal (C517, A and E), a 6-week MD (C299, B and F), a 9-day RLS following 4 weeks of MD (C425, C and G), and a 12-week RLS following 5 weeks of MD (C362, D and H). Scale bar A (A–D) = 150 μm. Scale bar in E (E–H) = 1 mm.
For the short-term reverse occlusion animals, the initial period of MD extended to 4 or 5 weeks of age and subsequent RLS extended for 9 days. These rearing conditions resulted in reduced Cat-301 immunoreactivity in the dLGN but of varying severity. A similar variable reduction in Cat-301-positive neuronal density was observed in the visual cortex (Fig. 6D,E). Within each animal, however, the Cat-301 levels in area 17 and the dLGN (Kind et al. 1995) were highly correlated. Among the 3 MD animals that received the period of reverse-lid suture at 4 weeks, one (C425; Fig. 6D) exhibited very low Cat-301 expression in all cortical layers, another (C422) showed a moderate but reduced number of Cat-301-positive cells, while the third (C450) exhibited normal numbers of Cat-301-positive cells (Supplementary Table 1). The results from the 3 MD animals that received 9 days of reverse occlusion at 5 weeks were also diverse, and the level of Cat-301 immunoreactivity in area 17 of one animal (C426) did not correlate as closely with levels in the dLGN. The lowest level of Cat-301 immunoreactivity was observed in the visual cortex of C426, an animal that exhibited normal overall levels of immunoreactivity in the dLGN, albeit with higher levels in laminae innervated by the initially nondeprived eye (Kind et al. 1995). A second animal (C427; Fig. 6E) exhibited a slight reduction in Cat-301-positive cells in the extragranular layers, but no reduction at all in layer IV. This particular animal did not participate in the earlier study of the dLGN, but, qualitatively, Cat-301 levels in the dLGN appeared normal. Finally, C451 exhibited normal levels of Cat-301 immunoreactivity in all cortical layers. The dLGN of this animal exhibited a slight overall reduction in the number of Cat-301-positive cells (Kind et al. 1995). The diversity of Cat-301 immunoreactivity in area 17 amongst the 2 short-term RLS is apparent in the microphotographs of Figure 6. The results of laminar counts in the visual cortex of all 3 of these animals are displayed in the form of histograms in Figure 6F. To determine whether the decrease in Cat-301 immunoreactive neurons was accompanied by an overall decrease in Cat-301 levels, we performed optical density measurements. These measurements were in agreement that the qualitative observations and cell density measurements with animals with reduced Cat-301 neurons showing less overall staining intensity (Supplementary Fig. 3). Furthermore, they also revealed the laminar specificity, with the largest decreases in layer 2/3, followed by the infragranular layers and little or no effect in layer 4.
Strabismus
A stable strabismus was induced in 4 animals by tenotomy of one of the extraocular muscles. Although stable, the degree of misalignment was variable across animals. Estimates of the angle of deviation were made on all animals at the time of electrophysiological recording when animals were at least 1 year old (Sengpiel et al. 1994) based upon the differences between their paralyzed eye positions and the mean values observed in normal animals under similar conditions. The 2 animals on which the lateral rectus had been sectioned were both esotropic with a misalignment of either 4° (CS1) or 34° (CS2), while the animals that had their medial rectus sectioned (DS1 and DS2) exhibited exotropia of, respectively, 30° and 8°. Electrophysiological recordings from the 4 animals revealed an abnormal distribution of OD (reported in Sengpiel et al. 1994) from all cases with a substantial reduction from normal values in the proportion of neurons that could be excited through both eyes. For the 2 esotropic cats, the percentage of binocular cells (i.e., cells in OD classes 2-6) was 30.5% and 43.9% for CS1 and CS2, respectively. The percentage of binocular cells in the 2 exotropic cats DS1 and DS2 was, respectively, 28.6% and 26.8%.
Qualitatively, the level of Cat-301 immunoreactivity in the dLGNs of all 4 animals appeared normal. Photomicrographs of the dLGN contralateral to the operated eye of CS and DS are displayed in Figure 8 C,D and reveal both the normal overall levels of Cat-301 immunoreactivity as well as the absence of any laminar differences in staining. Cat-301 immunoreactivity appeared qualitatively normal in all 4 animals (see Fig. 7 A,B), a point confirmed by quantitative counts of Cat-301-positive cells (Table 1) in area 17 contralateral to the operated eye of CS1 and DS1. There was, however, a very slight reduction in the density of Cat-301-positive cells in the infragranular layers. Large stained pyramidal cells were clearly visible in layers III and V in all 4 strabismic cats.
Figure 8.
Photomicrographs through area 17 (A and B) and the dLGN (C and D) of an animal reared with esotropia (CS1; A and C) and another with exotropia (DS1; B and D). Strabismus had little effect on the final levels of Cat-301 expression in either the dLGN or area 17. Counts from these 2 animals can be found in Table 1. Scale bar in A (A,B) = 300 μm. Scale bar in D (C,D) = 1 mm.
Discussion
CSPGs have been shown to restrict visual cortical plasticity to MD in the adult visual cortex (Pizzorusso et al. 2002). It has been hypothesized that aggrecan, the CSPG recognized by the Cat-301 monoclonal antibody, is a key player in this process (Sur et al. 1988; Guimaraes et al. 1990; Hockfield 1990; Matthews et al. 2002). We now show that the temporal profile of expression of Cat-301 in cat area 17 is reciprocal to the profile of the sensitive period to MD (Cynader and Mitchell 1980; Olson and Freeman 1980; Daw et al. 1992). Furthermore, the loss of Cat-301 that results from dark rearing (Guimaraes et al. 1990) can be rescued by a subsequent period of normal visual experience that parallels the timing of the functional recovery of vision (Kaye et al. 1982). Finally, we demonstrated that while neither MD by itself, nor MD followed by long-term RLS, nor early induction of strabismus alter adult levels of Cat-301 in area 17, certain medium-length regimens of MD followed by reverse-lid suture, many of which lead to bilateral amblyopia, result in a large decrease in the number of aggrecan-positive cells. The normal levels of Cat-301 expression in monocularly deprived and long-term RLS animals clearly indicate that binocular vision is not necessary for the normal expression of aggrecan. However, the low levels of Cat-301 in the cortex of a subpopulation of short- and medium-length RLS animals (some of which result in bilateral amblyopia) indicate that certain binocular interactions during the sensitive period can dramatically reduce the density of aggrecan-positive neurons in primary visual cortex. It is not surprising that aggrecan expression in primary visual cortex does not necessarily correlate with final visual acuities since the latter likely depends on neural circuitry involving multiple cortical areas (Mitchell 1991). Instead, the low levels of Cat-301 appear to be most closely linked to conditions where neither eye has a clear territorial advantage in V1 following the termination of the rearing regiment. Indeed, Murphy and Mitchell (1987) showed that short periods of RLS that resulted in bilateral amblyopia resulted in normal OD histograms. It is tempting to speculate that following RLS paradigms that result in no clear dominant eye, the competitive interactions between the eyes prevent many neurons from reaching levels of activity that initiate Cat-301 expression.
In both the somatosensory cortex of rodents (McRae et al. 2007) and the deprived layers of a monocularly deprived cat dLGN (Supplementary Fig. 2), sensory deprivation causes a decrease of aggrecan mRNA as well as a loss of immunostaining. Matthews et al. (2002) showed that while the Cat-301 epitope is located on the protein core of aggrecan, carbohydrate moieties can occlude the Cat-301 epitope. Consequently, the decrease in Cat-301 staining reported in our study could arise from either a decrease in aggrecan expression or an increase or change in carbohydrate moieties that occlude the Cat-301 epitope. We believe that the former explanation accounts for the decrease in Cat-301 staining in the visual cortex reported here for 2 main reasons. First, if the loss of Cat-301 immunoreactivity resulted from an increase or change in a carbohydrate moieties leading to greater occlusion of the Cat-301 epitope, we would predict that immunostaining with either Cat-304, Cat-315, or Cat-316 antibodies, which recognize carbohydrate epitopes, would either increase or show no change in V1 to visual deprivation. However, dark rearing causes a reduction in the density of cells revealed by Cat-304 (Guimaraes et al. 1990), Cat-315, and Cat-316 antibodies (Lander et al. 1997). Second, treatment of dark-reared tissue with chondroinase ABC only increases staining intensity without an increase in the number of cells labeled for the Cat-301 epitope (Supplementary Fig. 4). However, if the loss of Cat-301 immunoreactivity resulted from occlusion of its epitope, we would expect that enzymatic digestion would increase both the intensity of Cat-301 staining and the density of cells labeled with Cat-301.
Aggrecan Expression Correlates with the Termination of the Sensitive Period
One of the most striking features of the maturation of the nervous system is the transition from a hyaluronan rich, highly soluble ECM, to a hyaluronan poor, insoluble ECM (Hockfield et al. 1990). This transition away from an environment permissive for plasticity and growth correlates well with the decline in sensitivity to MD (Hockfield et al. 1990). CSPGs are principal components of neuronal ECM, and their increase with age is experience dependent (Guimaraes et al. 1990). They are located perisynaptically in “perineuronal nets” that surround several cell types including the parvalbumin-positive GABAergic interneurons, where they have been hypothesized to stabilize synapses by binding hyaluronan and thereby increasing the insolubility of the ECM. Strong support for the hypothesis that CSPGs regulate the age-dependent decline in plasticity in visual cortex came when Pizzorusso et al. (2002) showed that enzymatic degradation of perineuronal nets with chondroitinase restored plasticity as assessed by the response to MD in adult rats, a result consistent with the idea that a mature ECM functions to restrict plasticity in adult animals. The very low expression patterns of aggrecan following certain regimens of RLS raises the possibility that the visual cortex of these animals may retain a higher degree of plasticity than age-matched normally reared animals. However, the fact that the functional recovery of vision of these animals was poor suggests that any enhanced plasticity might only be revealed by very specific manipulations or that complete loss of aggrecan in all layers, including layer 4 is necessary to promote functional recovery.
Based on the biochemical properties of aggrecan, its temporal and spatial expression patterns in both the cat dLGN and the rodent spinal cord, and the finding that CSPGs can inhibit neurite outgrowth, Hockfield et al. hypothesized that it is the key CSPG that regulates the termination of the sensitive period (Sur et al. 1988; Hockfield 1990; Hockfield et al. 1990). We now provide correlative evidence supporting this hypothesis. First, the developmental time course of aggrecan expression is distinct in different layers of cat area 17, in good agreement with layer-specific differences in the sensitive period to MD (Shatz and Stryker 1978; Cynader et al. 1980; Kalil 1980; Jones et al. 1984; Daw et al. 1992). Second, layer-specific effects of dark rearing (Guimaraes et al. 1990) on Cat-301 expression parallel effects seen in the response of neurons to dark rearing (Cynader and Mitchell 1980; Mower et al. 1985; Mower and Christen 1985; Mower 1991). Third, Cat-301 expression recovers to normal levels in animals reared in darkness from birth to 100 days followed immediately by an equal period of visual exposure. Previous work has demonstrated that cats dark reared from birth to around 100 days can recover near-normal visual acuities following a period of visual exposure of similar duration (Timney et al. 1978; Kaye et al. 1982).
The finding that certain periods of RLS result in a dramatic reduction in Cat-301 combined with the findings of Pizzorusso et al. (2002) that removal of CSPGs in adult rats restored OD plasticity to MD raises the intriguing possibility that animals raised with these regimes of RLS may maintain a higher degree of plasticity than their normally reared counterparts. A number of recent studies raise the possibility that certain early postnatal experiential manipulations can potentiate the effects of similar manipulations applied in adulthood. The quintessential example of facilitation of adult plasticity by early experience was provided by Knudsen's (1998) demonstration of the enhanced capacity of the auditory system of adult barn owls to adapt to altered sensory experience if they had received similar altered experience as juveniles. Recently, Hofer et al. (2006) showed that the susceptibility of visual cortical neurons in adult mice to MD was elevated in animals for which the same eye had been monocularly deprived when they were young. Moreover, another experiment on rodents suggests that certain experiential manipulations applied on adult animals may enhance adult plasticity. He et al. (2007) showed an enhancement of both behavioral and electrophysiological plasticity in rats that had been monocularly deprived when young immediately following a short (3–10 days) period of dark rearing in adulthood. Based on these findings in rodents, it is not unreasonable to hypothesize that certain early rearing regimens could maintain higher levels of plasticity in adult cat V1. Certainly, a key step in restoring or maintaining plasticity in the adult cortex would be to locally degrade the ECM (Berardi et al. 2004).
Cat-301 Labels GABAergic and Non-GABAergic Cells
The maturation of the GABAergic network has been demonstrated to play a crucial role in the temporal parameters of the sensitive period to MD in mice (Hensch et al. 1998; Hanover et al. 1999; Huang et al. 1999; and Fagiolini and Hensch 2000). Furthermore, dark rearing has been shown to affect numerous physiological properties of neurons in the cat striate cortex, such as orientation and direction selectivity, the maturation of which are believed to be dependent upon GABAergic input (Sillito 1975, 1977; Sillito et al. 1980 Blakemore and Van Sluyters 1975; Imbert and Buisseret 1975; Buisseret and Imbert 1976). GABA has also been implicated in mediating the effects of MD (Sillito et al., 1981). However, while light deprivation in adults has been shown to reduce glutamic acid decarboxylase (GAD) and GABA levels in area 17 of adult animals, neither MD nor dark rearing has any effect on the density of GAD- and GABA-positive cells (Bear et al. 1985; Mower et al. 1988) or on GABAA receptor levels (Shaw et al. 1987; Mower et al. 1988) and function (Tanaka et al. 1987; Tsumoto and Freeman 1987). Parvalbumin labels a subset of fast-spiking GABAergic basket cells in the cerebral cortex that play a key role in regulating plasticity (Hensch 2005). In rats, MD, but not dark rearing, reduces parvalbumin expression (Cellerino et al. 1992). In the mouse, dark rearing does reduce parvalbumin levels (Tropea et al. 2006). We now show normal density of parvalbumin expressing cells in area 17 following extended periods of dark rearing. The cellular basis of these species-specific effects of different rearing regimens on parvalbumin is not clear. However, it is clear that dark rearing in all species examined has a profound effect on GABAergic development (Morishita and Hensch 2008), which in cats can be clearly identified by the large reduction in Cat-301 levels. This reduction in Cat-301 represents the first biochemical correlate for the functional abnormalities observed in the inhibitory network in the cat striate cortex following early periods of altered visual experience. Our findings, and those of earlier studies (Guimaraes et al. 1990 and Lander et al. 1997), are consistent with the loss of functional properties of neurons in area 17 that rely on mature inhibitory connections and are also in agreement with recent findings that the GABAergic system regulates the timing of the sensitive period (for a review, see Hensch 2005). It is also important to note that we have used long periods of dark rearing; we did not examine whether shorter periods of deprivation would alter parvalbumin expression in area 17 of cats, but this would be interesting to examine in future experiments.
It should be noted that, in addition to the substantial effects of dark rearing on GABAergic cells in area 17, dark rearing also causes a complete loss of aggrecan-positive pyramidal cells. Following visual exposure, however, aggrecan expression reemerges on both pyramidal and GABAergic neurons, a result consistent with the reappearance of orientation-selective and to a lesser extent directionally selective cells (e.g., Imbert and Buisseret 1975; Cynader et al. 1976; Buisseret et al. 1978; Cynader and Mitchell 1980).
Cat-301 Expression and Cortical Binocularity
Several of our findings clearly indicate that Cat-301 expression does not correlate well with the degree of cortical binocularity. To test this hypothesis more directly, we induced strabismus surgically in 4 kittens at the time eye opening. Despite a near-complete breakdown of cortical binocularity in these animals (Sengpiel et al. 1994), Cat-301 levels in the visual cortex of both esotropes and exotropes were normal or near normal.
It should be noted that despite the dissociation between the levels of Cat-301 and the level of binocularity of cortical cells, it is nevertheless apparent that the former can be influenced to a considerable extent by an animal’s early history of interactions between the two eyes. This influence is best exemplified by comparing the results obtained in cats C418 and C362. Although both animals were monocularly deprived until 5 weeks of age, at which time they experienced a period of reverse occlusion, C418 had binocular vision restored at 10 weeks of age (i.e., during the sensitive period), while C362 had binocular vision restored at 17 weeks of age. As seen in Figure 7, overall Cat-301 levels were dramatically reduced in C418, but not C362, indicating that the relatively early initiation of binocular vision for C418 had a detrimental effect on Cat-301 expression. This was so despite the fact that the outcomes for the 2 animals were quite similar with respect to the visual acuities of the 2 eyes. The visual acuity recovered by the deprived eye of both animals during the period of reverse occlusion was largely lost following introduction of binocular visual exposure. The apparent influence of binocular interactions on Cat-301 expression in the dLGN led us to hypothesize that cortical events dictate Cat-301 levels in the dLGN (Kind et al. 1995). It is possible that restoration of binocular visual input at an earlier age did not permit consolidation of mature physiological properties through the initially deprived eye and that during the subsequent period of binocular visual exposure, the activity patterns of individual neurons provided inappropriate conditions for full expression of the CPSG recognized by Cat-301.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This work was supported by grants from National Institutes of Health (EY 06511 to Susan Hockfield), the Medical Research Council (G97 06008 to C. Blakemore), and the Natural Science and Engineering Research Council of Canada (A7660 to D.E.M.).
Supplementary Material
Acknowledgments
We would like to dedicate this paper to Prof. Susan Hockfield. This work would not have been possible without her vision, constant encouragement, guidance, and mentorship. We would also like to thank Prof. Colin Blakemore for his support and Prof. Nigel Daw for his generous donation of tissue. Conflict of Interest: None declared.
References
- Bear MF. Bidirectional synaptic plasticity: from theory to reality. Philos Trans R Soc Lond B Biol Sci. 2003;358:649–655. doi: 10.1098/rstb.2002.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Rittenhouse CD. Molecular basis for induction of ocular dominance plasticity. J Neurobiol. 1999;41:83–91. doi: 10.1002/(sici)1097-4695(199910)41:1<83::aid-neu11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Bear MF, Schmechel DE, Ebner FF. Glutamic acid decarboxylase in the striate cortex of normal and monocularly deprived kittens. J Neurosci. 1985;5:1262–1275. doi: 10.1523/JNEUROSCI.05-05-01262.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Curr Opin Neurobiol. 2000;10:138–145. doi: 10.1016/s0959-4388(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Ratto GM, Maffei L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003;26:369–378. doi: 10.1016/S0166-2236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Maffei L. Extracellular Matrix and Visual Cortical Plasticity: Freeing the Synapse. Neuron. 2004;6:905–908. doi: 10.1016/j.neuron.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Van Sluyters RC. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol (Lond) 1975;248:663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhoeffer T. Neurotrophins and activity-dependent development of the neocortex. Curr Opin Neurobiol. 1996;6:119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- Buisseret P, Imbert M. Visual cortical cells: their developmental properties in normal and dark-reared kittens. J Physiol (Lond) 1976;255:511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisseret P, Gary-Bobo E, Imbert M. Ocular motility and recovery of oritentational properties of visual cortical neurons in dark-reared kittens. Nature. 1978;272:816–817. doi: 10.1038/272816a0. [DOI] [PubMed] [Google Scholar]
- Cellerino A, Siciliano R, Domenici L, Maffei L. Parvalbumin immunoreactivity: a reliable marker for the effects of monocular deprivation in the rat visual cortex. Neuroscience. 1992;51:749–753. doi: 10.1016/0306-4522(92)90514-3. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Cynader M. Prolonged sensitivity to monocular deprivation in dark-reared cats: effects of age and visual exposure. Dev Brain Res. 1983;8:155–164. doi: 10.1016/0165-3806(83)90002-0. [DOI] [PubMed] [Google Scholar]
- Cynader M, Berman N, Hein A. Recovery of function in cat visual cortex following prolonged deprivation. Exp Brain Res. 1976;25:139–156. doi: 10.1007/BF00234899. [DOI] [PubMed] [Google Scholar]
- Cynader M, Mitchell DE. Prolonged sensitivity to monocular deprivation in dark-reared cats. J Neurophysiol. 1980;43:1026–1040. doi: 10.1152/jn.1980.43.4.1026. [DOI] [PubMed] [Google Scholar]
- Cynader M, Timney B, Mitchell DE. Period of susceptibility of kitten visual cortex to the effects of monocular deprivation extends beyond six months of age. Brain Res. 1980;191:545–550. doi: 10.1016/0006-8993(80)91303-7. [DOI] [PubMed] [Google Scholar]
- Daw NW. Visual development. New York: Plenum Press; 2005. [Google Scholar]
- Daw NW, Fox K, Sato H, Czepita D. Critical period for monocular deprivation in the cat visual cortex. J Neurophysiol. 1992;67:197–202. doi: 10.1152/jn.1992.67.1.197. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Hockfield S, Garren H, Van Essen DC. Antibody labeling of functional subdivisions in visual cortex: cat-301 immunoreactivity in striate and extrastriate cortex of the macaque. Vis Neurosci. 1990;5:67–81. doi: 10.1017/s0952523800000080. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in the primary cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Guimaraes A, Zaremba S, Hockfield S. Molecular and morphological changes in the cat lateral geniculate nucleus and visual cortex induced by visual deprivation are revealed by monoclonal antibodies Cat-304 and Cat-301. J Neurosci. 1990;10:3014–3024. doi: 10.1523/JNEUROSCI.10-09-03014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JL, Huang ZH, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci. 1999;19:RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Ray B, Dennis K, Quinlan EM. Experience-dependent recovery of vision following chronic deprivation. Nat Neurosci. 2007;10:1134–1136. doi: 10.1038/nn1965. [DOI] [PubMed] [Google Scholar]
- Hendry SC, Hockfield S, Jones EG, McKay R. Monoclonal antibody that identifies subsets of neurons in the central visual system of monkey and cat. Nature. 1984;307:267–270. doi: 10.1038/307267a0. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hensch T, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockfield S. Proteoglycans in neural development. Semin Dev Biol. 1990;1:55–63. [Google Scholar]
- Hockfield S, Kalb RG, Zaremba S, Fryer HJ. Expression of neural proteoglycans correlates with the acquisition of mature neuronal properties in the mammalian brain. Cold Spring Harbor Symp Quant Biol. 1990;55:505–514. doi: 10.1101/sqb.1990.055.01.049. [DOI] [PubMed] [Google Scholar]
- Hockfield S, McKay RD, Hendry SHC, Jones EG. A surface antigen that identifies ocular dominance columns in the visual cortex and laminar features of the lateral geniculate nucleus. Cold Spring Harbor Symp Quant Biol. 1983;48:877–889. doi: 10.1101/sqb.1983.048.01.090. [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. Prior experience enhances plasticity in adult visual cortex. Nat Neurosci. 2006;9:127–213. doi: 10.1038/nn1610. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron. 2007;56:312–326. doi: 10.1016/j.neuron.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in the mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Humphrey AL, Sur M, Uhlrich DJ, Sherman SM. Projection patterns of individual X- and Y- cell axons from the lateral geniculate nucleus to cortical area 17 in the cat. J Comp Neurol. 1985;233:159–189. doi: 10.1002/cne.902330203. [DOI] [PubMed] [Google Scholar]
- Imbert M, Buisseret P. Receptive field characteristics and plastic properties of visual cortical cells in kittens reared with or without visual experience. Exp Brain Res. 1975;22:25–36. doi: 10.1007/BF00235409. [DOI] [PubMed] [Google Scholar]
- Jones KR, Spear PD, Tong L. Critical periods for effects of monocular deprivation: differences between striate and extrastriate cortex. J Neurosci. 1984;4:2543–2552. doi: 10.1523/JNEUROSCI.04-10-02543.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil R. A quantitative study of the effects of monocular enucleation and deprivation on cell growth in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol. 1980;189:483–524. doi: 10.1002/cne.901890305. [DOI] [PubMed] [Google Scholar]
- Kaye M, Mitchell DE, Cynader M. Depth perception, eye alignment, and cortical ocular dominance of dark-reared cats. Dev Brain Res. 1982;2:37–53. doi: 10.1016/0165-3806(81)90057-2. [DOI] [PubMed] [Google Scholar]
- Kind PC, Beaver CJ, Mitchell DE. Effects of early periods of monocular deprivation and reverse lid suture on the development of Cat-301 immunoreactivity in the dorsal lateral geniculate nucleus (dLGN) of the cat. J Comp Neurol. 1995;359:523–536. doi: 10.1002/cne.903590402. [DOI] [PubMed] [Google Scholar]
- Kind PC, Blakemore C, Fryer H, Hockfield S. Identification of proteins downregulated during postnatal development of the cat visual cortex. Cereb Cortex. 1994;4:361–375. doi: 10.1093/cercor/4.4.361. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Capacity for plasticity in the adult owl auditory system expanded by juvenile experience. Science. 1998;279:1531–1533. doi: 10.1126/science.279.5356.1531. [DOI] [PubMed] [Google Scholar]
- Lachance PE, Chaudhuri A. Microarray analysis of developmental plasticity in monkey primary visual cortex. J Neurochem. 2004;88:1455–1469. doi: 10.1046/j.1471-4159.2003.02274.x. [DOI] [PubMed] [Google Scholar]
- Lander C, Kind P, Maleski M, Hockfield S. A family of activity-dependent neuronal cell-surface chondroitin sulfate proteoglycans in cat visual cortex. J Neurosci. 1997;17:1926–1939. doi: 10.1523/JNEUROSCI.17-06-01928.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL, Maurer D. Multiple sensitive periods in human visual development: evidence from visually derived children. Dev Psychobiol. 2005;46:163–183. doi: 10.1002/dev.20055. [DOI] [PubMed] [Google Scholar]
- Majdan M, Shatz CJ. Effects of visual experience on activity-dependent gene regulation in cortex. Nat Neurosci. 2006;9:650–659. doi: 10.1038/nn1674. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Kelly GM, Zerillo CA, Gray G, Tiemeyer M, Hockfield S. Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J Neurosci. 2002;22:7536–7547. doi: 10.1523/JNEUROSCI.22-17-07536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae PA, Rocco MM, Kelly G, Brumberg JC, Matthews RT. Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex. J Neurosci. 2007;27:5405–5411. doi: 10.1523/JNEUROSCI.5425-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DE. The long-term effectiveness of different regimens of occlusion on recovery from early monocular deprivation in kittens. Philos Trans R Soc Ser B. 1991;333:51–79. doi: 10.1098/rstb.1991.0060. [DOI] [PubMed] [Google Scholar]
- Mitchell DE, Timney B. Postnatal development of function in the mammalian visual system. In: Darian-Smith I, editor. Handbook of physiology Section I: the nervous system. Vol. 3. Part 1 sensory processes. Bethesda (Maryland): American Physiological Society; 1984. pp. 507–555. [Google Scholar]
- Morishita H, Hensch TK. Critical period revisited: impact on vision. Curr Opin Neurobiol. 2008;18:101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Movshon JA, Kiorpes L. The role of experience in visual development. In: Coleman JR, editor. Development of sensory systems in mammals. New York: Wiley; 1990. pp. 155–202. [Google Scholar]
- Movshon JA, Van Sluyters RC. Visual neuronal development. Annu Rev Psychol. 1981;32:477–522. doi: 10.1146/annurev.ps.32.020181.002401. [DOI] [PubMed] [Google Scholar]
- Mower GD. The effect of dark-rearing on the time course of the critical period in cat visual cortex. Dev Brain Res. 1991;58:151–158. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- Mower GD, Berry D, Burchfiel JL, Duffy FH. Comparison of the effects of dark-rearing and binocular suture on development and plasticity of cat visual cortex. Brain Res. 1981;220:255–267. doi: 10.1016/0006-8993(81)91216-6. [DOI] [PubMed] [Google Scholar]
- Mower GD, Caplan C, Christen WG, Duffy FH. Dark rearing prolongs physiological but not anatomical plasticity of the cat visual cortex. J Comp Neurol. 1985;235:448–466. doi: 10.1002/cne.902350404. [DOI] [PubMed] [Google Scholar]
- Mower GD, Christen WG. Role of visual experience in activating critical period in cat visual cortex. J Neurophysiol. 1985;53:572–589. doi: 10.1152/jn.1985.53.2.572. [DOI] [PubMed] [Google Scholar]
- Mower GD, Rustsad R, White WF. Quantitative comparisons of gamma-aminobutyric acid neurons and receptors in the visual cortex of normal and dark-reared cats. J Comp Neurol. 1988;272:293–302. doi: 10.1002/cne.902720211. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Mitchell DE. Reduced visual acuity in both eyes of monocularly deprived kittens following a short or a long period of reverse occlusion. J Neurosci. 1987;7:1526–1536. doi: 10.1523/JNEUROSCI.07-05-01526.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CR, Freeman RD. Profile of the sensitive period for monocular deprivation in kittens. Exp Brain Res. 1980;39:17–21. doi: 10.1007/BF00237065. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Prasad SS, Kojic LZ, Li P, Mitchell DE, Hachisuka A, Sawada J, Gu Q, Cynader MS. Gene expression patterns during enhanced periods of visual cortex plasticity. Neuroscience. 2002;111:35–45. doi: 10.1016/s0306-4522(01)00570-x. [DOI] [PubMed] [Google Scholar]
- Sengpiel F, Blakemore C, Kind PC, Harrad R. Interocular suppression in the visual cortex of strabismic cats. J Neurosci. 1994;11:6855–6871. doi: 10.1523/JNEUROSCI.14-11-06855.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. Ocular dominance in layer IV of the cat’s visual cortex and the effects of monocular deprivation. J Physiol (Lond) 1978;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw C, Aoki C, Wilkinson M, Prusky G, Cynader M. Benzodiazepine ([sH] flunitrazepam) binding in cat visual cortex: ontogenesis of normal characteristics and the effects of dark rearing. Dev Brain Res. 1987;37:67–76. doi: 10.1016/0165-3806(87)90229-x. [DOI] [PubMed] [Google Scholar]
- Sillito AM. The contribution of inhibitory mechanisms to the receptive field properties of neurons in the striate cortex of the cat. J Physiol (Lond) 1975;250:305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito AM. Inhibitory processes underlying the directional specificity of simple, complex and hypercomplex cells in the cat's visual cortex. J Physiol (Lond) 1977;271:699–720. doi: 10.1113/jphysiol.1977.sp012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito AM, Kemp JA, Patel H. Inhibitor interactions contributing to the ocular dominance of monocularly dominated cells in the normal cat striate cortex. Exp Brain Res. 1980;41:1–10. doi: 10.1007/BF00236673. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Kemp JA, Blakemore C. The role of GABAergic inhibition in the cortical effects of monocular deprivation. Nature. 1981;272:816–817. doi: 10.1038/291318a0. [DOI] [PubMed] [Google Scholar]
- Sur M, Frost DO, Hockfield S. Expression of a surface-associated antigen on Y-cells in the cat lateral geniculate nucleus is regulated by visual experience. J Neurosci. 1988;8:874–882. doi: 10.1523/JNEUROSCI.08-03-00874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Freeman RD, Ramoa AS. Dark-reared kittens: GABA sensitivity of cells in the visual cortex. Exp Brain Res. 1987;65:673–675. doi: 10.1007/BF00235991. [DOI] [PubMed] [Google Scholar]
- Timney B, Mitchell DE, Giffin F. The development of vision in cats after extended periods of dark-rearing. Exp Brain Res. 1978;31:547–560. doi: 10.1007/BF00239811. [DOI] [PubMed] [Google Scholar]
- Tropea D, Kreiman G, Lyckman A, Mukherjee S, Yu H, Horng S, Sur M. Gene expression changes and molecular pathways mediating activity-dependent plasticity in visual cortex. Nat Neurosci. 2006;9:660–668. doi: 10.1038/nn1689. [DOI] [PubMed] [Google Scholar]
- Tsumoto T, Freeman RD. Dark-reared cats: responsivity of cortical cells influenced pharmacologically by an inhibitory antagonist. Exp Brain Res. 1987;65:666–672. doi: 10.1007/BF00235990. [DOI] [PubMed] [Google Scholar]
- Wiesel TN. The postnatal development of the visual cortex and the influence of the environment. Nature. 1982;299:583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.