Abstract
Plasticity of the human primary motor cortex (M1) has a critical role in motor control and learning. The cerebellum facilitates these functions using sensory feedback. We investigated whether cerebellar processing of sensory afferent information influences the plasticity of the primary motor cortex (M1). Theta-burst stimulation protocols (TBS), both excitatory and inhibitory, were used to modulate the excitability of the posterior cerebellar cortex and to condition an ongoing M1 plasticity. M1 plasticity was subsequently induced in 2 different ways: by paired associative stimulation (PAS) involving sensory processing and TBS that exclusively involves intracortical circuits of M1. Cerebellar excitation attenuated the PAS-induced M1 plasticity, whereas cerebellar inhibition enhanced and prolonged it. Furthermore, cerebellar inhibition abolished the topography-specific response of PAS-induced M1 plasticity, with the effects spreading to adjacent motor maps. Conversely, cerebellar excitation had no effect on the TBS-induced M1 plasticity. This demonstrates the key role of the cerebellum in priming M1 plasticity, and we propose that it is likely to occur at the thalamic or olivo-dentate nuclear level by influencing the sensory processing. We suggest that such a cerebellar priming of M1 plasticity could shape the impending motor command by favoring or inhibiting the recruitment of several muscle representations.
Keywords: cerebellum, human, modulation, motor cortex, plasticity, repetitive transcranial magnetic stimulation, thalamus
Introduction
Central nervous system plasticity is crucial for motor control, learning, memory, and functional reorganization of the damaged cortex. Studies in animals have demonstrated that sensory feedback arising from movements and interactions with the environment are processed by the cerebellum to facilitate motor control and promote motor learning (Nixon 2003; Ben Taib et al. 2005; Chen and Wolpaw 2005; Wolpaw and Chen 2006). In humans too, imaging studies point to the involvement of the cerebellum (CB) in sensory processing, ranging from active discrimination of texture (Gao et al. 1996) and shape (Roland et al. 1989) to monitoring limb movement (Miall et al. 2001) and sensory tasks (Jueptner et al. 1997). Based on this and taking advantage of the noninvasive cerebellar stimulation, we investigated whether cerebellar processing of sensory afferent information influences the plasticity of the primary motor cortex (M1) that potentially underlies motor adaptation/learning in humans. We hypothesized that cerebellar conditioning (i.e., excitation or inhibition) would either facilitate or block the response of M1 to a plasticity induction protocol that was dependent on sensory afferent stimulation, but not the response to a protocol that was independent of sensory afferent stimulation. To test this, we altered the functioning of the CB by exciting or inhibiting it with an appropriate plasticity induction protocol (Popa et al. 2010). The effect of cerebellar conditioning was evaluated by subsequently measuring the response of M1 to a plasticity induction protocol applied to M1, which was dependent on or independent of peripheral sensory input. We used paired associative stimulation (PAS) as the plasticity induction protocol that was dependent on peripheral afferent input (Quartarone et al. 2006) and theta-burst stimulation (TBS) as the plasticity induction protocol independent of it (Huang et al. 2005). Cerebellar conditioning was achieved using intermittent TBS to excite the cerebellar cortex and continuous TBS to inhibit it.
Materials and Methods
Subjects
Twenty-four healthy volunteers (15 women and 8 men; mean age 32.6 ± 6.6 years) participated in the study. All subjects were right handed. Experimental procedures were approved by the local Ethics Committee and performed according to the ethical standards laid down in the Declaration of Helsinki. All subjects gave their written informed consent before the experiments.
Electromyographic Recordings
The subjects were seated comfortably in an armchair, with the 2 hands resting symmetrically on a pillow placed on their lap. They were asked to visually fix a point 1 m in front of them. Motor evoked potentials (MEPs) were recorded from the right Abductor pollicis brevis (APB) and Abductor digiti minimi (ADM), using disposable Ag/AgCl surface electrodes in a muscle belly–tendon montage. Responses were amplified (1000×) and filtered (100–3000 Hz) with a Digitimer D360 amplifier (Digitimer Ltd, Welwyn Garden City, UK), then digitally transformed at a sampling rate of 10 000 Hz (CED Power 1401 MkII, Cambridge Electronic Design (CED) Ltd, Cambridge, UK), and stored off-line for analysis (Signal 4.02, CED Ltd, Cambridge, UK).
Experimental Paradigm
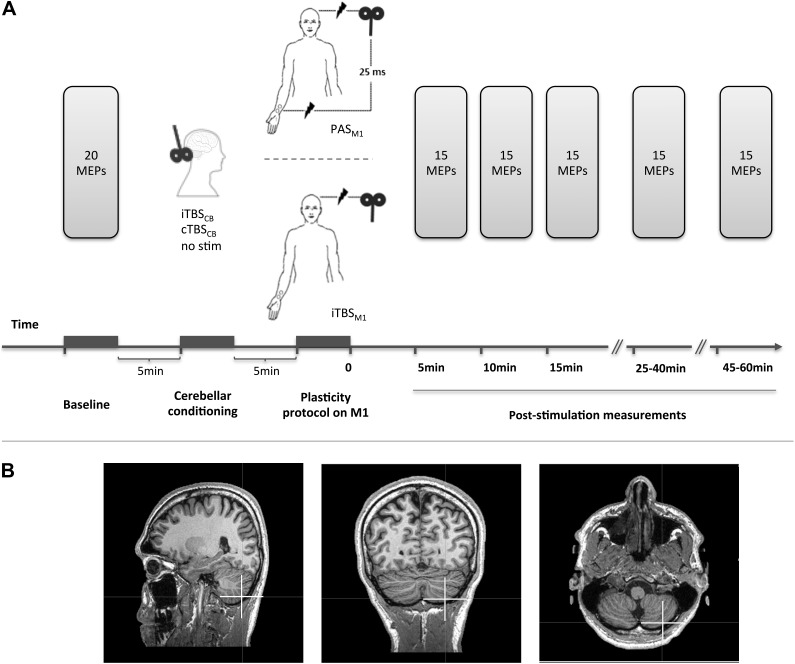
The study consisted of 3 sessions involving 25 ms PAS delivered at 5 Hz on the M1 (PAS) and 3 sessions involving intermittent TBS on the M1 (iTBSM1), as the plasticity inducing protocols at the level of M1. The 2 protocols were chosen in such a way as to have both facilitatory effects on M1 and similar durations (i.e., 2 min for the 5 Hz PAS and 3 min 20 s for the iTBS), but to be dependent and independent of peripheral input, respectively.
The PAS sessions were: 1) a PAS session preceded by cerebellar excitation (iTBSCB → PAS), 2) a PAS session preceded by cerebellar inhibition (cTBSCB → PAS), 3) a PAS session alone, not preceded by any cerebellar stimulation (PAS). The iTBSM1 sessions were: 4) a facilitatory TBS session preceded by cerebellar excitation (iTBSCB → iTBSM1), 5) a facilitatory TBS session preceded by cerebellar inhibition (cTBSCB → iTBSM1), and 6) a facilitatory TBS session alone, not preceded by any cerebellar stimulation (iTBSM1). Any 2 successive sessions were conducted at least 1 week apart. The order of interventions was pseudorandomized across the subjects.
The same 14 subjects underwent sessions 1–5 (PAS, iTBSCB → PAS, cTBSCB → PAS, iTBSM1, iTBSCB → iTBSM1), 9 subjects (of which 7 new) participated in the session 6 (cTBSCB → iTBSM1). The new subjects who participated to the session 6 had their own control (iTBSM1) recorded separately. The change in the excitability of the M1 before and after each intervention was measured by using single-pulse transcranial magnetic stimulation (TMS) to evoke EMG responses (i.e., MEPs) in the APB and ADM. The APB, innervated by median nerve, which is stimulated during the PAS protocol, was the target muscle; the ADM, innervated by the unstimulated ulnar nerve, was the reference muscle to assess the topographic specificity of cortical changes induced by each intervention. TMS pulses were delivered above the motor threshold over the APB's “motor hot-spot” (the point within M1 where evoked MEPs have maximum amplitude for APB). The cortical representation of APB and ADM are close enough for consistent measurable MEPs to be evoked simultaneously in both muscles. This allows exploring the topographic specificity of the effects (Weise et al. 2006, 2011; Quartarone et al. 2008).
We also performed a control set of experiments in 6 subjects to explore the effect of excitatory cerebellar conditioning preceding PAS (iTBSCB → PAS) on the response of primary somatosensory cortex (S1). This was compared with the response to PAS alone without cerebellar conditioning (PAS). We used the P14/N20 amplitude of the somatosensory evoked potentials (SEPs) as the measure of the subcortical relays and the N20/P25 amplitude of the SEPs as the measure of the S1 response. Seven subjects (of which 3 new) participated in the SEP sessions.
TMS Sessions
Evaluation of Corticospinal Output Excitability
The TMS pulses were applied over the left motor cortex with a 70-mm figure-of-eight coil connected to a Bistim magnetic stimulator (The Magstim Company, Whitland, UK). The magnetic stimuli had a nearly monophasic pulse configuration, with a rise time of approximately 0.1 ms, decaying back to zero over approximately 0.8 ms. The optimal position and coil tilt for eliciting MEPs from the right APB muscle were recorded and maintained throughout the experimental sessions with the help of an magnetic resonance imaging (MRI)-based neuronavigation system (eXimia 2.2.0, Nextim Ltd, Helsinki, Finland) or were marked on a head bonnet for the subjects whose MRI was not available. The direction of the induced current was posterior to anterior at an approximately 45° from the midline, for optimal trans-synaptic activation of the motor cortex (Werhahn et al. 1994; Kaneko et al. 1996).
After identifying and recording the positions of the stimulation spot, the resting motor threshold (RMT) was calculated for APB. The RMT was defined as the lowest intensity that produced MEPs of ≥50 μV in at least 5 of 10 trials with the muscles relaxed (Rossini et al. 1994). The active motor threshold (AMT) was also measured. The AMT was defined as the lowest intensity that produced MEPs of ≥0.2 mV in at least 5 of 10 trials when the subject exerted 10% of maximum voluntary contraction using visual feedback (Rothwell 1997).
Twenty MEPs were averaged prior to the intervention and 15 MEPs were averaged 5, 10, 15, 25–40, and 45–60 min after the end of the intervention. The intensity was adjusted and kept at 130% of the RMT.
Cerebellar Stimulation Target
Previous studies have correlated motor tasks with activation in lobules V, VI, VIIIa, and VIIIb of the CB (Stoodley and Schmahmann 2009). Tactile stimulation is also reported to activate sensorimotor hand areas in the cerebellar cortex (lobules VI and VIIIb), as well as in the inferior olive (Bushara et al. 2001; Grodd et al. 2001; Wu et al. 2010). Lobules V and VI are very deep and not readily accessible to TMS stimulation. We chose lobule VIII of CB as the target. In the subjects having their own MRI (N = 5), lobule VIII was identified on the MRI, and the TMS coil was placed and maintained over the target with the help of the neuronavigation system. In these subjects, we measured, on the vertical and horizontal axis, the distance relative to the inion of the cerebellar spot. The mean distances were 2 cm lower and 4 cm lateral to the inion (Fig. 1), that is, lower and more lateral than the classical surface landmarks for cerebellar stimulation (Theoret et al. 2001). These latter landmarks were used for the subjects who did not have their own MRI. The current induced by stimulation of this location has a vertical and caudal to rostral orientation, a direction previously found to be optimal for inducing a measurable effect (Ugawa et al. 1995; Theoret et al. 2001).
Figure 1.
Experimental setup: (A) The MEP amplitudes were measured before and after each plasticity inducing protocol. The protocols consisted of PAS or facilitatory TBS (iTBSM1) of the left primary motor cortex (M1). PAS was delivered alone, or preceded by facilitatory (iTBSCB → PAS) or inhibitory (cTBCCB → PAS) stimulation of the right posterior CB. Facilitatory TBS was delivered to left M1 alone, or preceded by facilitatory (iTBSCB → iTBSM1) or inhibitory (cTBSCB → iTBSM1) stimulation of the right posterior CB. (B) The cerebellar stimulation targeted the posterior part of lobule VIII of the right CB (white cross).
TBS of M1 and CB
A 70-mm figure-of-eight cooled coil connected to a SuperRapid2 magnetic stimulator (Magstim Company, Whitland, Wales, UK) was used to deliver the repetitive stimulation to right CB and left M1. The target in left M1 was APB's hotspot. The magnetic stimulus had a biphasic waveform with a pulse width of 0.3 ms.
For excitatory protocols targeting the CB (iTBSCB) or the motor cortex (iTBSM1), 600 stimuli were delivered at 80% of the AMT in 3-pulse bursts at 50 Hz repeated every 200 ms within 2-s trains and separated by 8-s pauses (i.e., intermittent TBS). For cerebellar inhibition (cTBSCB), 600 stimuli were delivered at 80% of the AMT in 3-pulse bursts at 50 Hz repeated every 200 ms (i.e., continuous TBS) (Huang et al. 2005). Such stimulations can modulate the cerebellar output for at least 30 min (Popa et al. 2010). The stimulation intensities used in this study are well below the maximum limit recommended by the current guidelines for delivering repeated transcranial magnetic stimulation (rTMS) (Wassermann 1998; rediscussed and updated by Rossi et al. 2009).
PAS of M1
For PAS, electric stimulation pulses were delivered over the median nerve at the wrist at 2.5× the sensory threshold. If it induced any twitch, the intensity was decreased until the electromyographically monitored twitch disappeared. Each pulse was followed 25 ms later by a magnetic pulse delivered over the APB's hotspot at 90% AMT. Six hundred pairs of stimuli were delivered at 5 Hz. This stimulation was meant to increase the excitability of M1 when delivered alone (Quartarone et al. 2006).
All experiments were performed in the afternoon in order to maximize plastic effects and to reduce variability (Sale et al. 2007).
The SEPs
In this experimental paradigm, the SEPs were tested before and after PAS and before and after PAS preceded by excitatory cerebellar conditioning (iTBSCB → PAS). SEPs were measured in 6 blocks every 5 min until 30 min after the end of the interventions. Each block of SEPs consisted of 500 pulses delivered over the right median nerve at the wrist with a frequency of 3 Hz, at 3× the perceptual threshold or immediately below the electromyographically measured motor threshold, whichever of the 2 was lower. Online EMG monitoring assured that no muscle twitch was evoked in any of the recorded subjects. The SEPs were recorded with Ag/AgCl surface electrodes in a P3–to–earlobe montage. Responses were amplified (100 000×) and filtered (20–3000 Hz) with a Digitimer D360 amplifier, then digitally transformed at a sampling rate of 1024 Hz (CED Power 1401 MkII) and stored off-line for analysis (Signal 4.02).
Data Analysis
The effects of the interventions 1–6 on the excitability of M1 neurons were evaluated by comparing the mean peak-to-peak amplitude of MEPs from APB and ADM before and after each intervention. To avoid possible differences due to intersession variability of individual MEP amplitudes, we analyzed the postintervention mean MEPs normalized to the preintervention mean MEP. The effects of the interventions 1 and 3 (i.e., iTBSCB → PAS and PAS) on the SEPs were evaluated by comparing the mean P14-N20 and N20–P25 amplitudes before and after each intervention, normalized to their baseline. Each parameter was submitted to repetitive-measures analysis of variance (ANOVA), with TIME as the main within-group factor and INTERVENTION as the main between-group factor. When a significant main effect was seen, Fisher's post hoc test was used to characterize the time course of the parameters after each type of intervention and to compare the interventions 2 × 2. As the group of subjects undergoing intervention 1–5 did not completely match the group undergoing intervention 6, the data from the 2 groups were submitted to separate analysis.
For all statistical analyses, a P value of <0.05 was assumed to denote significance. Stat View software (SAS Institute Inc, Cary, NC, USA) was used for all statistical analyses.
Results
The subjects did not report any adverse effects after any of the interventions. There was also no clinically evident motor impairment (e.g., cerebellar tremor) at the end of any session.
Effect of Cerebellar Conditioning on PAS
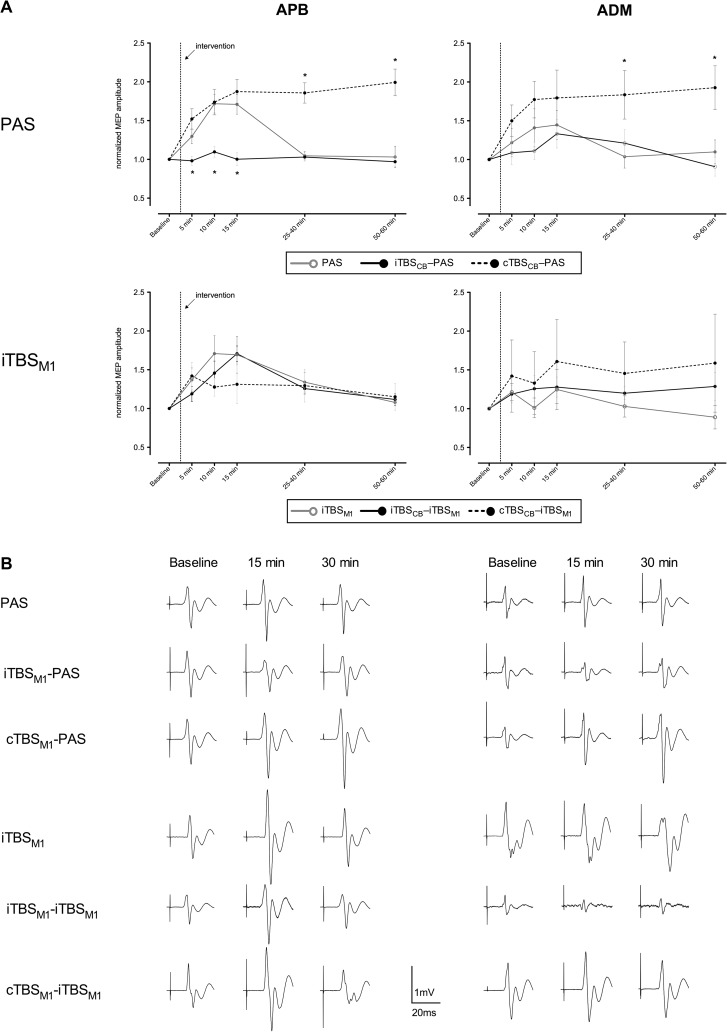
There were no statistically significant differences between the MEP amplitudes at baseline of all session (Supplementary Table 1). PAS delivered without cerebellar conditioning led to MEP facilitation only in the APB, lasting for more than 15 min (Fig. 2A, PAS/APB panel: continuous gray line). Interestingly, cerebellar inhibition (cTBSCB → PAS) enhanced the effect of the subsequent PAS. Indeed MEP facilitation was larger and prolonged, lasting more than 50 min post intervention (P < 0.001) (Fig. 2A, PAS/APB panel: dashed black line). In contrast, cerebellar excitation (iTBSCB → PAS) prevented the subsequent PAS from inducing any MEP facilitation: MEPs maintained the same level as before the intervention (Fig. 2A, PAS/APB panel: continuous black line). On the other hand, cerebellar excitation had no effect on M1 plasticity evoked by facilitatory TBS (iTBSCB → iTBSM1). Indeed, the MEP profile was similar to the profile evoked by the facilitatory TBS of M1 without cerebellar conditioning (iTBSM1). This was confirmed by repeated-measures ANOVA that revealed a significant effect of INTERVENTION (i.e., PAS, iTBSCB → PAS, cTBSCB → PAS, iTBSM1, iTBSCB → iTBSM1) (F4,55 = 13.4, P < 0.0001), TIME (F4,55 = 18.9, P < 0.0001), as well as their interaction (F16,55 = 4.4, P < 0.0001), with significant post hoc differences at 10 and 15 min postintervention (Fisher's test: post5 vs. post10 P < 0.0001, post5 vs. post15 P < 0.0001). Cerebellar excitation as well as cerebellar inhibition modified the PAS-induced effect (Fisher's test: iTBSCB → PAS vs. PAS P < 0.002, cTBSCB → PAS vs. PAS P < 0.0002, iTBSCB → PAS vs. cTBSCB → PAS P < 0.0001). In contrast, cerebellar excitation did not modify the effect of the facilitatory TBS of M1 (Fisher's test: iTBSCB → iTBSM1 vs. iTBSM1 P = 0.2). The 2 excitatory protocols on M1 without the cerebellar excitatory conditioning had similar effects (PAS vs. iTBSM1 P = 0.3). The repeated-measures ANOVA on the group that had both cTBSCB → iTBSM1 and iTBSM1 alone revealed no significant influence of the cerebellar inhibition on the M1 excitation (INTERVENTON: P = 0.64), both having a similar time profile (TIME: F4,16 = 3.4, P < 0.014). The repeated-measures ANOVA did not reveal any significant changes for the MEPs of ADM in this group: no effect of INTERVENTION (P = 0.99) or TIME (P = 0.1). In conclusion, cerebellar conditioning has an effect only on PAS-induced plasticity of M1.
Figure 2.
Mean MEPs after each intervention: (A) The “PAS” panels show the effects of PAS and of the cerebellar priming on PAS in the same group of subjects. The iTBSM1 panels show the effects of iTBSM1 alone and of the cerebellar priming on iTBSM1 in the same group of subjects, except for the cTBSM1 → iTBSM1 protocol that was performed as a control experiment on a separate set of subjects (analyzed separately vs. their own iTBSM1 session). The MEPs were averaged at several time points before and after each plasticity inducing protocol, then normalized to the prestimulation mean values. Data are presented as means ± standard error. (*) indicates normalized mean MEP amplitudes significantly different from the values obtained after PAS alone (in the “PAS” panels) and after iTBSM1 alone (in the “iTBSM1” panels) at corresponding time points. (B) Examples of nonnormalized mean MEPs from the same subject (except the cTBSCB → iTBSM1 session) representative for each session.
In order to explore the topographic specificity of the 5 interventions, we analyzed the MEPs of the nontarget muscle ADM. We found that the inhibitory cerebellar conditioning resulted in a long lasting facilitation of the PAS effect in ADM as in APB, indicating a loss of the topographic specificity. All the other 5 interventions did not bring any changes in the MEPs of ADM, indicating a preserved topographic specificity (Fig. 2A, ADM panels). This was confirmed by the repeated-measures ANOVA: INTERVENTION (F4,55 = 3.3, P < 0.02) and no effect of TIME (F4,55 = 1.4, P = 0.3) or INTERVENTION × TIME interaction (F16,55 = 1.4, P = 0.1). Only cerebellar inhibition affected the PAS-induced effect on ADM (Fisher's test: cTBSCB → PAS vs. PAS P < 0.02, iTBSCB → PAS vs. PAS P = 0.6, cTBSCB → PAS vs. iTBSCB → PAS P < 0.007, iTBSCB → iTBSM1 vs. iTBSM1 P = 0.2).
Effect of Cerebellar Conditioning of PAS on SEPs
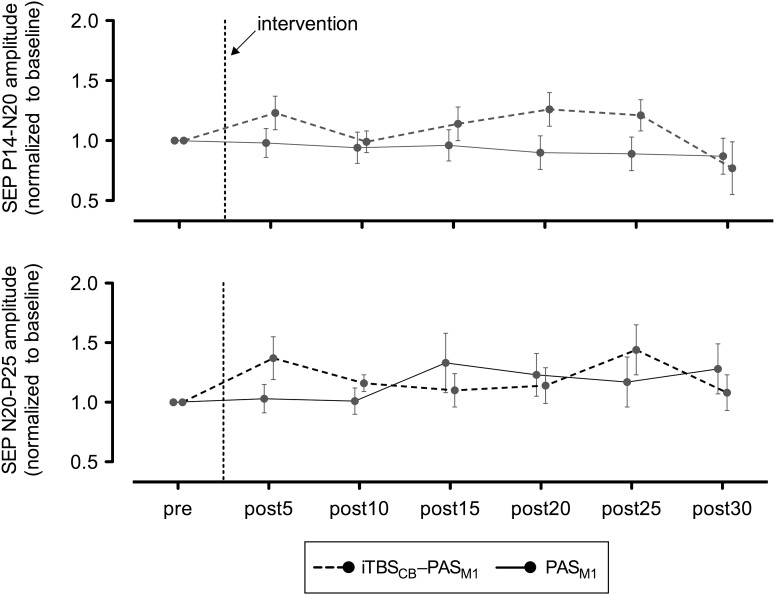
Contrasting with the capability of cerebellar excitatory conditioning to block the enhancement of MEPs induced by PAS, cerebellar excitation did not significantly modify the effect of PAS (Fig. 3) on the P14/N20 subcortical components of SEPs (repeated-measures ANOVA: INTERVENTION F1,10 = 1.4, P = 0.2; TIME F6,10 = 0.4, P = 0.89), or the N20/P25 cortical components of SEPs (repeated-measure ANOVA: INTERVENTION F1,10 = 0.83, P = 0.4; TIME F6,10 = 0.34, P = 0.9). There were no statistically significant differences between the 2 interventions regarding the latencies of the peaks that mark the discharge of the subcortical and of the cortical sensory relays: the P14 latency at baseline was 14.8 ± 0.3 ms, after PAS 14.7 ± 0.4 ms (P = 0.8), and after iTBSCB → PAS 14.7 ± 0.5 ms (P = 0.9), while the N20 latency at baseline was 20.0 ± 0.6 ms, after PAS it was 20.3 ± 0.6 ms (P = 0.4), and after iTBSCB → PAS, it was 20.1 ± 0.5 ms (P = 0.6). Further exploration of the effect of cerebellar stimulation on SEPs is outside of the scope of the present study and needs additional experiments.
Figure 3.
Mean SEPs before and after PAS alone or iTBSCB → PAS, normalized to baseline. Data are presented as means ± standard error.
Discussion
The results of our study support our initial hypothesis that cerebellar excitation or inhibition would alter the response of the motor cortex to different plasticity induction protocols depending on the presence or the absence of a sensory afferent component in the protocol. The cerebellar excitation caused a topographically specific loss of induction of plastic changes in M1 (only in the target muscle) when PAS was applied, while the cerebellar inhibition led to an enhanced response of the M1 to PAS along with a loss in topographic specificity (changes both in the target and the reference muscle). Furthermore, the cerebellar excitation did not alter the response of the M1 to excitatory TBS. From this, we infer that the role of CB in shaping and scaling plasticity of the motor cortex is exerted through a modulation of the peripheral sensory afferents.
Homeostatic Changes within M1 versus Subcortical Sensory Gating
Previous studies that have examined the effect of cerebellar stimulation on corticospinal tract excitability using low-frequency (1 Hz) rTMS of the lateral CB have found contrasting results showing either no changes in MEP amplitudes (Fierro et al. 2007; Popa et al. 2010) or facilitation (Gerschlager et al. 2002; Oliveri et al. 2005). These studies viewed the response of M1 as an almost direct chain effect from the cerebellar nuclei through the thalamus to the motor cortex. One single study using TBS to modulate the cerebellar output has shown that excitatory TBS led to a facilitation of MEPs, while inhibitory TBS led to an inhibition of MEPs, both up to 15 min (Koch et al. 2008). The authors acknowledge that the result is apparently counterintuitive with respect to the 1 Hz stimulation and speculate, for the first time, that the TBS might have an effect on the intermediate synapses of the dentato-thalamo-cortical pathway, rather than at the M1 level. Our present results bring further support to this hypothesis, even if the findings regarding the effects of cerebellar TBS on MEP remain debatable (Popa et al. 2010).
Homeostatic mechanisms contribute to the regulation of human M1 plasticity, in agreement with the Bienenstock–Cooper–Munro rule of a sliding threshold for long-term potentiation (LTP) or depression (LTD) induction (Bienenstock et al. 1982). According to this principle, any change in the M1 homeostatic state (even if remotely induced) would condition the effect of any subsequent intervention. Such a mechanism would explain, for instance, why priming of motor cortex with an LTP-inducing protocol prevents further enhancement of M1 excitability in response to a second LTP-inducing protocol, while priming of motor cortex with an LTD-inducing protocol enhances the M1 excitability in response to a second LTP-inducing protocol (Muller et al. 2007). Several studies (Iyer et al. 2003; Lang et al. 2004; Siebner et al. 2004) have shown that after priming M1 with an LTD-inducing protocol, a subsequent stimulation can have a reverse effect, thus underscoring the importance of a priming phenomenon on any subsequent event impinging on the same stimulated structure. In our study, both PAS and iTBS, being excitatory for M1, should have modified the MEPs in the same way, that is, enhance the MEPs if they find M1 excitability decreased by the cerebellar priming and diminish the MEPs if they find M1 excitability increased by the cerebellar priming. We have found that iTBSCB influences PAS and the facilitatory TBS applied over M1 differently (Fig. 2A: continuous black line vs. dotted black line), suggesting that a “splitting” of the effect occurs upstream of CB before M1. It is therefore reasonable to consider the M1 response to PAS after cerebellar stimulation more as a change in the way the information is conveyed to M1, rather than homeostatic phenomenon within the M1. From this perspective, the targets modulated by the cerebellar output might be the structures that process or relay the afferent information before reaching M1.
There are 5 possible main sites where this influence of cerebellar modulation might occur: 1) primary somatosensory cortex, 2) premotor cortex (PMC), 3) thalamus, 4) cerebello-olivary complex, and 5) spinal cord.
Effect of Cerebellar Modulation Acting at the Somatosensory Cortex
We performed additional control experiments using SEPs to investigate whether cerebellar modulation can influence the primary somatosensory cortex output that could explain the subsequent divergent response within the motor cortex. We found that cerebellar excitation did not significantly modify the effect of PAS on the cortical components of SEPs (N20/P25), although a trend to enhance them was observed only from 15 min onwards. Even if this enhancement were significant, it would not explain the very early alteration of the M1 response, which was already evident at 5 min postintervention (Fig. 2A: continuous black line). The depressant effect of cerebellar conditioning on M1 response to PAS is thus unlikely to primarily involve the somatosensory cortex.
Effect of Cerebellar Modulation Acting at the PMC
It was recently shown in macaque monkeys that Purkinje cells from lobules III–VIII project to the F2r area (Hashimoto et al. 2010), the equivalent of the dorsal PMC in humans. These lobules include sections (lobules IV–VI, VIIB, and VIII) linked to the arm area of M1 (Kelly and Strick 2003). Since our stimulation for cerebellar conditioning was targeting lobule VIII, it is reasonable to think that it might have simultaneously modulated output toward both M1 and PMC. Previous studies have shown that facilitatory rTMS conditioning of the PMC can invert the effects of a facilitatory PAS delivered over the M1, while an inhibitory conditioning of the PMC can invert the effects of an inhibitory PAS (Pötter-Nerger et al. 2009). In order to have a facilitation of the PMC output, there should be an increased facilitatory output in the dentato-thalamo-cortical pathway, which would occur after an inhibition of the cerebellar cortex. Instead, in our study, the blocking of the facilitatory PAS effect was seen after iTBSCB.
There are no reports in the literature about the effect of an inhibitory stimulation of the PMC on a facilitatory PAS delivered over M1. However, inhibitory 1 Hz rTMS of PMC is reported to decrease the excitability of M1 (Gerschlager, Neurology 2001), which would favor PAS applied to M1 though a metaplastic effect (Muller et al. 2007). An inhibition of PMC could follow a reduction in dentate-thalamo-cortical output triggered by iTBSCB. Yet, we observed the opposite: iTBSCB blocked PAS, and it was cTBSCB that enhanced it.
These evidences suggest that the cerebellar conditioning is unlikely to be through the PMC.
Effect of Cerebellar Modulation Acting at the Thalamic Nuclei
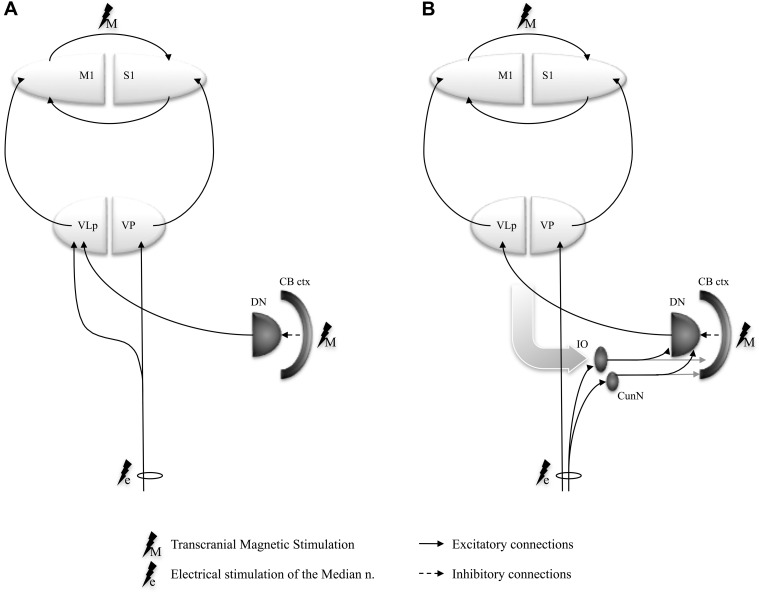
Cerebellar projections have an obligatory relay in the posterior part of the ventrolateral (VLp) thalamic nucleus (ventral intermediate nucleus in the classification of Hassler 1959) before reaching cortical motor areas (Asanuma et al. 1983; Sakai et al. 1996), while somatosensory inputs traveling through the spinothalamic pathways toward S1 relay in the ventral posterior nucleus. There is evidence that some spinothalamic terminals end in clusters around the neurons in the VLp nucleus projecting to motor cortex, making them a more likely route for short-latency somatosensory inputs to be relayed to the motor cortex (Hirai and Jones 1988). Moreover, thalamic neurons, which respond to kinesthetic stimuli in awake humans (Ohye et al. 1989) and monkeys (Vitek et al. 1994), seem to be in a close functional relationship with magnocellular neurons receiving cerebellar projections in VLp (Butler et al. 1992). This evidence suggests that at least some of the cerebellar control of proprioceptive information might occur at the level of thalamic neurons before reaching cortical motor areas. Thalamic neurons responsive to kinesthetic stimuli, if controlled by cerebellar projections, seem ideally positioned to fine-tune a motor command within the motor cortex (Fig. 3A). If this were true, then cerebellar excitatory stimulation could induce LTP-like effects in cerebellar cortex and subsequently augment the normal inhibitory output of cerebellar Purkinje cells to cerebellar deep nuclei, which would result in a reduction of the deep cerebellar nuclear excitatory output to the thalamic neurons. This in turn would increase the threshold necessary to facilitate the transmission of kinesthetic information to M1, manifesting as reduced facilitation of MEP in response to a sensory afferent-dependent LTP-induction protocol on M1 (i.e., PAS). In contrast, cerebellar inhibition can induce LTD-like effects within the cerebellar cortex (Popa et al. 2010), thus reducing the normal cerebellar cortical inhibition of dentate nuclear neurons and facilitating excitatory output toward the thalamic relay, which will promote M1 plasticity. The changes in M1 plasticity by cerebellar excitation and inhibition found in our study are congruent with such a gating effect of sensory information by the CB at the thalamus.
Effect of Cerebellar Modulation on Normal Olivo-Dentate Sensory Processing
Sensory information, such as the one from the median nerve stimulation in PAS, is conveyed directly to the thalamic nuclei, but it can concomitantly activate afferent pathways projecting to the CB though the spino-inferior olivary (IO) fasciculus and the spino-cuneo-cerebellar tract. Both these pathways send excitatory projections to both the cerebellar cortex and the cerebellar nuclei in a somatotopic manner, which ensures that the spinal modulatory inputs within the deep cerebellar and the cerebellar cortical maps on the ipsilateral side correspond with the spinal projection to thalamic and cerebral cortical maps on the contralateral side (Alisky and Tolbert 1997; De Zeeuw et al. 1998). Additionally, the IO nucleus responds to unexpected stimuli that, by definition, are not self-generated by active movements (Eccles et al. 1972; Gellman et al. 1985). This property of IO nucleus, coupled with the convergence of ascending peripheral and descending cortical inputs on it, suggest that the IO-cerebellar complex might work as an unexpected-event detector that modulates responses to peripheral inputs not anticipated by the previously generated movement model (Ekerot 1999; Llinas 2009). The peripheral electrical stimulation in PAS could act as a stream of non–self-generated afferent impulses that activate the olivo-dentato-thalamo-cortical (Fig. 4B) system and keep it in a hyperresponsive state. A similar state could result from a direct activation of the dentate nucleus via the spinocerebellar tracts (Allen et al. 1977, 1978). The TMS stimuli applied to the motor cortex during PAS could utilize this hyperresponsive state to facilitate an LTP in M1. An artificial excitation by TBS of the cerebellar cortex could depress the response of the dentate nucleus, which could then not mediate the PAS response efficiently; on the other hand, an inhibition of the cerebellar cortex by the appropriate TBS protocol could facilitate the dentate nucleus and the dentato-thalamo-cortical relay and enhance PAS response (Fig. 4B).
Figure 4.
Schematic representation of the spino-cerebello-thalamo-cortical circuit models controlling the peripheral afferent information flow to M1: (A) afferents are modulated at thalamic level (VLp) by the cerebellar efferents; (B) afferent inputs are conveyed through inferior olive to the dentate nuclei to interact with the cerebello-thalamo-cortical system. The gray curved arrow stands for all nonspinal inputs to the inferior olive. (CB ctx, stimulated cerebellar cortex; DN, dentate nuclus; IO, inferior olive; VLp, posterior part of the ventrolateral thalamic nucleus [VIM in Hassler's nomenclature]; VP, ventral posterior thalamic nucleus, pars caudalis).
Effect of Cerebellar Modulation Acting at the Spinal Level via the Rubrospinal Tract
Modulation of cerebellar output might also cause facilitation of MEP by inducing plastic changes in spinal motor neurons through the cerebello-rubro-spinal relay (Nathan and Smith 1982; Cheney et al. 1991; Ralston 1994). Indeed, Meunier et al. (2007) have shown that PAS by itself can induce plastic changes in humans not only at the cortical level, as reflected by an increase in MEP amplitude elicited by cortical stimulation, but also at the spinal level. The authors raised the possibility that this MEP facilitation could be due more to the development of spinal plasticity than to genuine cortical plasticity. Lamy et al. (2010) further demonstrated that PAS affects the H-reflex by acting at a presynaptic level. If there were a possible direct influence of cerebellar excitation or inhibition on spinal circuits, it would arrive at the spinal level via the cerebellar nucleorubral tract (Ralston 1994) and then the rubrospinal tract (Nathan and Smith 1982; Cheney et al. 1991), which projects at a presynaptic level on the spinal motor circuit (Rudomin et al. 1981; Jankowska 1988). If the cerebellar conditioning interferes with the descending volley in the cerebellorubral and rubrospinal tracts and their second-order relay in spinal motor neurons, then cerebellar conditioning should influence the M1 response to both the TBS-induced as well as PAS-induced plasticity. On the contrary, we found that cerebellar excitation had no effect on M1 plasticity induced by excitatory TBS to M1, while it attenuated M1 plasticity induced by PAS. We can therefore exclude an alteration in the descending cerebello-rubro-spinal output secondary to cerebellar conditioning as a potential site of cerebellar modulation of motor plasticity.
What Might Be the Physiological Role of Cerebellar Conditioning of M1 Plasticity?
Interestingly, the contrasting effects of cerebellar excitation and inhibition observed in our study on the MEPs from APB and ADM, 2 muscles with topographically close cortical representations, support a highly discriminating role of cerebellar excitatory and inhibitory functional outputs to M1. A similar phenomenon has been observed by Kassavetis et al. (2011) in 2 intrinsic hand muscles. The authors found that while MEP is facilitated only in the activated muscle, the cerebellar inhibition of the contralateral M1 (Ugawa et al. 1995) is suppressed for both the active and the inactive muscle during the initiation of voluntary movement. They postulated that this loss of topographic specificity affecting both the active and surrounding muscles at onset of the movement might be responsible for bringing the motor system to a state of preparedness for the impending voluntary movement. This would allow efficient subsequent corrections to be performed. We extend this postulation to our observations. It may explain why inhibitory cerebellar conditioning (i.e., cTBSCB) resulted in a nonfocal heightened response of M1 to PAS with spread to adjacent cortical maps. According to the rules of metaplasticity, in such a preexcited system, the muscle contraction sequence during a movement would be more easily “trimmed” by intracortical inhibitory mechanisms. On the contrary, excitatory cerebellar conditioning totally suppressed any response to PAS. Again, according to metaplasticity rules, the recruitment of a specific neuronal population may be facilitated in a preinhibited system, enabling a quick reaction to a given sensory context.
We propose that the paradigms used in our experiments for inducing cerebellar excitation and inhibition are equivalent to artificial prolongations of physiological phenomena that normally occur on a millisecond scale during movement planning. In particular, excitation of the cerebellar cortex would inhibit the ipsilateral dentate output (Ito et al. 2007), which could then weaken the excitatory control of thalamic or the olivo-dentate sensory relay and their effects on M1 plasticity, leaving M1 in a metaplastic state less amenable to further plastic modifications. Physiologically, such a phenomenon in M1 could help prevent the acquisition of elements of a new motor program from sources external to M1. In contrast, inhibition of the cerebellar cortex would disinhibit the dentate output, which would either enhance the excitatory thalamic relay or facilitate the olivo-nuclear complex control, permitting other inputs to induce a plastic change in M1, thus possibly contributing to the acquisition of elements of a new motor program. From this perspective, a complex movement could be seen as a succession of simple movements continuously anticipated and preplanned for efficient execution. This would explain the activation of the posterior neocerebellum (target in our experiment) during complex movements (Stoodley and Schmahmann 2009; Schlerf et al. 2010) and during the learning of a new motor task (Orban et al. 2006; Olsson et al. 2008; Seidler and Noll 2008).
It is worth observing that the loss of PAS specificity to the target muscle after cerebellar inhibition closely resembles that observed in dystonic patients (Weise et al. 2006, 2011; Quartarone et al. 2008). This could bring additional support to the increasingly recognized role of the CB in the pathophysiology of dystonia (Argyelan et al. 2009; Wu et al. 2009).
In conclusion, we show that modulation of the cerebellar cortex by noninvasive stimulation can affect the response of M1 cortex to a subsequent plasticity induction protocol that involves sensory afferent input but not otherwise. This remote cerebellar effect on M1 could be mediated by gating of sensory information at the thalamic or olivo-nuclear level. We propose that cerebellar processing of sensory inputs can prime the motor cortex plasticity in a topographically specific manner. This could help organize the impending motor command by favoring or inhibiting the recruitment of several muscle representations. These observations represent a starting point both for noninvasive studies of deep-structure physiology and for therapeutic manipulation of subcortical brain plasticity.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This research was conducted within the framework of an Institute National de la Santé et de la Recherche Médicale (INSERM)–Indian Council of Medical Research (ICMR) collaborative project. INSERM supported the research through grant #C10-01. ICMR supported the research at the Sree Chitra Tirunal Institute for Medical Science and Technolgy (SCTIMST), Kerala, India. SCTIMST supported the research through internal research fund project #5040. This work was also supported by the patient association Alliance France Dystonie and the Dystonia Coalition. The Dystonia Coalition is part of the National Institutes of Health (NIH) Rare Diseases Clinical research Network. Funding and/or programmatic support for this project has been provided by NS065701 from the NIH Office of Rare Diseases Research and the National Institute of Neurological Disorders and Stroke. The views expressed in written materials or publications do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the US Government. T.P. was the recipient of grants from Université Pierre et Marie Curie (UPMC) and Fondation Motrice. C.H. was the recipient of a scholarship from Fondation Groupama pour la Santé. S.M. and M.V. were the beneficiaries of a Contrat d'interface INSERM/APHP.
Supplementary Material
Acknowledgments
We thank Clement Lena and Chantal François for their insightful comments. Conflict of Interest : None declared.
References
- Alisky JM, Tolbert DL. Quantitative analysis of converging spinal and cuneate mossy fibre afferent projections to the rat cerebellar anterior lobe. Neuroscience. 1997;80:373–388. doi: 10.1016/s0306-4522(97)00082-1. [DOI] [PubMed] [Google Scholar]
- Allen GI, Gilbert PF, Marini R, Schultz W, Yin TC. Integration of cerebral and peripheral inputs by interpositus neurons in monkey. Exp Brain Res. 1977;27:81–99. doi: 10.1007/BF00234827. [DOI] [PubMed] [Google Scholar]
- Allen GI, Gilbert PF, Yin TC. Convergence of cerebral inputs onto dentate neurons in monkey. Exp Brain Res. 1978;32:151–170. doi: 10.1007/BF00239724. [DOI] [PubMed] [Google Scholar]
- Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss HU, Bressman SB, Dhawan V, Eidelberg D. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci. 2009;29:9740–9747. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma C, Thach WT, Jones EG. Distribution of cerebellar terminations and their relation to other afferent terminations in the ventral lateral thalamic region of the monkey. Brain Res. 1983;286:237–265. doi: 10.1016/0165-0173(83)90015-2. [DOI] [PubMed] [Google Scholar]
- Ben Taib NO, Manto M, Pandolfo M, Brotchi J. Hemicerebellectomy blocks the enhancement of cortical motor output associated with repetitive somatosensory stimulation in the rat. J Physiol (Lond) 2005;567:293–300. doi: 10.1113/jphysiol.2005.088229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushara KO, Wheat JM, Khan A, Mock BJ, Turski PA, Sorenson J, Brooks BR. Multiple tactile maps in the human cerebellum. Neuroreport. 2001;12:2483–2486. doi: 10.1097/00001756-200108080-00039. [DOI] [PubMed] [Google Scholar]
- Butler EG, Horne MK, Rawson JA. Sensory characteristics of monkey thalamic and motor cortex neurones. J Physiol (Lond) 1992;445:1–24. doi: 10.1113/jphysiol.1992.sp018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Ablation of cerebellar nuclei prevents H-reflex down-conditioning in rats. Learn Mem. 2005;12:248–254. doi: 10.1101/lm.91305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE, Mewes K. Neural mechanisms underlying corticospinal and rubrospinal control of limb movements. Prog Brain Res. 1991;87:213–252. doi: 10.1016/s0079-6123(08)63054-x. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Simpson JI, Hoogenraad CC, Galjart N, Koekkoek SK, Ruigrok TJ. Microcircuitry and function of the inferior olive. Trends Neurosci. 1998;21:391–400. doi: 10.1016/s0166-2236(98)01310-1. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Sabah NH, Schmidt RF, Taborikova H. Cutaneous mechanoreceptors influencing impulse discharges in cerebellar cortex. 3. In Purkyne cells by climbing fiber input. Exp Brain Res. 1972;15:484–497. doi: 10.1007/BF00236404. [DOI] [PubMed] [Google Scholar]
- Ekerot CF. Climbing fibres—a key to cerebellar function. J Physiol (Lond) 1999;516:629. doi: 10.1111/j.1469-7793.1999.0629u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro B, Giglia G, Palermo A, Pecoraro C, Scalia S, Brighina F. Modulatory effects of 1 Hz rTMS over the cerebellum on motor cortex excitability. Exp Brain Res. 2007;176:440–447. doi: 10.1007/s00221-006-0628-y. [DOI] [PubMed] [Google Scholar]
- Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science. 1996;272:545–547. doi: 10.1126/science.272.5261.545. [DOI] [PubMed] [Google Scholar]
- Gellman R, Gibson AR, Houk JC. Inferior olivary neurons in the awake cat: detection of contact and passive body displacement. J Neurophysiol. 1985;54:40–60. doi: 10.1152/jn.1985.54.1.40. [DOI] [PubMed] [Google Scholar]
- Gerschlager W, Christensen LOD, Bestmann S, Rothwell JC. rTMS over the cerebellum can increase corticospinal excitability through a spinal mechanism involving activation of peripheral nerve fibres. Clin Neurophysiol. 2002;113:1435–1440. doi: 10.1016/s1388-2457(02)00156-6. [DOI] [PubMed] [Google Scholar]
- Grodd W, Hulsmann E, Lotze M, Wildgruber D, Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp. 2001;13:55–73. doi: 10.1002/hbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Takahara D, Hirata Y, Inoue K, Miyachi S, Nambu A, Tanji J, Takada M, Hoshi E. Motor and non-motor projections from the cerebellum to rostrocaudally distinct sectors of the dorsal premotor cortex in macaques. Eur J Neurosci. 2010;31:1402–1413. doi: 10.1111/j.1460-9568.2010.07151.x. [DOI] [PubMed] [Google Scholar]
- Hassler R. Anatomy of the thalamus. In: Schaltenbrand G, Bailey P, editors. Introduction to stereotaxis with an atlas of the human brain. Stuttgart (Germany): Thieme; 1959. pp. 230–290. [Google Scholar]
- Hirai T, Jones EG. Segregation of lemniscal inputs and motor cortex outputs in cat ventral thalamic nuclei: application of a novel technique. Exp Brain Res. 1988;71:329–344. doi: 10.1007/BF00247493. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Ito M, Yoshida M, Obata K. Monosynaptic inhibition of the intracerebellar nuclei induced from the cerebellar cortex. Cerebellum. 2007;6:103–104. [PubMed] [Google Scholar]
- Iyer MB, Schleper N, Wassermann EM. Priming stimulation enhances the depressant effect of low-frequency repetitive transcranial magnetic stimulation. J. Neurosci. 2003;23:10867–10872. doi: 10.1523/JNEUROSCI.23-34-10867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Target cells of rubrospinal tract fibres within the lumbar spinal cord. Behav Brain Res. 1988;28:91–96. doi: 10.1016/0166-4328(88)90083-6. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Ottinger S, Fellows SJ, Adamschewski J, Flerich L, Müller SP, et al. The relevance of sensory input for the cerebellar control of movements. Neuroimage. 1997;5:41–48. doi: 10.1006/nimg.1996.0249. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Kawai S, Fuchigami Y, Morita H, Ofuji A. The effect of current direction induced by transcranial magnetic stimulation on the corticospinal excitability in human brain. Clin Neurophysiol. 1996;101:478–482. doi: 10.1016/s0013-4694(96)96021-x. [DOI] [PubMed] [Google Scholar]
- Kassavetis P, Hoffland BS, Saifee TA, Bhatia KP, van de Warrenburg BP, Rothwell JC, Edwards MJ. Cerebellar brain inhibition is decreased in active and surround muscles at the onset of voluntary movement. Exp Brain Res. 2011;209:437–442. doi: 10.1007/s00221-011-2575-5. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Mori F, Marconi B, Codecà C, Pecchioli C, Salerno S, Torriero S, Lo Gerfo E, Mir P, Olivieri M, et al. Changes in intracortical circuits of the human motor cortex following theta burst stimulation of the lateral cerebellum. Clin Neurophysiol. 2008;119:2559–2569. doi: 10.1016/j.clinph.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Lamy JC, Russmann H, Shamim EA, Meunier S, Hallett M. Paired associative stimulation induces change in presynaptic inhibition of Ia terminals in wrist flexors in humans. J Neurophysiol. 2010;104:755–764. doi: 10.1152/jn.00761.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ernst D, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol Psychiatry. 2004;56:634–639. doi: 10.1016/j.biopsych.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Llinas RR. Inferior olive oscillation as the temporal basis for motricity and oscillatory reset as the basis for motor error correction. Neuroscience. 2009;162:797–804. doi: 10.1016/j.neuroscience.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Russmann H, Simonetta-Moreau M, Hallett M. Changes in spinal excitability after PAS. J Neurophysiol. 2007;97:3131–3135. doi: 10.1152/jn.01086.2006. [DOI] [PubMed] [Google Scholar]
- Miall RC, Reckess GZ, Imamizu H. The cerebellum coordinates eye and hand tracking movements. Nat Neurosci. 2001;4:638–644. doi: 10.1038/88465. [DOI] [PubMed] [Google Scholar]
- Muller JF, Orekhov Y, Liu Y, Ziemann U. Homeostatic plasticity in human motor cortex demonstrated by two consecutive sessions of paired associative stimulation. Eur J Neurosci. 2007;25:3461–3468. doi: 10.1111/j.1460-9568.2007.05603.x. [DOI] [PubMed] [Google Scholar]
- Nathan PW, Smith MC. The rubrospinal and central tegmental tracts in man. Brain. 1982;105:223–269. doi: 10.1093/brain/105.2.223. [DOI] [PubMed] [Google Scholar]
- Nixon PD. The role of the cerebellum in preparing responses to predictable sensory events. Cerebellum. 2003;2:114–122. doi: 10.1080/14734220309410. [DOI] [PubMed] [Google Scholar]
- Ohye C, Shibazaki T, Hirai T, Wada H, Hirato M, Kawashima Y. Further physiological observations on the ventralis intermedius neurons in the human thalamus. J Neurophysiol. 1989;61:488–500. doi: 10.1152/jn.1989.61.3.488. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Koch G, Torriero S, Caltagirone C. Increased facilitation of the primary motor cortex following 1 Hz repetitive transcranial magnetic stimulation of the contralateral cerebellum in normal humans. Neurosci Lett. 2005;376:188–193. doi: 10.1016/j.neulet.2004.11.053. [DOI] [PubMed] [Google Scholar]
- Olsson CJ, Jonsson B, Nyberg L. Learning by doing and learning by thinking: an FMRI study of combining motor and mental training. Front Hum Neurosci. 2008;2:5. doi: 10.3389/neuro.09.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, Wardak C, Durand JB, Vanduffel W. Mapping the parietal cortex of human and non-human primates. Neuropsychologia. 2006;44:2647–2667. doi: 10.1016/j.neuropsychologia.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Popa T, Russo M, Meunier S. Long-lasting inhibition of cerebellar output. Brain Stimul. 2010;3:161–169. doi: 10.1016/j.brs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Pötter-Nerger M, Fischer S, Mastroeni C, Groppa S, Deuschl G, Volkmann J, Quartarone A, Münchau A, Siebner HR. Inducing homeostatic-like plasticity in human motor cortex through converging corticocortical inputs. J Neurophysiol. 2009;102:3180–3190. doi: 10.1152/jn.91046.2008. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Morgante F, Sant'Angelo A, Rizzo V, Bagnato S, Terranova C, Siebner HR, Berardelli A, Girlanda P. Abnormal plasticity of sensorimotor circuits extends beyond the affected body part in focal dystonia. J Neurol Neurosurg Psychiatry. 2008;79:985–990. doi: 10.1136/jnnp.2007.121632. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Rizzo V, Bagnato S, Morgante F, Sant'Angelo A, Girlanda P, Siebner HR. Rapid-rate paired associative stimulation of the median nerve and motor cortex can produce long-lasting changes in motor cortical excitability in humans. J Physiol. 2006;575:657–670. doi: 10.1113/jphysiol.2006.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston DD. Cerebellar terminations in the red nucleus of Macaca fascicularis: an electron-microscopic study utilizing the anterograde transport of WGA: hRP. Somatosens Res. 1994;11:101–107. doi: 10.3109/08990229409028863. [DOI] [PubMed] [Google Scholar]
- Roland PE, Eriksson L, Widén L, Stone-Elander S. Changes in regional cerebral oxidative metabolism induced by tactile learning and recognition in man. Eur J Neurosci. 1989;1:3–18. doi: 10.1111/j.1460-9568.1989.tb00769.x. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A The Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods. 1997;74:113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Engberg I, Jimenez I. Mechanisms involved in presynaptic depolarization of group I and rubrospinal fibers in cat spinal cord. J Neurophysiol. 1981;46:532–548. doi: 10.1152/jn.1981.46.3.532. [DOI] [PubMed] [Google Scholar]
- Sakai ST, Inase M, Tanji J. Comparison of cerebellothalamic and pallidothalamic projections in the monkey (Macaca fuscata): a double anterograde labeling study. J Comp Neurol. 1996;368:215–228. doi: 10.1002/(SICI)1096-9861(19960429)368:2<215::AID-CNE4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, Nordstrom MA. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp Brain Res. 2007;181:615–626. doi: 10.1007/s00221-007-0960-x. [DOI] [PubMed] [Google Scholar]
- Schlerf JE, Verstynen TD, Ivry RB, Spencer RM. Evidence of a novel somatopic map in the human neocerebellum during complex actions. J Neurophysiol. 2010;103:3330–3336. doi: 10.1152/jn.01117.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Noll DC. Neuroanatomical correlates of motor acquisition and motor transfer. J Neurophysiol. 2008;99:1836–1845. doi: 10.1152/jn.01187.2007. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24:3379–3385. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Theoret H, Haque J, Pascual-Leone A. Increased variability of paced finger tapping accuracy following repetitive magnetic stimulation of the cerebellum in humans. Neurosci Lett. 2001;306:29–32. doi: 10.1016/s0304-3940(01)01860-2. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995;37:703–713. doi: 10.1002/ana.410370603. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Ashe J, DeLong MR, Alexander GE. Physiologic properties and somatotopic organization of the primate motor thalamus. J Neurophysiol. 1994;71:1498–1513. doi: 10.1152/jn.1994.71.4.1498. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Weise D, Schramm A, Beck M, Reiners K, Classen J. Loss of topographic specificity of LTD-like plasticity is a trait marker in focal dystonia. Neurobiol Dis. 2011;42:171–176. doi: 10.1016/j.nbd.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Weise D, Schramm A, Stefan K, Wolters A, Reiners K, Naumann M, Classen J. The two sides of associative plasticity in writer's cramp. Brain. 2006;129:2709–2721. doi: 10.1093/brain/awl221. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JK, Meyer BU, Priori A, Rothwell JC, Day BL, Thompson PD. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Clin Neurophysiol. 1994;93:138–146. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Chen XY. The cerebellum in maintenance of a motor skill: a hierarchy of brain and spinal cord plasticity underlies H-reflex conditioning. Learn Mem. 2006;13:208–215. doi: 10.1101/lm.92706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Fairhall SL, McNair NA, Hamm JP, Kirk IJ, Cunnington R, Anderson T, Lim VK. Impaired sensorimotor integration in focal hand dystonia patients in the absence of symptoms. J Neurol Neurosurg Psychiatry. 2009;81:659–665. doi: 10.1136/jnnp.2009.185637. [DOI] [PubMed] [Google Scholar]
- Wu X, Nestrasil I, Ashe J, Tuite P, Bushara K. Inferior olive response to passive tactile and visual stimulation with variable interstimulus intervals. Cerebellum. 2010;9:598–602. doi: 10.1007/s12311-010-0203-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.