Abstract
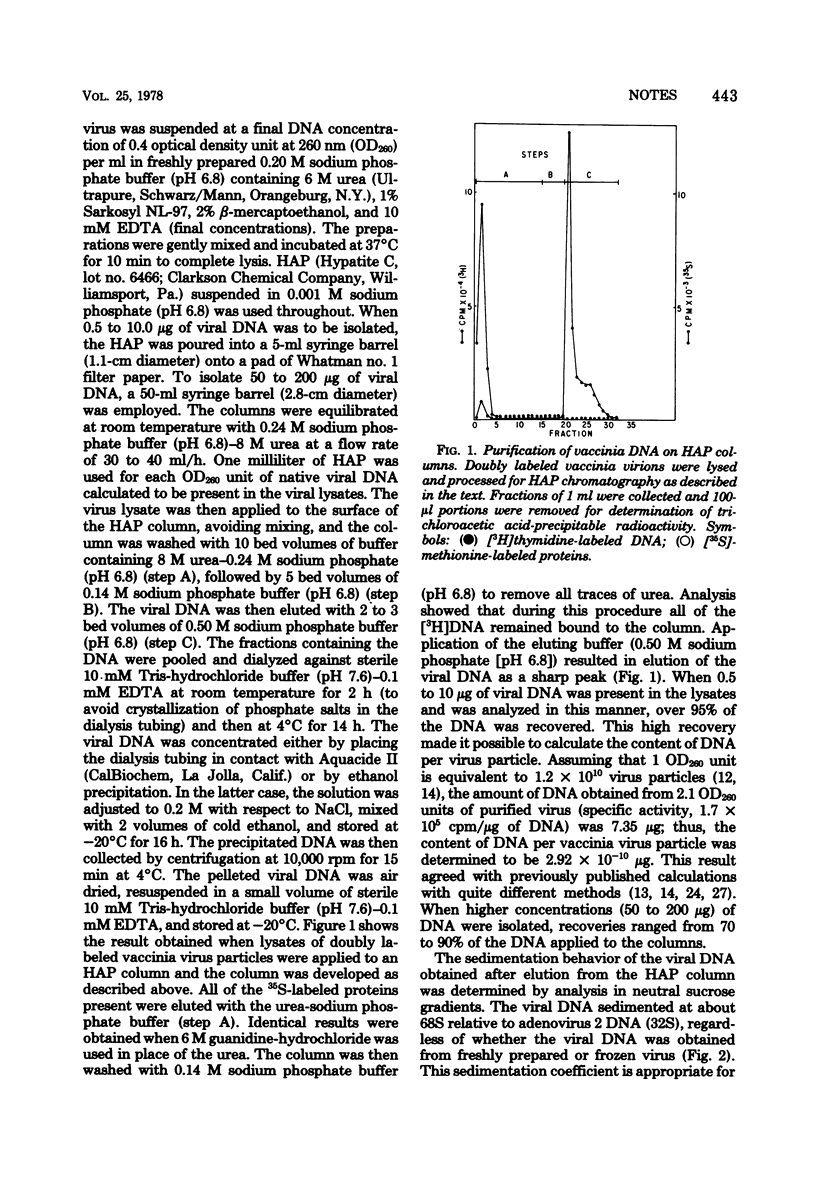
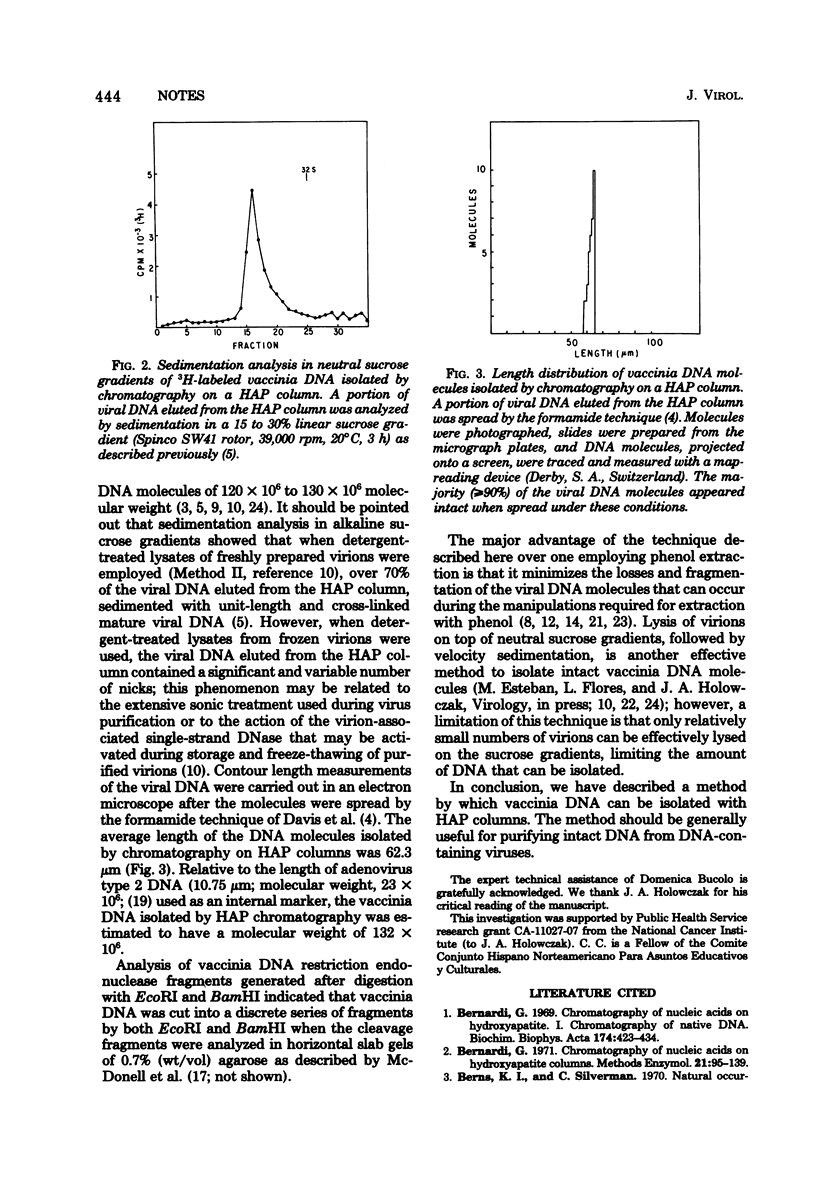
A procedure for the isolation of intact vaccinia DNA molecules by chromatography on hydroxyapatite in the presence of 6 M urea is described. When lysates of virions containing 0.5 to 10 microgram of DNA were employed, over 95% of the viral DNA could be recovered free of poteins. Vaccinia DNA molecules isolated in this manner sedimented at 68S in neutral sucrose gradients and had an average contour length of 62.3 micrometer when examined in an electron microscope, and the DNA could be cleaved with the restriction endonuclease EcoRI and BamHI. The results of these analyses showed that intact vaccinia DNA molecules of 120 X 10(6) to 130 X 10(6) molecular weight could be obtained by the procedures described.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardi G. Chromatography of nucleic acids on hydroxyapatite. I. Chromatography of native DNA. Biochim Biophys Acta. 1969 Feb 18;174(2):423–434. doi: 10.1016/0005-2787(69)90273-1. [DOI] [PubMed] [Google Scholar]
- Esteban M., Holowczak J. A. Replication of vaccinia DNA in mouse L cells. I. In vivo DNA synthesis. Virology. 1977 May 1;78(1):57–75. doi: 10.1016/0042-6822(77)90078-2. [DOI] [PubMed] [Google Scholar]
- Esteban M., Metz D. H. Early virus protein synthesis in vaccinia virus-infected cells. J Gen Virol. 1973 May;19(2):201–206. doi: 10.1099/0022-1317-19-2-201. [DOI] [PubMed] [Google Scholar]
- Gafford L. G., Randall C. C. The high molecular weight of the fowlpox virus genome. J Mol Biol. 1967 Jun 14;26(2):303–310. doi: 10.1016/0022-2836(67)90299-9. [DOI] [PubMed] [Google Scholar]
- Geshelin P., Berns K. I. Characterization and localization of the naturally occurring cross-links in vaccinia virus DNA. J Mol Biol. 1974 Oct 5;88(4):785–796. doi: 10.1016/0022-2836(74)90399-4. [DOI] [PubMed] [Google Scholar]
- Holowczak J. A. Poxvirus DNA. I. Studies on the structure of the vaccinia genome. Virology. 1976 Jul 1;72(1):121–133. doi: 10.1016/0042-6822(76)90317-2. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., BECKER Y. THE REPLICATION AND COATING OF VACCINIA DNA. J Mol Biol. 1964 Dec;10:452–474. doi: 10.1016/s0022-2836(64)80066-8. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Anderson C. W., Baum P. R., Gesteland R. F. Mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1344–1348. doi: 10.1073/pnas.72.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Meinke W., Goldstein D. A., Hall M. R. Rapid isolation of mouse DNA from cells in tissue culture. Anal Biochem. 1974 Mar;58(1):82–88. doi: 10.1016/0003-2697(74)90444-8. [DOI] [PubMed] [Google Scholar]
- Murray R. E., Green M. Adenovirus DNA. IV. Topology of adenovirus genomes. J Mol Biol. 1973 Mar 15;74(4):735–738. doi: 10.1016/0022-2836(73)90061-2. [DOI] [PubMed] [Google Scholar]
- Nathans D., Smith H. O. Restriction endonucleases in the analysis and restructuring of dna molecules. Annu Rev Biochem. 1975;44:273–293. doi: 10.1146/annurev.bi.44.070175.001421. [DOI] [PubMed] [Google Scholar]
- Oda K. I., Joklik W. K. Hybridization and sedimentation studies on "early" and "late" vaccinia messenger RNA. J Mol Biol. 1967 Aug 14;27(3):395–419. doi: 10.1016/0022-2836(67)90047-2. [DOI] [PubMed] [Google Scholar]
- PFAU C. J., MCCREA J. F. STUDIES ON THE DEOXYRIBONUCLEIC ACID OF VACCINIA VIRUS. III. CHARACTERIZATION OF DNA ISOLATED BY DIFFERENT METHODS AND ITS RELATION TO VIRUS STRUCTURE. Virology. 1963 Nov;21:425–435. doi: 10.1016/0042-6822(63)90204-6. [DOI] [PubMed] [Google Scholar]
- Parkhurst J. R., Heidelberger C. Rapid lysis of vaccinia virus on neutral sucrose gradients with release of intact DNA. Acta Pathol Microbiol Scand Suppl. 1976 Mar;71(1):53–59. doi: 10.1016/0003-2697(76)90010-5. [DOI] [PubMed] [Google Scholar]
- Sarov I., Becker Y. Studies on vaccinia virus DNA. Virology. 1967 Nov;33(3):369–375. doi: 10.1016/0042-6822(67)90112-2. [DOI] [PubMed] [Google Scholar]
- Smith M. J., Hough B. R., Chamberlin M. E., Davidson E. H. Repetitive and non-repetitive sequence in sea urchin heterogeneous nuclear RNA. J Mol Biol. 1974 May 5;85(1):103–126. doi: 10.1016/0022-2836(74)90132-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- ZWARTOUW H. T. THE CHEMICAL COMPOSITION OF VACCINIA VIRUS. J Gen Microbiol. 1964 Jan;34:115–123. doi: 10.1099/00221287-34-1-115. [DOI] [PubMed] [Google Scholar]


