Summary
Background
The mechanisms of salt sensitivity as an important intermediate phenotype of essential hypertension remain elusive. A novel theory proposes that lymphatic vessels regulate sodium and fluid homeostasis. Since vascular endothelial growth factor C (VEGF-C) plays a vital role in lymphatic capillary hyperplasia, we hypothesized that VEGF-C was involved in salt-sensitive hypertension. We therefore investigated its plasma concentration in salt-sensitive subjects.
Material/Methods
Twenty-seven subjects (BP ≤160/100 mmHg; age range 25–50 years) from a rural community of northern China were enrolled in this study. The baseline BP of volunteers was monitored for 3 days, followed by a low-salt diet for 7 days (3 g/day, NaCl) and a high-salt diet for 7 days (18 g/day, NaCl). Those who exhibited a BP increase of 10% from low-salt period to high-salt period were diagnosed as salt-sensitive subjects. The concentration of plasma VEGF-C was measured by an immunoenzyme method (ELISA).
Result
High salt intake significantly increased the plasma VEGF-C level. It was higher in the salt-sensitive subjects (3642.2±406.1 pg/ml) than in the salt-resistant subjects (2249.8±214.6 pg/ml). The comparison of VEGF-C levels between the 2 groups had significant statistical difference (P<0.01).
Conclusions
The VEGF-C level increases significantly in the salt-sensitive subjects after high salt intake. VEGF-C could be used as a biomarker of salt sensitivity.
Keywords: salt-sensitive, lymphatic system, vascular endothelial growth factor C, diet intervention
Background
Several epidemiological and interventional studies have demonstrated a clear relationship between salt intake and hypertension [1]. A heterogeneous blood pressure (BP) response to changes in dietary sodium chloride (NaCl) intake, a phenomenon generally referred to as salt sensitivity, is observed in both hypertensive patients and normotensive individuals [2,3]. Several prospective studies have found that salt sensitivity is associated with an increased incidence of death caused by cardiovascular complications [4,5]. Although salt sensitivity is well-established in experimental and human hypertension, the pathophysiological mechanisms leading to such individual susceptibility remain unclear.
Vascular endothelial growth factor C (VEGF-C), which stimulates the lymphatic formation and the expression of endothelial nitric oxide synthase, plays a vital role in hyperplasia of the lymphatic capillary network [6]. Inhibition of the VEGF-C expression in skin can aggravate the BP elevation during high salt diet [7]. However, the change of VEGF-C in salt-induced hypertension in humans has not been reported.
In the present study, we hypothesized that VEGF-C was involved in salt-sensitive hypertension. In order to verify this hypothesis, 27 subjects sequentially took a low-salt diet and a high-salt diet and the plasma VEGF-C concentration was measured to assess the relationship between VEGF-C level and salt sensitivity in humans.
Material and Methods
Subjects
Twenty-seven normotensive or untreated mild hypertensive subjects were enrolled in this study. Subjects came from a rural community of northern China and had a similar diet. Hypertension was defined as a mean systolic BP (SBP) equal to or above 140 mmHg and/or mean diastolic BP (DBP) equal to or above 90 mmHg. Subjects whose BP was equal to or above 160/100 mmHg were excluded, as well as those who had diabetes mellitus or had a history of liver or renal disease. All the subjects who finally participated in this study had no symptoms of coronary or peripheral arterial diseases and were nonsmokers. The ethics committee of the medical school of Xi’an Jiaotong University approved the study protocol. Each subject signed the informed consent. All of the procedures were performed in accordance with the institutional guidelines.
Protocol
The protocol consisted of a series of investigations, including a baseline history and the physical examinations (height, weight, and BP) that were taken daily. During the 3 days of baseline investigation, each subject was given detailed dietary instructions to avoid table salt, cooking salt, high-sodium foods, and food rich in nitrite/nitrate in the subsequent 14 days. Then they had a low-salt diet (51.3 mmol or 3 g of NaCl per day) for 7 days and a high-salt diet (307.7 mmol or 18 g of NaCl per day) for 7 days. All of the meals were prepared in the research kitchens and consumed by the subjects in the same place.
BP measurement
Three random-zero BP measurements with a 1-minute interval were obtained using a Hawksley random-zero sphygmomanometer (Hawksley & Sons Ltd., Lancing, UK; zero range 0–20 mmHg) in the morning during the 3-day baseline observation, as well as on the second, fifth, sixth and seventh days of the low-salt and high-salt diet periods. It was measured by trained and certified observers according to the procedures recommended by the American Heart Association. The subjects had a minimal 5-minute rest in the sitting position before BP was measured. In addition, participants were advised to avoid drinking alcohol, coffee or tea, smoking, and doing exercise 30 minutes prior to their BP measurement. SBP and DBP were respectively determined by the first and fifth phases of the Korotkoff sounds. Pulse pressure (PP) was calculated as the following formula: PP=SBP–DBP. Mean blood pressure was obtained as the following formula: MBP=DBP+1/3×PP. Because of the lack of universal consensus in the definition of salt sensitivity of BP, subjects with an increase of MBP equal to or above 10% from the low-salt diet to the high-salt diet were considered as salt-sensitive. Those below 10% were defined as salt-resistant [8,9].
Biochemical analyses
Blood samples for fasting serum were obtained from the peripheral veins and centrifuged immediately at 3000 g for 5 minutes. The supernatants were stored at −80°C until the assay was performed. Blood glucose and serum lipids [total cholesterol (TC), TG, HDL-C] of each sample were measured. The concentration of plasma VEGF-C was determined using a validated sandwich ELISA, in which a VEGF-C-specific antibody (Uscnlife Science Inc., China) was used to bind the serum VEGF-C. The intra- and inter-assay variation coefficients of VEGF-C concentration were obtained from 5 plasmatic samples and they ranged from 4.4% to 7.6% (mean 5.8%) and from 5.4% to 7.2% (mean 6.8%), respectively.
Measurement of sodium and potassium values in 24-hour urine
The sodium and potassium concentrations in the urine were measured by a flame photometer. The total values of sodium and potassium excreted by urine in 24 hours were calculated by multiplying their concentrations and the total volume of urine in 24 hours.
Statistical analyses
The data were shown as mean ±SD. The parameters of the salt-sensitive and salt-resistant hypertensive subjects were compared by 1-way ANOVA. Differences between biochemical markers obtained at low and high salt intakes in salt-sensitive and salt-resistant patients were calculated by analysis of variance with repeated measures design. Age, sex and body-mass index (BMI) were adjusted by multivariable analysis. The relationship between the VEGF-C concentration and blood pressure was determined by Pearson’s correlation coefficient or Spearman’s correlation. If the residuals were normally distributed, Pearson’s correlation coefficient was chosen; otherwise Spearman’s correlation was used. All calculations were performed with SPSS (version 16). P value less than 0.05 was statistically significant.
Results
Salt-sensitive and salt-resistant subjects
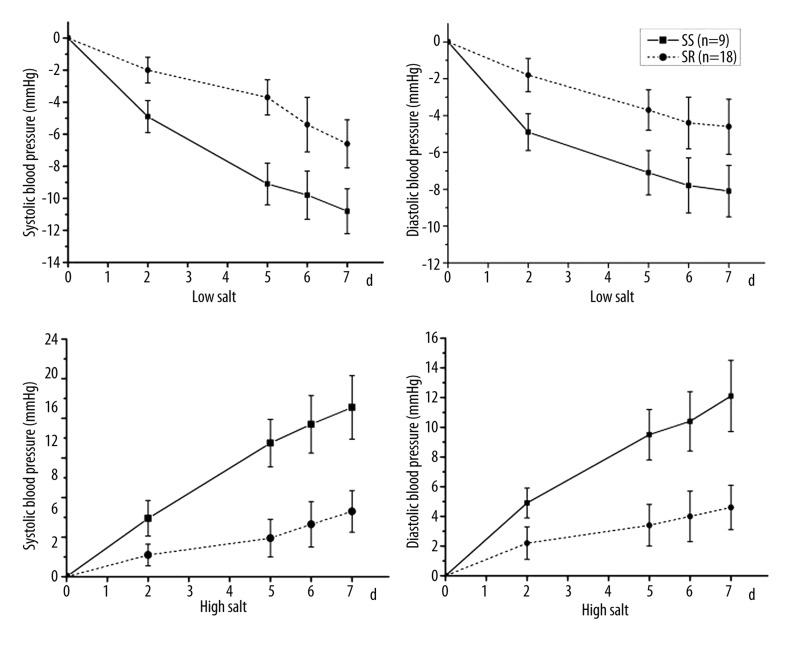
All of the 27 subjects completed the intervention trial. The mean BP of 9 subjects increased by 10% when the diet was changed from low-salt to high-salt, therefore they were considered as salt-sensitive subjects. The remaining 18 subjects were regarded as salt-resistant. No significant differences in age, BMI, SBP and DBP were observed between the salt-sensitive and salt-resistant subjects (Table 1). The BP changes of the 27 subjects during the low-salt and high-salt diet periods are shown in Figure 1.
Table 1.
Characteristics of salt-sensitive and salt-resistant subjects.
| Parameters | SS(n=9) | SR(n=18) | P |
|---|---|---|---|
| Age, y | 43.3±4.8 | 45.1±7.2 | 0.63 |
| Sex, male/female | 6/3 | 11/7 | – |
| Waist circumference, cm | 79.7±8.7 | 83.9±7.9 | 0.08 |
| BMI,kg/m2 | 23.7±1.8 | 25.3±2.5 | 0.75 |
| Systolic blood pressure, mmHg | 129.4±15.8 | 120.1±11.5 | 0.09 |
| Diastolic blood pressure, mmHg | 83.7±10.6 | 80.8±8.2 | 0.44 |
| Mean blood pressure, mmHg | 98.9±12.0 | 93.9±8.9 | 0.23 |
| Glucose, mg/dl | 100.6±23.9 | 99.8±17.8 | 0.91 |
| CHOL, mg/dl | 159.6±42.0 | 144.6±26.6 | 0.24 |
| Triglycerides, mg/dl | 178.6±99.3 | 144.5±86.1 | 0.33 |
| HDL cholesterol, mg/dl | 46.5±12.3 | 44.9±11.6 | 0.72 |
| 24 h Urinary sodium, mmol | 235.1±121.0 | 267.2±158.3 | 0.73 |
| 24 h Urinary potassium, mmol | 36.2±15.2 | 39±13.1 | 0.72 |
Figure 1.
The BP change on the second, fifth, sixth and seventh days of the low-salt and high-salt diet periods.
Influence of different salt intake to urinary sodium excretions
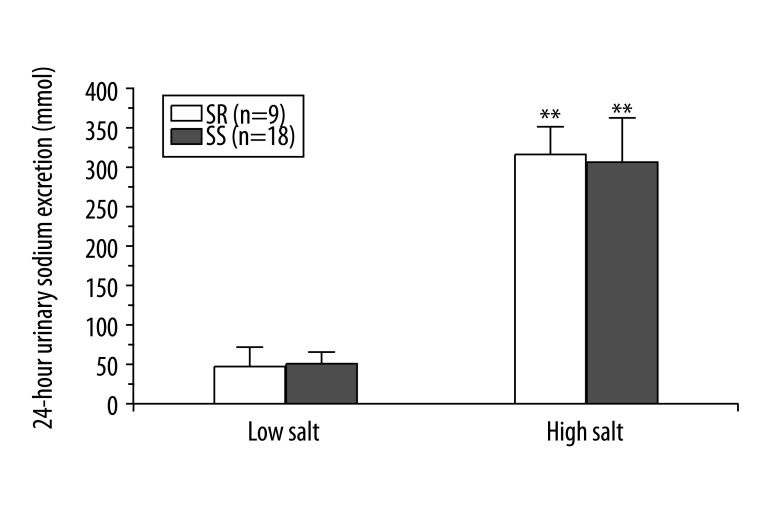
In order to observe the influence of salt intake on urinary sodium excretions, the total volume of sodium in the urine was calculated at the end of each diet period. Sodium level was remarkably higher during the high-salt diet than during the low-salt diet; however, there was no significant difference between the salt-sensitive subjects and the salt-resistant subjects (Figure 2).
Figure 2.
Influence of salt loading on BP and sodium excretion by urine. ** P<0.01 vs. low salt diet
Influence of high salt intake on the plasma VEGF-C levels
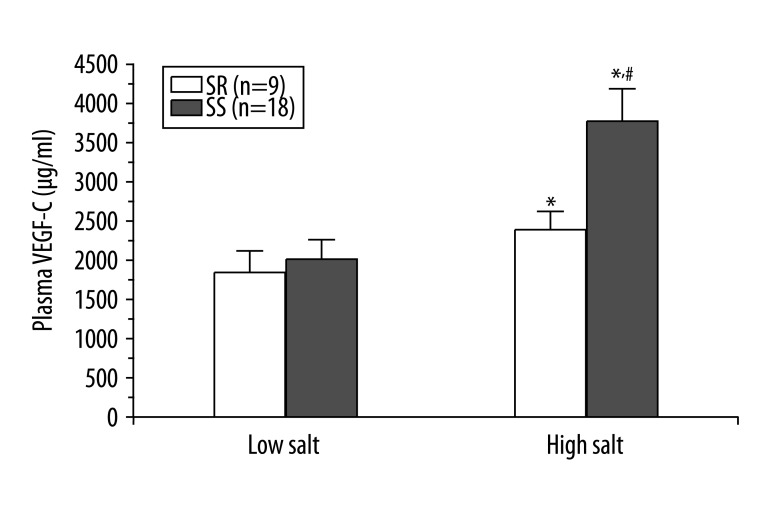
As shown in Figure 3, the statistical difference was not significant in the plasma VEGF-C levels between the salt-sensitive subjects and the salt-resistant subjects during low salt intake (1921.1±238.2 μg/ml vs. 1804.2±206.4 μg/ml, P=0.59). However, the high salt intake clearly enhanced the plasma VEGF-C levels in both the salt-sensitive subjects (3642.2±406.1 μg/ml) and the salt-resistant subjects (2249.8±214.6 μg/ml). The increase was significantly higher in the salt-sensitive subjects than in the salt-resistant subjects (P=0.0038). Compared with the low salt intake, the salt-sensitive subjects had significantly increased plasma VEGF-C level after high salt intake (P<0.001). In contrast, the salt-resistant subjects did not show this trend (P=0.041). Moreover, there was no correlation between the plasma VEGF-C and BP (r=0.412, P=0.29).
Figure 3.
The plasma VEGF-C concentration in the salt sensitive and salt resistant subjects during low-salt and high-salt diet. * P<0.05 vs. low salt diet, #P<0.05 vs. SR group.
Discussion
We report for the first time that high salt intake can increase the plasma VEGF-C level in humans. Moreover, the VEGF-C level rose more in the salt-sensitive than in the salt-resistant subjects.
The subjects enrolled in this study had 3 special characteristics. First, they came from a rural community and had similar lifestyle including diet and physical activity, which reduced the data variation caused by individual life-style differences. Second, the participants with BMI greater than 28 kg/m2 were excluded, which eliminated the variation caused by BMI. Thirdly, all the subjects completed their various diets and cooperated very well with the urine collection.
Traditionally, the regulation of fluid homeostasis is based on the “two-compartment” model: interstitial Na+ is readily equilibrated with plasma Na+ and can be absorbed into the blood, and the kidney indirectly adjusts the concentration and total volume of interstitial Na+ by blood purification. Therefore, salt-sensitive hypertension is commonly believed to be a “renal affair”. Recently, Titze et al proposed a new theory of fluid homeostasis regulation: sodium (Na+) can be stored in the interstitial sites by binding to the proteoglycans there and becomes osmotically inactive [10–13]. Moreover, the subcutaneous lymphatic system under the skin can create a third fluid compartment to modulate the BP rise in the state of local hypertonicity, acting as a fluid buffering system to regulate BP [13–15]. The novel theory provides new insights in exploring the potential mechanisms of salt-induced hypertension.
Some studies have shown that VEGF-C induces selective hyperplasia of the lymphatic vasculature, which is involved in the draining of interstitial fluids and in immune function, inflammation, and tumor metastasis [2,16–18]. Machnik et al demonstrated that the cells in the mononuclear phagocyte system acted as extrarenal regulators of interstitial Na+ and water homeostasis and could activate the tonicity-responsive enhancer binding protein, which bound to the VEGF-C gene. By binding to the VEGF receptor 2, VEGF-C stimulates the hyperplasia of the lymphatic capillary network and increases the expression of endothelial nitric oxide synthase. When the macrophage cells of rats are depleted or the VEGF-C signaling pathway is blocked, the blood pressure will dramatically increase during high salt intake [7,19,20]. These new findings might also explain why antiangiogenesis drugs, which can block the VEGF signaling pathway and are commonly used for cancer treatment, often cause hypertension [21,22]. Interestingly, the salt-sensitive subjects with refractory hypertension also exhibit significantly higher levels of plasma VEGF-C in the circulating system compared with normal subjects. Our results also show that the increased salt intake results in significant enhancement of the plasma VEGF-C level in the salt-sensitive subjects. According to all the results, it is also possible that those people predisposed to salt-sensitive hypertension may have a dysfunctional Na+ buffering system in the skin interstitium, and VEGF-C can be considered as a biomarker of salt sensitivity.
Our study has several limitations. Firstly, VEGF-C is only a surrogate parameter of lymphatic capillary hyperplasia or density. The actual lymphatic capillary hyperplasia and tissue sodium content were not studied. Secondly, sex is an important factor influencing BP. Subjects were not divided by sex due to the small size of our study. Thirdly, the mechanisms that result in the increase of VEGF-C level in salt sensitivity status were not studied. Finally, since all the subjects enrolled in this study were Chinese, it is not clear whether our results also occur in other races. In order to eliminate these limitations, further studies are required.
Conclusions
Subjects were divided into salt-sensitive and salt-resistant groups according to their BP change from low-salt diet to high-salt diet. The plasma VEGF-C level increased significantly in the salt-sensitive subjects after high salt intake. Therefore, plasma VEGF-C level could be used as a biomarker of salt sensitivity and to diagnose potential hypertension. The inhibition of VEGF-C may be useful in hypertension therapy in the future.
Footnotes
Source of support: Natural Science Foundation of China ( NO: 81070218 and 30671160)
References
- 1.Muntzel M, Drüeke T. A comprehensive review of the salt and blood pressure relationship. Am J Hypertens. 1992;5:1S–42S. doi: 10.1093/ajh/5.4s.1s. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–90. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 3.Adrogue HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med. 2007;356(19):1966–78. doi: 10.1056/NEJMra064486. [DOI] [PubMed] [Google Scholar]
- 4.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–32. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 5.Morimoto A, Uzu T, Fujii T, et al. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–37. doi: 10.1016/S0140-6736(97)05189-1. [DOI] [PubMed] [Google Scholar]
- 6.Jeltsch M, Kaipainen A, Joukov V, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276(5317):1423–25. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 7.Machnik A, Dahlmann A, Kopp C, et al. Mononuclear phagocyte system depletion blocks interstitial tonicity-responsive enhancer binding protein/vascular endothelial growth factor C expression and induces salt-sensitive hypertension in rats. Hypertension. 2010;55(3):755–61. doi: 10.1161/HYPERTENSIONAHA.109.143339. [DOI] [PubMed] [Google Scholar]
- 8.Fang Y, Mu JJ, He LC, et al. Salt loading on plasma asymmetrical dimethylarginine and the protective role of potassium supplement in normotensive salt-sensitive asians. Hypertension. 2006;48:724–29. doi: 10.1161/01.HYP.0000238159.19614.ce. [DOI] [PubMed] [Google Scholar]
- 9.Wright JT, Rahman M, Scarpa A, et al. Determinants of salt sensitivity in black and white normotensive and hypertensive women. Hypertension. 2003;42:1087–92. doi: 10.1161/01.HYP.0000101687.89160.19. [DOI] [PubMed] [Google Scholar]
- 10.Titze J. Water-free sodium accumulation. Semin Dial. 2009;22(3):253–55. doi: 10.1111/j.1525-139X.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 11.Titze J, Machnik A. Sodium sensing in the interstitium and relationship to hypertension. Curr Opin Nephrol Hypertens. 2010;19(4):385–92. doi: 10.1097/MNH.0b013e32833aeb3b. [DOI] [PubMed] [Google Scholar]
- 12.Titze J, Shakibaei M, Schafflhuber M, et al. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Am J Physiol Heart Circ Physiol. 2004;287(1):H203–8. doi: 10.1152/ajpheart.01237.2003. [DOI] [PubMed] [Google Scholar]
- 13.Titze J, Lang R, Ilies C, et al. Osmotically inactive skin Na+ storage in rats. Am J Physiol Renal Physiol. 2003;285(6):F1108–17. doi: 10.1152/ajprenal.00200.2003. [DOI] [PubMed] [Google Scholar]
- 14.Marvar PJ, Gordon FJ, Harrison DG. Blood pressure control: salt gets under your skin. Nat Med. 2009;15(5):487–88. doi: 10.1038/nm0509-487. [DOI] [PubMed] [Google Scholar]
- 15.Rabelink TJ, Rotmans JI. Salt is getting under our skin. Nephrology Dialysis Transplantation. 2009;24(11):3282–83. doi: 10.1093/ndt/gfp399. [DOI] [PubMed] [Google Scholar]
- 16.Mandriota SJ, Jussila L, Jeltsch M, et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20(4):672–82. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karpanen T, Egeblad M, Karkkainen MJ, et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61(5):1786–90. [PubMed] [Google Scholar]
- 18.Yoo SY, Lee SY, Yoo NC. Cytokine expression and cancer detection. Med Sci Monit. 2009;15(3):RA49–56. [PubMed] [Google Scholar]
- 19.Machnik A, Neuhofer W, Jantsch J, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C – dependent buffering mechanism. Nature Medicine. 2009;15(5):545–52. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 20.Titze J, Luft FC, Bauer K, et al. Extrarenal Na+ balance, volume, and blood pressure homeostasis in intact and ovariectomized deoxycorticosterone-acetate salt rats. Hypertension. 2006;47(6):1101–17. doi: 10.1161/01.HYP.0000221039.17735.1a. [DOI] [PubMed] [Google Scholar]
- 21.Wu S, Chen JJ, Kudelka A, et al. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. Lancet Oncol. 2008;9(2):117–23. doi: 10.1016/S1470-2045(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 22.Yeh E, Bickford CL. Cardiovascular Complications of Cancer Therapy: Incidence, Pathogenesis, Diagnosis, and Management. J Am Coll Cardiol. 2009;53(24):2231–47. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]