Summary
Background
This article examines the effectiveness of differentiated rehabilitation programs for a patient with frontal syndrome after severe TBI and long-term coma. We hypothesized that there would be a small response to relative beta training, and a good response to rTMS, applied to regulate the dynamics of brain function.
Case Report
M. L-S, age 26, suffered from anosognosia, executive dysfunction, and behavioral changes, after a skiing accident and prolonged coma, rendering him unable to function independently in many situations of everyday life. Only slight progress was made after traditional rehabilitation. The patient took part in 20 sessions of relative beta training (program A) and later in 20 sessions of rTMS (program B); both programs were combined with behavioral training. We used standardized neuropsychological testing, as well as ERPs before the experiment, after the completion of program A, and again after the completion of program B. As hypothesized, patient M.L-S showed small improvements in executive dysfunction and behavioral disorders after the conclusion of program A, and major improvement after program B. Similarly, in physiological changes the patient showed small improvement after relative beta training and a significant improvement of the P300 NOGO component after the rTMS program.
Conclusions
The rTMS program produced larger physiological and behavioral changes than did relative beta training. A combination of different neurotherapeutical approaches (such as neurofeedback, rTMS, tDCS) can be suggested for similar severe cases of TBI. ERPs can be used to assess functional brain changes induced by neurotherapeutical programs.
Keywords: TBI, executive dysfunctions, behavioral changes, neurotherapy, ERP’s
Background
Every year, in Europe, 1 million people suffer a traumatic brain injury (TBI). 80% of these are mild, but 10 to 15% of these patients are left, 3 months after the accident, with somatic, cognitive and behavioral disorders, often thought of as psychogenic, and therefore disregarded [1–3].
In a previous longitudinal cohort study it was found that patients encountered problems in the physical (40%), cognitive (62%), behavioral (55%), and social domains (49%) of the Differentiated Outcome Scale (DOS), with higher frequency related to severity of injury. Even those with mild TBI experienced cognitive (43%) and behavioral problems (33%) [4]. Due to the multidimensional nature of symptom complaints within the brain injury population, emotional and behavioral problems are usually neglected [5,6]. The current study used the Personality Assessment Inventory (PAI) to detect emotional and behavioral profiles in a sample of 440 adult TBI patients. Using a rigorous three-step cluster analysis approach, seven clusters were identified, indicating that half of the sample (50%) showed clinically significant affective and behavioral symptoms, typified by multiple features listed in the Diagnostic and Statistical Manual of Mental Disorders (DSM), Axis I and/or II. Two of the subtypes showed severe and diverse affective symptoms, but were distinguished from each other by antisocial features and substance use. Two other subtypes, with predominantly internalized presentations, were characterized by mainly depressive and somatic features, and the second by cognitive disturbance and mild anxiety. One group of the sample (50%) had no significant affective or behavioral complaints but were characterized by two profile types classified as essentially normal, but distinguishable by one having an increased tendency to minimize symptoms. The other, predominantly externalized presentation, showed high substance use and antisocial features in behavior [5]. The identified profiles taken in the context of important demographic information can provide descriptive insight into the nature of postinjury affective and behavioral symptoms, facilitating more comprehensive conceptualization of the client’s needs that can be addressed through more tailored interventions.
It should be emphasized at this point, however, that it is nearly impossible to use such self-reporting methods to evaluate personality dysfunction or anti-social behavior in the case of patients with disturbances of awareness (such as anosognosia) or “frontal syndrome.” As a general rule these patients are not aware of the problems resulting from brain damage.
This is perhaps the one of the most important reasons why little attention has been given to the neurotherapy of behavioral changes in recent neuropsychological literature. It is difficult to justify this relative neglect, however, since behavioral changes subsequent to traumatic brain injury (TBI) cause serious therapeutic difficulties [7–13].
Hence the problems encountered by our patient, M.L-S, who had a severe TBI, and long-term coma, are described in the present study.
Case Report
M. L-S, age 26, suffered a brain injury in January of 2005 (while skiing he collided with a tree). He was initially hospitalized, comatose, in a clinic in Bolzano, Italy; two months later he was transported to Poland, where he awakened from coma. He had post-traumatic amnesia for a period of one year.
The brain MRI made two years after the accident (in 2007) showed gliotic posttraumatic changes in the right hemisphere with dilatation of the right lateral ventricle (Figure 1). A follow-up MRI made one year later showed that gliotic posttraumatic changes in the right hemisphere were more prominent than in 2007 with atrophy of brain parenchyma (external porencephaly) (Figure 2).
Figure 1.
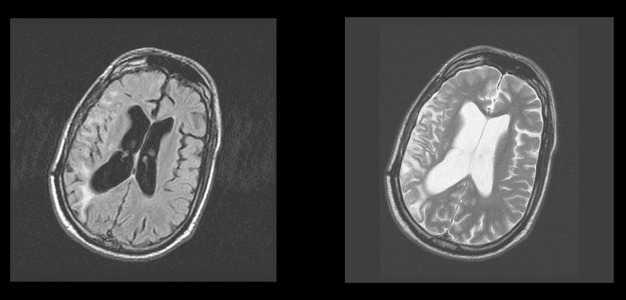
Brain MRI. 2007. FLAIR and frFSET2 sequences, axial plane. Gliotic posttraumatic changes in the right hemisphere with dilatation of right lateral ventricle.
Figure 2.
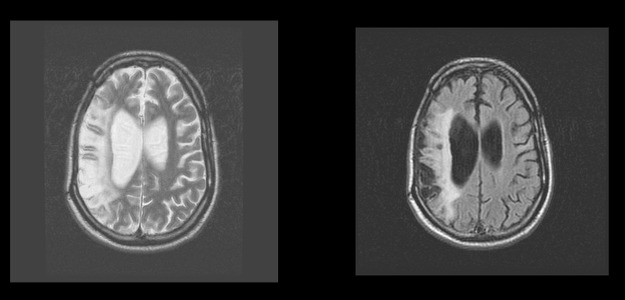
Brain MRI. 2008. FrFSET2 and FLAIR sequences, axial plane. Gliotic posttraumatic changes in the right hemisphere more prominent than in 2007 with atrophy of brain parenchyma (external porencephaly).
In neuropsychological testing he showed anosognosia, executive dysfunction, and behavioral changes, also called frontal syndrome. These difficulties made him dependent upon others and unable to function by himself in many situations of everyday life.
Only a little progress was made after traditional rehabilitation. The patient showed perseverations in the performance of the Trail Making Test, part A (TMT [A]) and in a writing sample (B) (Figure 3).
Figure 3.
Perseverations in the performance of the TMT (A) and in writing (B) in Exam. 2
Before the experiment he was prone to fits of uncontrolled laughter, and was sporadically aggressive and impulsive; he showed no motivation for any treatment and refused to participate in his own care. He incessantly complained of fatigue. It should be noted that he was enthusiastic for neurotherapy when it was proposed, but. he was very resistant to advice of any kind, and would not revise or reconsider a decision once he had made up his mind.
The Program of Neurotherapy
The patient took part in two differentiated rehabilitation programs of neurotherapy in crossover design:
-
Program A administered in 2 modules.
Module 1 – 20 sessions of relative beta training; the goal of the training was to activate the frontal cortex by enhancing beta activity recorded over the frontal electrodes. In more detail the procedure was as follows. Electrodes were placed at Fz and Cz – bipolar recording. The procedure was to increase the ratio of beta EEG power/EG power in theta and alpha frequency bands. The beta frequency band was from 13 to 21 Hz. The combined theta and alpha frequency bands were from 4 to 12 Hz. Each session included approximately 20 min of neurofeedback training.
Module 2 – 20 sessions of behavioral training combined with relative beta training (the procedure is described in more detail in Pachalska 2008 [14]).
-
Program B, administered in 2 modules:
Repetitive transcranial magnetic stimulation (rTMS), which is a non-invasive brain stimulation technique that modulates cortical activity [15]. During rTMS a fluctuating magnetic field is used to induce an electrical current discharged through a coil held to the scalp over a brain region of interest. The magnetic field penetrates the scalp over a brain region of interest. The magnetic field also penetrates the skull and induces a depolarizing electrical current in the underlying cortical surface. Repetitive strings of stimulation at a given frequency can either decrease (low frequency TMS) or enhance (high frequency TMS) the excitability of the underlying cortical areas [for review see 16]. 20 sessions of rTMS intervention (25 repetitions) with low frequency rTMS (1 Hz) were used to reduce the excitability of left frontal and temporal brain regions, and high frequency (5 Hz) to stimulate right frontal and temporal brain regions. This was based on functional imaging studies of M.L-S’s brain, suggesting that over-activation of left frontal and temporal cortices may reduce the recovery potentials by inhibiting (perilesional) right frontal and temporal areas.
Module 2 – 20 sessions of behavioral training combined with rTMS intervention (the procedure is described in more detail in Pachalska 2008 [14]).
The therapy was administered by the same therapist team, but not simultaneously, as he was hospitalized in different institutions at different times. We used neuropsychological testing as well as ERPs before the entire experiment, as well as after the completion of program A and after the completion of program B. The basic clinical background is provided in Table 1.
Table 1.
Neuropsychological testing of the patient ML-S in examination 1, 2 and 3.
| Measure | Exam. 1 | Exam. 2 | Exam. 3 |
|---|---|---|---|
| WAIS-R | |||
| IQ – Full | 61.5/100 | 64.5/100 | 94.5/100 |
| IQ – Verbal | 65.5/100 | 69.5/100 | 99.5/100 |
| IQ – Nonverbal | 57.5/100 | 61.5/100 | 89.5/100 |
| Attention | |||
| WMS-III Spatial span | 3 (1st%ile) | 3 (1st%ile) | 12 (75th%ile) |
| Visuospatial ability | |||
| WAIS-III block design | 3 (1st%ile) | 3 (1st%ile) | 8 (25th%ile) |
| Logical Memory | |||
| WMS-III Immediate logical memory | 5/24 | 7/24 | 20/24 |
| WMS-III Delayed logical memory | 3/24 | 4/24 | 18/24 |
| WMS-III Immediate visual recall | 9/41 | 12/41 | 37/41 |
| WMS-III Delayed visual recall | 4/41 | 6/41 | 26/41 |
| Verbal memory | |||
| CVLT Short Delay Free Recall | 0/9(<1st%ile) | 1/9 | 2/9 (<1st%ile) |
| CVLT Long Free Recall | 0/9(<1st%ile) | 0/9 (<1st%ile) | 2/9 (<1st%ile) |
| CVLT Long Delay Cue Recall | 0/9(<1st%ile) | 0/9 (<1st%ile) | 2/9 (<1st%ile) |
| Executive Functions | |||
| TMT – number sequencing | Discontinued | 150s. (<1st%ile) | 54s. (10th%ile) |
| TMT – number letter sequencing | Discontinued | Discontinued | 150s. (<1st%ile) |
| Stroop | |||
| Color | 90 s. (<1st%ile) | 89 s. (<1st%ile) | 41 s. (16th%ile) |
| Word | 29 s. (25th%ile) | 29 s. (25th%ile) | 42 s. (63rd%ile) |
| Interferences | Discontinued | Discontinued | 128 s. (<1th%ile) |
| WCST | |||
| Categories | 0 (2–5th%ile) | 0 (2–5th%ile) | 2 (>16th%ile) |
| Perseverative errors | 46 (<1th%ile) | 45 (<1th%ile) | 19 (37th percentile) |
| Conceptual level responses | 63 (<19th%ile) | 63 (<19th%ile) | 48 (45th%ile) |
| Fail to maintain sets | Discontinued | Discontinued | 4 (2–5th%ile) |
TMT – TrialMaking Test. Level of performance corresponding to the percentiles: 98–99%ile – very superior; 91–97 %ile – superior; 75–90%ile – high average; 25–74%ile – average; 9–24%ile – low average; 3–8%ile – borderline; 2nd%ile and below – impaired.
The experiment was reviewed and approved by the respective medical ethics committees, and the patient gave written informed consent for the anonymous publication of his case history.
Cognitive Functions
Neuropsychological testing in examination 1st showed multiple deficits (see Table 1). Over the course of the entire neurotherapy program, ML-S’s verbal and non-verbal IQ increased significantly (cf. Table 1), though most of the improvement took place after program B. Most of his cognitive dysfunctions also resolved, including immediate and delayed logical and visual recall on the WMS-III (cf. Table 1). His results for maintaining attention on the WMS-III also improved (34/40 points). In other cognitive functions ML-S’s results also improved in the 3rd examination. On the auditory learning task, he had forgotten all the words after a 15-minute filled delay in the 1st and 2nd examinations, and got 5 words in recognition; however, in the 3rd examination he remember 2 words after the delay, and got all the words at recognition. This general pattern was repeated in nearly all neuropsychological parameters.
However, as hypothesized, patient M.L-S showed small improvements in executive dysfunction after conclusion of program A (Exam 2), and large improvement after program B (Exam 3), even those these were the most disturbed of all his neuropsychological functions.
Characteristics of Frontal Syndrome
In order to evaluate the qualitative disturbances occurring in ML-S’s behavior, we used the Frontal Behavioral Inventory [17–19], adapted for Polish [20]. This questionnaire consists of 24 questions that can be answered by a layman who has regular contact with the patient (usually a close family member), and has proven to be a sensitive and specific measure of frontal syndrome [17,18]. Each of the questions simply asks whether or not a particular behavior has been occurring or has changed since the injury, with four possible answers:
– No, never (0 points);
– Yes, but only occasionally or slightly (1 point);
– Yes, rather often (2 points);
– Very much so, all the time (3 points).
If the person answering the questions is uncertain or does not understand the question, the person administering the inventory can amplify or clarify. The questionnaire itself labels each question with the name of the symptom that the behavior presumably exemplifies, but in our own experience with this test we have found that the labels often confuse the examinee. For example, the first question on the questionnaire reads as follows
Apathy
Has she/he lost interest in friends or daily activities?
If we read the question exactly as written, the examinee often focuses on the word “apathy,” which they may or may not understand, whereas the simple question “Has she/he lost interest in friends or daily activities?” elicits a more concrete answer, which is what the interpretation of the Inventory really requires.
For purposes of analysis the 24 questions can be grouped into four categories:
impaired social conduct (social inappropriateness, impulsivity, poor judgement and inappropriate jocularity);
impaired personal conduct (perseverations and obsessive/compulsive behavior, inflexibility, and concreteness);
mood disorders (irritability, aggression, restlessness);
control disorders (hyperorality, hypersexuality, utilization syndrome).
In the present study, the authors asked ML-S’s mother to complete the questionnaire 3 times: before the commencement of program A (Exam 1), once again immediately after its completion (Exam 2), and again immediately after completion of the rTMS program (Exam 3).
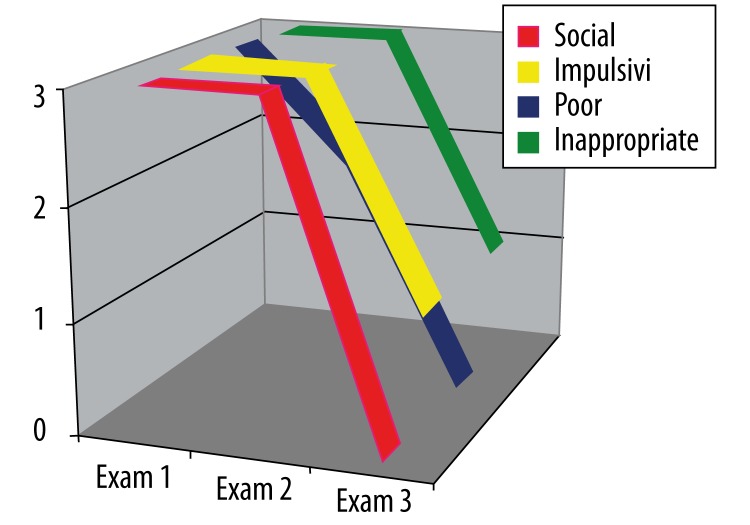
Impaired social conduct
As can be seen in Figure 4, ML-S showed severe disturbances in this category in the first examination. The second examination showed no change in any aspect except for “poor judgement” (which went down from 3 to 2), but in the third examination the scores had fallen to zero in every category except impulsivity (Figure 4).
Figure 4.
ML-S’s results on the Frontal Behavioral Inventory in the category “Impaired social conduct” over three examinations (see text).
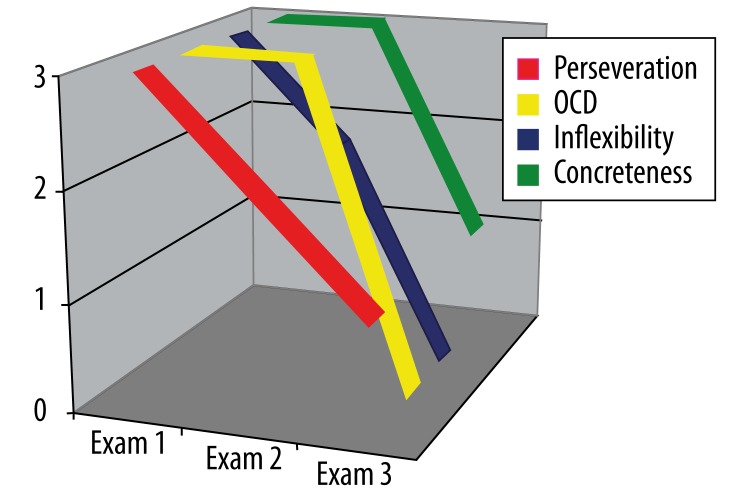
Impaired personal conduct
ML-S’s mother reported severe symptoms in all four parameters of impaired personal conduct in the first examination. The obsessive/compulsive behavior and the tendency to concreteness had not improved by the second examination, but there was some improvement in perseveration and inflexibility. All four parameters showed improvement in the third examination, with obsessive/compulsive behavior and inflexibility rated at zero (Figure 5).
Figure 5.
ML-S’s results on the Frontal Behavioral Inventory in the category “Impaired personal conduct” over three examinations (see text).
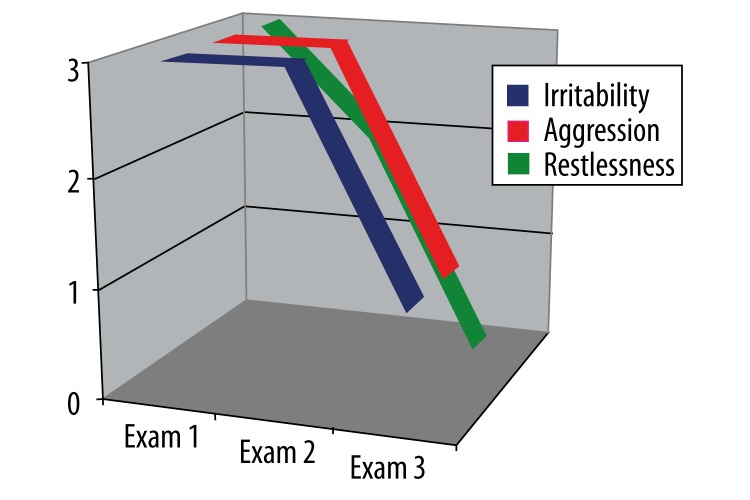
Mood disorders
In the first examination, the patient scored a maximum of three points in each of the three parameters of the category “mood disorders.” The second examination showed no improvement in irritability and aggression, but some improvement in restlessness. By the third examination, irritability and aggression were at the level of one point, and restlessness at zero (Figure 6).
Figure 6.
ML-S’s results on the Frontal Behavioral Inventory in the category “Mood disorders” over three examinations (see text).
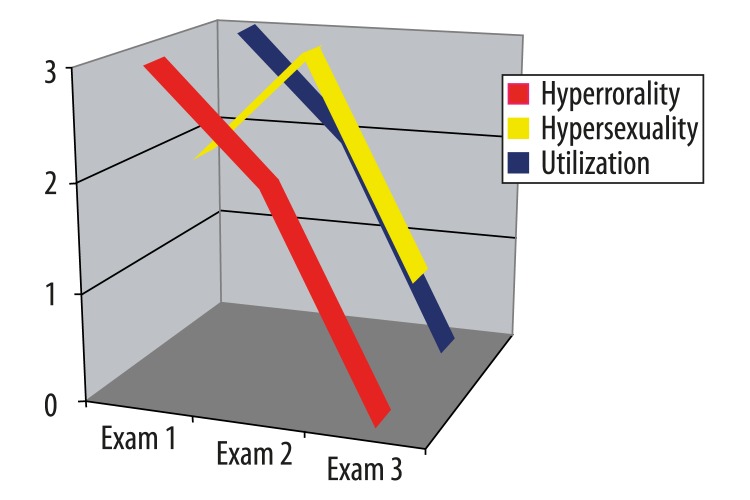
Control disorders
In the first examination, ML-S had severe symptoms of hyperorality and utilization behavior (which were prominent features of the patient’s behavior during therapy as well), but received only two points for hypersexuality. By the time of the second examination, there had been improvement in hyperorality and utilization behavior (two points each), but a marked increase in hypersexuality (3 points), this being the only parameter that actually deteriorated between the first and second examinations. On the third examination, there were still traces of the hypersexuality, but hyperorality and utilization behavior had both dropped to zero (Figure 7).
Figure 7.
ML-S’s results on the Frontal Behavioral Inventory in the category “Control disorders” over three examinations (see text)
To sum up the neuropsychological testing, patient M.L-S, as hypothesized, showed small improvements in behavioral changes after the conclusion of program A, and large improvement after the conclusion of program B.
Event Related Potentials (ERPs)
Event related potentials (ERPs) were used to assess functional changes in the patient induced by rehabilitation programs. We used this approach for the following reasons. First, ERPs have a superior temporal resolution (on the order of milliseconds) among other imaging methods, such as fMRI and PET (which have time resolution of 6 seconds and more) [21], Secondly, ERPs have been proven to be a powerful tool for detecting changes induced by neurofeedback training in ADHD children [22,23]. And finally, in contrast to spontaneous EEG oscillations, ERPs reflect stages of information flow within the brain [21,22,24].
The diagnostic power of ERPs has been enhanced by the recent emergence of new methods of analysis, such as Independent Component Analysis (ICA) and Low Resolution Electromagnetic Tomography (LORETA) [21].
A modification of the visual two-stimulus GO/NO GO paradigm was used (Figure 8). Three categories of visual stimuli were selected:
Figure 8.
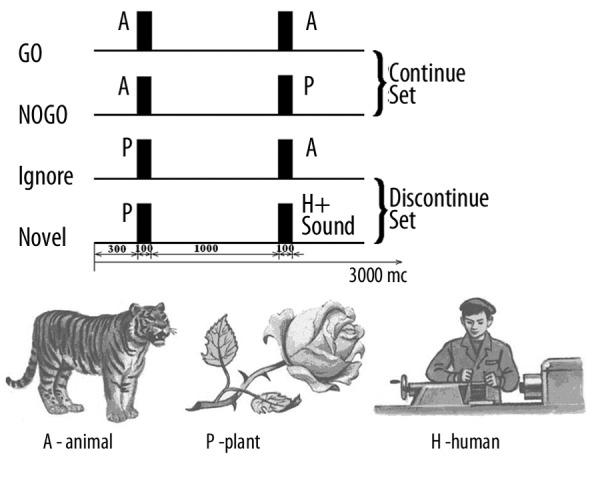
Schematic representation of the two stimulus GO/NOGO task. From top to bottom: time dynamics of stimuli in four categories of trials. Abbreviations: A, P, H stimuli are “Animals”, “Plants” and “Humans” respectively. GO trials are when A-A stimuli require the subject to press a button. NOGO trials are A-P stimuli, which require suppression of a prepared action. GO and NOGO trials represent “Continue set” in which subjects have to prepare for action after the first stimulus presentation (A). Ignore trials are stimuli pairs beginning with a P, which require no preparation for action. Novel trials are pairs requiring no action, with presentation of a novel sound as the second stimuli. Ignore and Novel trials represent “Discontinue set”, in which subjects do not need to prepare for action after the first stimulus presentation. Time intervals are depicted at the bottom.
20 different images of animals, referred to later as “A”;
20 different images of plants, referred to as “P”;
20 different images of people of different professions, presented along with an artificial “novel” sound, referred to as “H+Sound”.
All visual stimuli were selected to have a similar size and luminosity. The randomly varying novel sounds consisted of five 20-ms fragments filled with tones of different frequencies (500, 1000, 1500, 2000, and 2500 Hz). Each time a new combination of tones was used, while the novel sounds appeared unexpectedly (the probability of appearance was 12.5%).
The trials consisted of presentations of paired stimuli with inter-stimulus intervals of 1 s. The duration of stimuli was 100 ms. Four categories of trials were used (Figure 8): A-A, A-P, P-P, and P-(H+Sound). The trials were grouped into four blocks with one hundred trials each. In each block a unique set of five A, five P, and five H stimuli were selected. Participants practiced the task before the recording started.
The patient sat upright in an easy chair looking at a computer screen. The task was to press a button with the right hand in response to all A-A pairs as fast as possible, and to withhold button pressing in response to other pairs: A-P, P-P, P-(H+Sound). According to the task design, two preparatory sets were distinguished: a “Continue set,” in which A is presented as the first stimulus and the subject is presumed to prepare to respond; and a “Discontinue set,” in which P is presented as the first stimulus, and the subject does not need to prepare to respond. In the “Continue set” A-A pairs will be referred to as “GO trials,” A-P pairs as “NO GO trials.” Averages for response latency and response variance across trials were calculated. Omission errors (failure to respond in GO trials) and commission errors (failure to suppress a response to NO GO trials) were also computed.
EEGs were recorded from 19 scalp sites. The electrodes were applied according to the International 10–20 system. The EEG was recorded referentially to linked ears, allowing computational re-referencing of the data (remontaging).
Results
Behavior in the GO/NOGO task
The behavioral parameters in the GO/NOGO task measured in the patient before the rehabilitation programs (first recording), after rehabilitation program A – neurofeedback training (second recording), and after rehabilitation program B – rTMS (third recording) are presented in Table 2. As one can see, the omission errors normalized substantially after program B. The patient’s performance in the two stimulus GO/NOGO task was abnormal at the first recording: namely, the number of omission errors (indicator of attention) and variance of response (indicator of performance consistency) were significantly different from the norm (see Table 2; p values below). Rehabilitation program A did not change the behavioral parameters significantly. In contrast, substantial changed occurred after the rehabilitation program B. It should be stressed here that in spite of dramatic changes the variance of response of the patient still remained deviant from the corresponding parameter in the healthy controls.
Table 2.
Behavioral parameters in the GO/NOGO task before rehabilitation programs (1st recording), after rehabilitation program A (2nd recording), and after rehabilitation program B (3rd recording). P-values of deviations from mean values of the healthy controls are presented in separate rows.
| ERP’s | Omission | Commission | RT1 | var(RT1) |
|---|---|---|---|---|
| 1strecording | 26% | 4% | 596 | 27.7 |
| p- of deviation from norms for first recording | 0.000 | 0.01 | 0.11 | 0.000 |
| 2ndrecording | 21% | 1% | 563 | 21.7 |
| p- of deviation from norms for second recording | 0.000 | 0.67 | 0.21 | 0.03 |
| 3rdrecording | 3% | 0 | 527 | 19.0 |
| p- of deviation from norms for third recording | 0.12 | 0.71 | 0.23 | 0.001 |
| Mean values for a group of healthy subjects of the same age (N=74) | 1.8% | 0.5% | 414 | 9.1 |
ERPs in the GO/NOGO task
It should stressed here that EEG spectra did not change significantly during the course of the two rehabilitation programs. In contrast, ERPs changed substantially after Program B. Figure 9 depicts the results of ERP recordings before treatment (first recording), after rehabilitation program A (second recording), and after rehabilitation program B (third recording). As one can see, the amplitude of spatial distribution of the NOGO ERPs differed for the corresponding parameters of healthy controls at the first recording. No visible changes occurred after rehabilitation program A. Large and statistically significant changes occurred after rehabilitation program B. It should be stressed, however, that even after substantial change in the course of program B, the NOGO ERPs in the patient were still much different from the norm.
Figure 9.
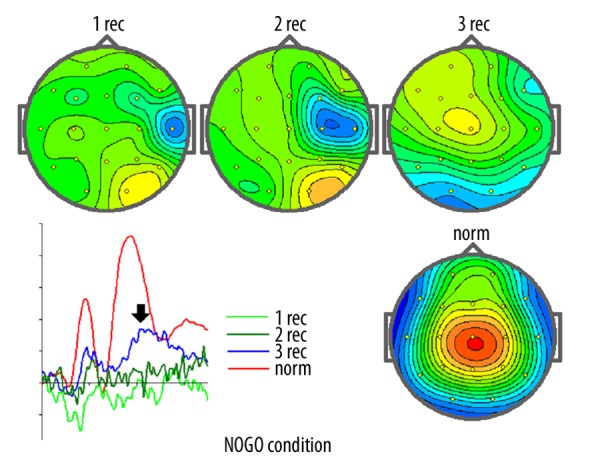
ERP recordings in examination 1st, 2nd and 3rd in comparison to norm.
In Figure 9, maps of the evoked potential measured at 360 ms and ERPs recorded at Cz in the NOGO condition of the GO/NOGO task are presented for the pre-treatment state (1 rec), after program A (2 rec), and after program B (3 rec). They are contrasted to the corresponding parameters recorded in a group of healthy controls of the same age. In ERP recodings: X-axis – time (the whole range is 700 ms), Y-axis – averaged voltage measured in μV (each bin corresponds to 2 μV).
To sum up the neurophysiological testing, patient M.L-S, as hypothesized, showed some slight improvement after relative beta training (program A) and a improvement of ERPs after administration of rTMS (program B).
Discussion
Traditional therapies for functional brain recovery after traumatic brain injury are still not satisfactory [11,12]. To date the best approach seems to be intensive physical and cognitive therapy [9]; however, the results are limited and functional gains are often minimal [13]. Therefore, adjunct interventions that can augment the response of the brain to the behavioral and cognitive training might be useful to enhance therapy-induced recovery in TBI patients. In this context, neurofeedback self-regulation and noninvasive brain stimulation appear to be options as additional interventions to standard physical therapies.
In the case of neurofeedback in TBI patients, quantitative electroencephalography (qEEG) patterns are assessed and then compared to a database obtained from a normative population [21]. Deviations in qEEG patterns from the normative group form the basis for an intervention plan [25]. The deficiency of relative beta EEG activity found in our TBI patient prompted us to suggest relative neurofeedback training for him. This training was intended to activate the hypofunctioning frontal lobes by means of self-regulation, using the EEG neurofeedback parameter (the relative beta EEG power) as an index of hypofrontality. It should be stressed here that neurofeedback alone did not have any significant effect on either neuropsychological or neurophysiological parameters of brain functioning in our patient, as reflected in neuropsychological and neurophysiological parameters recorded after 20 sessions of neurofeedback.
Two non-invasive methods of injecting electrical currents into the brain have proved to be promising for inducing long-lasting plastic changes in motor systems. They are transcranial magnetic stimulation (TMS) [27,28] and transcranial direct current stimulation (tDCS) [29]. These techniques represent powerful methods for priming cortical excitability for subsequent motor or cognitive training. Thus their combined use can optimize the plastic changes induced by motor-cognitive practice, leading to more remarkable and long-lasting clinical gains in rehabilitation [28].
TMS is delivered to the brain by passing a strong brief electrical current through an insulated wire coil placed on the skull. The current generates a transient magnetic field, which in turn induces a secondary current in the brain that is capable of depolarising neurons [27]. Depending on the frequency and duration of the stimulation, the shape of the coil and the strength of the magnetic field, TMS can stimulate or suppress activity in cortical regions [28].
tDCS delivers weak polarizing direct currents to the cortex via two electrodes placed on the scalp: an active electrode is placed on the site overlying the cortical target, and a reference electrode is usually placed over the contralateral supraorbital or mastoid area. tDCS acts by inducing sustained changes in neural cell membrane potential: cathodal tDCS leads to brain hyperpolarization (inhibition), whereas anodal results in brain depolarization (excitation) [29].
TMS and tDCS employ different mechanisms of actions on the brain, with TMS acting as a neuro-stimulator and tDCS as a neuro-modulator. TMS has better spatial and temporal resolution, and its protocols are better established. tDCS has the advantage of being easier to use in double-blind or sham-controlled studies and easier to apply concurrently with behavioural tasks [29]. Despite their differences, both TMS and tDCS can induce long-term after-effects on cortical excitability that can last for months [31,32]. These long-term after-effects are believed to engage mechanisms of neural plasticity, making these techniques ideally suited in rehabilitation of stroke and TBI [33].
In our patient, in program A, relative beta training was applied, according to recent findings in the literature [26,34], but it was not effective. The patient did not improve in attention, which is a bad sign for recovery in general [35].
In program B, however, rTMS was applied in order to activate the hypofunctioning areas of the frontal lobe. Five sessions of rTMS appeared to produce clinically significant changes in neuropsychological parameters, as well as statistically reliable changes in physiological parameters of brain functioning. We did not use tDCS, but on the basis of literature we can suggest that combination of brain stimulation techniques, such as TMS and tDCS, might have beneficial consequences for TBI patients.
Conclusions
As hypothesized, patient M.L-S showed small improvements in executive dysfunction and behavioral disorders after conclusion of program A, and large improvement after conclusion of program B. Specifically, the patient improved in social functioning: we found decreased impulsivity, and improved functioning in many situations of everyday life. He also became more self-dependent in social situations.
Similarly, the patient showed small improvement in neurophysiological parameters after conclusion of program A (relative beta training). ERPs showing differences from norms remained, with no major changes between pre and post recordings. However, we found a significant increase after conclusion of program B (rTMS) of the P300 NOGO component.
The ERP recording made after rTMS showed improvement, which would imply the usefulness of rTSM even for such patients with severe brain damage, after long term coma.
The need for a deeper analysis of the patient’s problems in both personal and social context should be stressed, in order to adapt therapeutic procedures heuristically, consistent with a process-based approach, as well as further examination in neurometrics (ERPs). In this case the need for another approach (for example a combination of tDCS and NF) can be suggested. In both cases multi-center studies are needed.
Footnotes
Source of support: Departmental sources
References
- 1.Masson F, Thicoipe M, Aye P, et al. Epidemiology of severe brain injuries: a prospective population-based study. J Trauma. 2001;51:481–89. doi: 10.1097/00005373-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Ducrocq SC, Meyer PG, Orliaguet GA, et al. Epidemiology and early predictive factors of mortality and outcome in children with traumatic severe brain injury: experience of a French pediatric trauma center. Pediatr Crit Care Med. 2006;7(5):461–67. doi: 10.1097/01.PCC.0000235245.49129.27. [DOI] [PubMed] [Google Scholar]
- 3.Mauritz W, Wilbacher I, Majdan M, et al. Epidemiology, treatment and outcome of patients after severe traumatic brain injury in European regions with different economic status. Eur J Public Health. 2008;18(6):575–80. doi: 10.1093/eurpub/ckn079. [DOI] [PubMed] [Google Scholar]
- 4.Benedictus MR, Spikman JM, van der Naalt J. Cognitive and behavioral impairment in traumatic brain injury related to outcome and return to work. Arch Phys Med Rehabil. 2010;91(9):1436–41. doi: 10.1016/j.apmr.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Slawik H, Salmond CH, Taylor-Tavares JV, et al. Frontal cerebral vulnerability and executive deficits from raised intracranial pressure in child traumatic brain injury. J Neurotrauma. 2009:1891–903. doi: 10.1089/neu.2009.0942. [DOI] [PubMed] [Google Scholar]
- 6.Velikonja D, Warriner E, Brum C. Profiles of emotional and behavioral sequelae following acquired brain injury: cluster analysis of the Personality Assessment Inventory. J Clin Exp Neuropsychol. 2010;32(6):610–21. doi: 10.1080/13803390903401302. [DOI] [PubMed] [Google Scholar]
- 7.Velikonja D, Warriner E, Brum C. Profiles of emotional and behavioral sequelae following acquired brain injury: cluster analysis of the Personality Assessment Inventory. J Clin Exp Neuropsychol. 2010;32(6):610–21. doi: 10.1080/13803390903401302. [DOI] [PubMed] [Google Scholar]
- 8.Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med. 2011;30(1):179–88. doi: 10.1016/j.csm.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao V, Rosenberg P, Bertrand M, et al. Aggression after traumatic brain injury: prevalence and correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):420–29. doi: 10.1176/appi.neuropsych.21.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marvasti JA. Treatment of war trauma in veterans: pharmacotherapy and self-help proposal. Conn Med. 2011;75(3):133–41. [PubMed] [Google Scholar]
- 11.Milders M, Ietswaart M, Crawford JR, Currie D. Social behavior following traumatic brain injury and its association with emotion recognition, understanding of intentions, and cognitive flexibility. J Int Neuropsychol Soc. 2008;14(2):318–26. doi: 10.1017/S1355617708080351. [DOI] [PubMed] [Google Scholar]
- 12.Pachalska M, Moskała M, MacQueen BD, et al. Early neurorehabilitation in a patient with severe traumatic brain injury to the frontal lobes. Med Sci Monit. 2010;16(12):CS157–67. [PubMed] [Google Scholar]
- 13.Choi JH, Jakob M, Stapf C, et al. Multimodal early rehabilitation and predictors of outcome in survivors of severe traumatic brain injury. J Trauma. 2008;65(5):1028–35. doi: 10.1097/TA.0b013e31815eba9b. [DOI] [PubMed] [Google Scholar]
- 14.Pachalska M. Rehabilitacja neuropsychologiczna. [[Neuropsychological Rehabilitation]]. Lublin: Wydawnictwo UMCS; 2008. [Google Scholar]
- 15.Meinzer M, Harnish S, Conway T, Crosson B. Recent developments in functional and structural imaging of aphasia recovery after stroke. Aphasiology. 2011;25(3):271–90. doi: 10.1080/02687038.2010.530672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience –virtual lesion, chronometry, and functional connectivity. Current Opinion in Neurobiology. 2000;10(02):232–37. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- 17.Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci. 1997;24(1):29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- 18.Kertesz A, Nadkarni N, Davidson W, et al. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc. 2000;6(4):460–68. doi: 10.1017/s1355617700644041. [DOI] [PubMed] [Google Scholar]
- 19.Blair M, Kertesz A, Davis-Faroque, et al. Behavioural measures in frontotemporal lobar dementia and other dementias: the utility of the frontal behavioural inventory and the neuropsychiatric inventory in a national cohort study. Dement Geriatr Cogn Disord. 2007;23(6):406–15. doi: 10.1159/000101908. [DOI] [PubMed] [Google Scholar]
- 20.Pąchalska M, Talar J, Kurzbauer H, et al. Diagnostyka różnicowa zespołu czołowego u chorych po zamkniętych urazach czaszkowo-mózgowych. Ortopedia Traumatologia Rehabilitacja. 20021;4(1):81–87. [in Polish] [PubMed] [Google Scholar]
- 21.Kropotov JD. Quantitative EEG, event related potentials and neurotherapy. Academic Press, Elsevier; San Diego: 2009. p. 542. [Google Scholar]
- 22.Kropotov JD, Grin-Yatsenko VA, Ponomarev VA, et al. ERPs correlates of EEG relative beta training in ADHD children. Int J Psychophysiol. 2005;55(1):23–34. doi: 10.1016/j.ijpsycho.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Kropotov JD, Muller A. What can event related potentials contribute to neuropsychology. Acta Neuropsychologica. 2009;7(3):169–81. [Google Scholar]
- 24.Barry RJ, Clarke AR, McCarthy R, et al. Event-related potentials in adults with Attention-Deficit/Hyperactivity Disorder: an investigation using an inter-modal auditory/visual oddball task. Int J Psychophysiol. 2009;71(2):124–31. doi: 10.1016/j.ijpsycho.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Thatcher RW. EEG operant conditioning (biofeedback) and traumatic brain injury. Clin Electroencephalogr. 2000;31(1):38–44. doi: 10.1177/155005940003100110. [DOI] [PubMed] [Google Scholar]
- 26.Thornton KE, Carmody DP. Traumatic brain injury rehabilitation: qEEG biofeedback treatment protocols. Appl Psychophysiol Biofeedback. 2009;34(1):59–68. doi: 10.1007/s10484-009-9075-4. [DOI] [PubMed] [Google Scholar]
- 27.Pape TL, Rosenow J, Lewis G. Transcranial magnetic stimulation: a possible treatment for TBI. J Head Trauma Rehabil. 2006;21(5):437–51. doi: 10.1097/00001199-200609000-00063. [DOI] [PubMed] [Google Scholar]
- 28.Bashir S, Mizrahi I, Weaver K, et al. Assessment and modulation of neural plasticity in rehabilitation with transcranial magnetic stimulation. PMR. 2010;(Suppl 2):253–68. doi: 10.1016/j.pmrj.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. Neuroeng Rehabil. 2009:6–8. doi: 10.1186/1743-0003-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pascual-Leone A, Davey N, Rothwell JC, Wassermann EBP. Handbook of Transcranial Magnetic Stimulation. New York: Oxford University Press; 2002. [Google Scholar]
- 31.Nitsche MA, Doemkes S, Karakose T, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2007;97:3109–17. doi: 10.1152/jn.01312.2006. [DOI] [PubMed] [Google Scholar]
- 32.Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–65. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- 33.Fregni F, Boggio PS, Valle AC, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115–22. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- 34.Rossi S, Rossini PM. TMS in cognitive plasticity and the potential for rehabilitation. Trends Cogn Sci. 2004;8:273–79. doi: 10.1016/j.tics.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Schoenberger NE, Shif SC, Esty ML, et al. Flexyx Neurotherapy System in the treatment of traumatic brain injury: an initial evaluation. J Head Trauma Rehabil. 2001;16(3):260–74. doi: 10.1097/00001199-200106000-00005. [DOI] [PubMed] [Google Scholar]