Summary
Background
Some patients with right heart failure develop cardiac hepatopathy (CH). The pathophysiology of CH is thought to be secondary to hepatic venous congestion and arterial ischemia. We sought to define the clinical and hemodynamic characteristics associated with CH.
Material/Methods
A retrospective cross sectional analysis was performed in which subjects were identified from our institutional cardiology database if echocardiography showed either right ventricular (RV) hypokinesis or dilatation, and was performed within 30 days of right heart catheterization. A chart review was then performed to identify patient clinical characteristics and to determine if the patients had underlying liver disease. Subjects with non-cardiac causes for hepatopathy were excluded.
Results
In 188 included subjects, etiology for right heart dysfunction included left heart failure (LHF), shunt, pulmonary hypertension, mitral- tricuspid- and pulmonic valvular disease. On multivariate analysis, higher RV diastolic pressure and etiology for RV dysfunction other than LHF were both associated with CH. Low cardiac output was associated with CH only amongst those without LHF.
Conclusions
CH is most often seen in subjects with elevated RV diastolic pressure suggesting a congestive cause in most cases. CH associated with low cardiac output in patients without LHF suggests that low flow may be contributing to the patophysiology in some cases.
Keywords: cardiac hepatopathy, right heart failure, congestive heart failure
Background
Right heart failure causing cardiac hepatopathy (CH) is a distinct clinical entity in which the clinical and hemodynamic features are poorly defined [1–3]. Hepatocellular necrosis and dysfunction in the setting of right heart failure have been attributed to both hepatic congestion and hepatic ischemia [4]. The histopathologic appearance of a congested “nutmeg” liver is distinctive and well described. Microscopic examination shows sinusoidal congestion in the early stages; with subsequent hemorrhagic necrosis, hepatocyte degeneration, fatty changes and eventually fibrosis [2,4]. In hepatic ischemia, cardiac output and hence oxygen delivery is impaired [5]. Histological examination may reveal centrilobular fibrosis and subsequent portal fibrosis [4,6].
The finding of hepatopathy in patients with cardiac dysfunction has important clinical implications. Increased mortality has been demonstrated when transaminitis or hyperbilirubinemia [7,8] are present in patients with cardiac dysfunction. Since most patients with right heart dysfunction do not develop CH, we sought to define the clinical and hemodynamic characteristics associated with this entity.
Material and Methods
A retrospective cross sectional analysis was performed. Our hospital cardiology database was queried to identify all subjects who had right ventricular (RV) hypokinesis or dilatation on echocardiography. Patients were included if they also had right heart catheterization performed within 30 days of their echocardiograms. Charts were reviewed for demographic data, comorbid conditions, medications and serologic assessment of hepatic function. Subjects were excluded from analysis if potential causes of hepatic dysfunction other than cardiac disease were identified on chart review (Figure 1). Subjects with acute shock were excluded, namely those patients who presented with hypotension and/or cardiac arrest. Thereby, this excluded severe acute RV dysfunction and acute left ventricular (LV) dysfunction. Furthermore, subjects with malignancy, autoimmune disease, complex congenital cardiac disease, human immunodeficiency virus, substance abuse, and hemochromatosis were also excluded and labeled as other in Figure 1. These subjects were excluded because of the higher likelihood of noncardiac causes of hepatic dysfunction.
Figure 1.
Inclusions and exclusions.
CH was defined as an aspartate aminotransferase >100 U/L, an alkaline phosphatase >200 U/L, or a serum total bilirubin >2.0 mg/dL. These markers were chosen because they are often abnormal in CH. [9,10]. The cutoffs chosen represent values approximately twice the upper limit of normal. The chosen cutoffs also corresponded approximately with the 95th percentile observed in our study sample.
The etiology of right heart dysfunction was classified by a study cardiologist based on the clinical, echocardiographic, and hemodynamic data. This assessment was made while blinded to CH status. Etiology was classified as being due to one of the following: left heart failure, pulmonary arterial hypertension, shunt, tricuspid regurgitation, pulmonic regurgitation, or other etiology. Subjects were classified based on their primary cardiac pathology that was most likely to cause right heart dysfunction.
The study selection included patients who had evidence of right ventricular dilatation or hypokinesis who had both a cardiac catheterization and an echocardiogram within one month of each other during the period of December, 2000 through July, 2006. The criteria for the study were justified. Patients were included only with evidence of right heart dysfunction in our study population in order to evaluate the hepatic derangements seen with this type of cardiomyopathy.
Statistical analysis was done using Stata software, version 9.1 (College Station, TX). Normally distributed data were presented as the mean ± standard deviation (SD). Non-normal data were presented as the median [interquartile range (IR)]. Comparison of means was performed using the two-sample t-test. Comparison of categorical data was performed using χ2. Comparison of medians was performed using the Mann-Whitney or Kruskal Walllis tests as appropriate. Logistic regression analysis was used to examine for clinical, echocardiographic and hemodynamic variables associated with CH.
Results
From the study sample of 188 patients, 56 (30%) were found to have CH. Demographics and clinical variables are displayed in Table 1. No difference was seen in age, sex, body surface area, medication use, and prevalence of hypertension and diabetes between those with and without congestive hepatopathy. Renal function was similar between those with and without CH. Etiologies for RV dysfunction are shown in Table 2, stratified by the presence or absence of CH. Having left heart failure as the etiology for RV dysfunction was less common in the CH group, p=0.002.
Table 1.
Patient demographics and clinical variables.
| Patient demographics | All | Cardiac hepatopathy (n=56) | No cardiac hepatopathy (n=132) | p-value |
|---|---|---|---|---|
| Age (years) | 62±17 | 61±16 | 62±17 | 0.97 |
| Male (%) | 55 | 50 | 57 | 0.39 |
| Body surface area (m2) | 1.9±0.3 | 1.8±0.2 | 1.9±0.3 | 0.32 |
| Hypertension (%) | 81 | 86 | 79 | 0.26 |
| Diabetes (%) | 41 | 39 | 42 | 0.73 |
| Creatinine | 1.1 [1, 1.5] | 1.2 [1, 1.7] | 1.1 [1, 1.5] | 0.09 |
| Statin use (%) | 34 | 23 | 39 | 0.07 |
| Anti-platelet use (%) | 44 | 46 | 43 | 0.72 |
| ACEI or ARB use (%) | 46 | 46 | 45 | 0.90 |
| Beta blocker use (%) | 61 | 60 | 61 | 0.90 |
| Loop diuretic use (%) | 61 | 58 | 62 | 0.63 |
| Amiodarone use (%) | 2 | 5 | 1 | 0.14 |
| Warfarin use (%) | 24 | 17 | 27 | 0.22 |
| AST >100 mg/dL (%) | 13 | 45 | 0 | <0.001 |
| Alk-phos >200 mg/dL (%) | 14 | 48 | 0 | <0.001 |
| Total bilirubin >2.0 mg/dL (%) | 18 | 61 | 0 | <0.001 |
Normal data are presented as the mean ± standard deviation. Non-normal data are presented as the median [interquartile range]. ACEI – angiotensin-converting enzyme inhibitor; ARB – angiotensin receptor blocker; AST – aspartate aminotransferase; Alk-phos – alkaline phosphatase.
Table 2.
Etiology of right heart dysfunction.
| Patient demographics | All | Cardiac hepatopathy (n=56) | No cardiac hepatopathy (n=132) | p-value |
|---|---|---|---|---|
| Left heart failure (%) | 58 | 41 | 65 | 0.002 |
| Mitral valve disease (%) | 21 | 27 | 18 | 0.18 |
| Shunt (%) | 5 | 5 | 5 | 0.99 |
| Pulmonary hypertension (%) | 8 | 13 | 5 | 0.09 |
| Primary TR or PR (%) | 2 | 2 | 2 | 0.89 |
| Other etiology (%) | 7 | 13 | 5 | 0.05 |
TR – tricuspid regurgitation; PR – pulmonic regurgitation.
Echocardiographic and invasively measured data are shown in Table 3. Those with CH had similar LV ejection fraction, left atrial diameter, RV systolic pressure, and wedge pressure compared to those without CH. Prevalence of severe tricuspid regurgitation, RV hypokinesis and dilated RV were also similar in each group. Those with CH had a higher RV diastolic pressure than those without CH, p<0.001.
Table 3.
Echocardiographic and catheterization variables.
| Patient demographics | All | Cardiac hepatopathy (n=56) | No cardiac hepatopathy (n=132) | p-value |
|---|---|---|---|---|
| Echocardiography | ||||
| LV ejection fraction (%) | 30 [25, 50] | 30 [25, 50] | 30 [25, 50] | 0.48 |
| LA diameter (mm) | 48 ± 9 | 48±9 | 48±9 | 0.67 |
| Severe TR (%) | 23 | 21 | 24 | 0.68 |
| RV hypokinesis (%) | 80 | 73 | 83 | 0.11 |
| RV dilated (%) | 49 | 55 | 46 | 0.25 |
| Catheterization | ||||
| RV systolic pressure (mmHg) | 55±18 | 58±18 | 54±18 | 0.13 |
| RV diastolic pressure (mmHg) | 14±7 | 16±7 | 13±7 | <0.001 |
| Cardiac output (Liters/min) | 3.9 [3.1, 4.7] | 4.0 [3.3, 5.0] | 3.7 [3.0, 4.6] | 0.08 |
| Wedge pressure (mmHg) | 22 [16, 28] | 24 [16, 28] | 22 [16, 28] | 0.43 |
Normal data are presented as the mean ± standard deviation. Non-normal data are presented as the median [interquartile range]. LV – left ventricular; LA – left atrial; TR – tricuspid regurgitation; RV – right ventricular.
In a multivariate logistic regression model, the only variables found to be independent predictors of CH were etiology for RV dysfunction other than left heart failure, odds ratio (OR) 2.94 [95% confidence interval (CI): 1.51, 5.72] and increased RV diastolic pressure, OR 2.47 [95% CI: 1.11, 5.51].
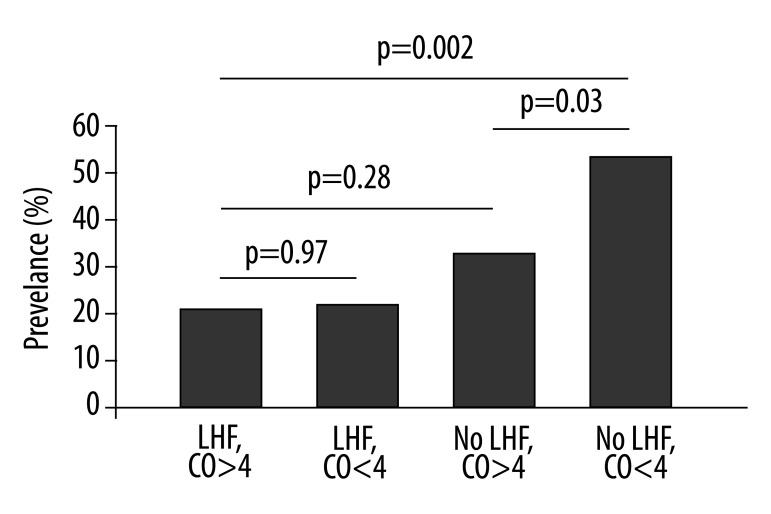
The relationship of cardiac output and congestive hepatopathy was complex. Overall, the cardiac output was similar in those with CH compared to those without CH, p=0.08, (Table 3). In the whole study sample, cardiac output was not an independent predictor of CH (OR 1.44 [95% CI: 0.73, 2.83], p=0.3). However, within the group of subjects whose RV dysfunction was not caused by left heart failure, low cardiac output was a significant predictor of CH (OR 2.74 [95% CI: 1.08, 6.95]). Accordingly, the prevalence of CH in those with left heart failure was 21%, as compared to 42% in those without left heart failure with preserved cardiac output and 56% in those without left heart failure with reduced cardiac output (Figure 2).
Figure 2.
Prevalence of cardiac hepatopathy in those with and without left heart failure (LHF) divided amongst different cardiac output (CO) subgroups.
Discussion
The main findings of our study were that CH was associated with increased RV diastolic pressure and with etiology for RV dysfunction not secondary to left heart failure. Reduced cardiac output was associated with CH only amongst those without left heart failure. This in contrast to the findings reported by Kubo, et al in which they found that patients with a lower cardiac index from left heart failure had worsening levels of liver function tests [9]. In their study, however, only patients with left heart failure were included. In addition, their study cohort had lower cardiac output compared with our study sample. This may account for the differences seen in those patients with left heart failure [9]. Furthermore, the finding of increased RV diastolic pressure in those with CH supports the hypothesized pathophysiology of hepatic congestion suggested from histological series. Increased RV diastolic pressure implies increased right atrial, central venous and hepatic venous pressure. This is similar to findings reported by Van Deursun whereby higher CVP was associated with liver function abnormalities [11]. Increased hepatic venous pressure is therefore a plausible explanation for sinusoidal congestion often observed in histological specimens of patients with CH [12].
It is not intuitively obvious that the etiology for RV dysfunction should be associated with CH. This finding was found to be independent of RV diastolic pressure and cardiac output on multivariate analysis. The cross-sectional nature of our study makes it impossible to draw conclusions regarding causality. We therefore cannot conclude that those with RV dysfunction who do not have left heart failure are at increased risk for developing CH independent of RV diastolic pressure and cardiac output. Our snapshot, one-time measurement of RV diastolic pressure may not reflect pressures chronically. This association should therefore be investigated further. Furthermore, the severity of tricuspid regurgitation was not found to be associated with CH. This is in contrast to previous reports by Lau showing an association between the severity of TR and liver function abnormalities [10].
The finding that lower cardiac output is associated with CH only in those without left heart failure is also intriguing. It is sensible that the measured cardiac output more truly reflects RV function when there is isolated RV dysfunction with normal LV output. When left heart dysfunction is also present, the measured cardiac output reflects the joint pathology of both chambers. Low cardiac output would be expected in those with chronic hepatic ischemia. Lack of association between low cardiac output and CH in both our whole cohort and in the group with left heart failure argues that hepatic ischemia was not often the cause of CH in our study. Since transient ischemic insult with hypotension or shock prior to right heart catheterization were excluded from this study, low cardiac output probably reflected a more chronic process.
Patients with cirrhotic cardiomyopathy have both elevated levels of NT-proBNP and LV diastolic dysfunction [13]. Using a value of 1000 pg/ml, the sensitivity of serum NT-proBNP in distinguishing ascites due to cirrhosis from ascites due to heart failure was 100% [14]. Most patients with acute decompensated heart failure have high liver stiffness values which, like NT-proBNP levels tend to decrease with clinical improvement [15]. Gamma-glutamyltransferase is also independently associated with an adverse outcome in patients with chronic heart failure [16].
Our study has some limitations. As previously mentioned, the cross sectional and retrospective nature of our investigation, limit inferences about causal relationships. Therefore, findings of associations between various parameters and CH do not imply that the parameters are the cause or the effect of CH. Further investigation into such mechanisms is warranted. It is also important to note that measurement of parameters on catheterization, serology and echocardiography were not simultaneous. This may have particular importance in congestive heart failure patients whose hemodynamics may vary substantially from day to day. Lastly, our study sample excluded those with acute RV or LV dysfunction and thereby limiting our analysis to chronic RV dysfunction.
Evolving technology in echocardiography has allowed for more sophisticated measures of right heart function. RV strain, strain rate and tricuspid annular plane systolic excursion are measures of RV contractility. 3-dimensional echocardiography may improve quantification of RV size, shape and function. Association between these parameters and CH may be an area for future investigation.
Conclusions
In conclusion, we found that CH was associated with higher RV diastolic pressure and etiology for RV dysfunction other than left heart failure. Low cardiac output was associated with CH only in those who did not have left heart failure. These findings help us to better understand the relationship between right heart dysfunction and CH.
Footnotes
Conflicts of interest
None of the authors have any conflicts of interest pertaining to this article.
Source of support: Departmental sources
References
- 1.Felder L, Mund A, Parker JG. Liver function tests in chronic congestive heart failure. Circulation. 1950;2(2):286–97. doi: 10.1161/01.cir.2.2.286. [DOI] [PubMed] [Google Scholar]
- 2.Myers RP, Cerini R, Sayegh R, et al. Cardiac hepatopathy: clinical, hemodynamic, and histologic characteristics and correlations. Hepatology. 2003;37(2):393–400. doi: 10.1053/jhep.2003.50062. [DOI] [PubMed] [Google Scholar]
- 3.Richman SM, Delman AJ, Grob D. Alterations in indices of liver function in congestive heart failure with particular reference to serum enzymes. Am J Med. 1961;30:211–25. doi: 10.1016/0002-9343(61)90093-6. [DOI] [PubMed] [Google Scholar]
- 4.Sherlock S. The liver in heart failure; relation of anatomical, functional, and circulatory changes. Br Heart J. 1951;13(3):273–93. doi: 10.1136/hrt.13.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn GD, Hayes P, Breen KJ, Schenker S. The liver in congestive heart failure: a review. Am J Med Sci. 1973;265(3):174–89. doi: 10.1097/00000441-197303000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Bynum TE, Boitnott JK, Maddrey WC. Ischemic hepatitis. Dig Dis Sci. 1979;24(2):129–35. doi: 10.1007/BF01324740. [DOI] [PubMed] [Google Scholar]
- 7.Allen LA, Felker GM, Pocock S, et al. Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail. 2009;11(2):170–77. doi: 10.1093/eurjhf/hfn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batin P, Wickens M, McEntegart D, et al. The importance of abnormalities of liver function tests in predicting mortality in chronic heart failure. Eur Heart J. 1995;16(11):1613–18. doi: 10.1093/oxfordjournals.eurheartj.a060785. [DOI] [PubMed] [Google Scholar]
- 9.Kubo SH, Walter BA, John DH, et al. Liver function abnormalities in chronic heart failure. Influence of systemic hemodynamics. Arch Intern Med. 1987;147(7):1227–30. [PubMed] [Google Scholar]
- 10.Lau GT, Tan HC, Kritharides L. Type of liver dysfunction in heart failure and its relation to the severity of tricuspid regurgitation. Am J Cardiol. 2002;90(12):1405–9. doi: 10.1016/s0002-9149(02)02886-2. [DOI] [PubMed] [Google Scholar]
- 11.van Deursen VM, Damman K, Hillege HL, et al. Abnormal liver function in relation to hemodynamic profile in heart failure patients. J Card Fail. 2010;16(1):84–90. doi: 10.1016/j.cardfail.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Naschitz JE, Slobodin G, Lewis RJ, et al. Heart diseases affecting the liver and liver diseases affecting the heart. Am Heart J. 2000;140(1):111–20. doi: 10.1067/mhj.2000.107177. [DOI] [PubMed] [Google Scholar]
- 13.Poliwczak AR, Bialkowska J, Broncel M, et al. Heart rhythm turbulence and NT-proBNP in decompensated liver cirrhosis – a pilot study. Med Sci Monit. 2011;17(6):PR5–11. doi: 10.12659/MSM.881788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheer TA, Joo E, Runyon BA. Usefulness of serum N-terminal-ProBNP in distinguishing ascites due to cirrhosis from ascites due to heart failure. J Clin Gastroenterol. 2010;44(1):e23–26. doi: 10.1097/MCG.0b013e318198113b. [DOI] [PubMed] [Google Scholar]
- 15.Colli A, Pozzoni P, Berzuini A, et al. Decompensated chronic heart failure: increased liver stiffness measured by means of transient elastography. Radiology. 2010;257(3):872–78. doi: 10.1148/radiol.10100013. [DOI] [PubMed] [Google Scholar]
- 16.Ess M, Mussner-Seeber C, Mariacher S, et al. Gamma-glutamyltransferase rather than total bilirubin predicts outcome in chronic heart failure. J Card Fail. 2011;17(7):577–84. doi: 10.1016/j.cardfail.2011.02.012. [DOI] [PubMed] [Google Scholar]