Summary
Background
The aim of this study was to screen molecular biomarkers for biodosimetry from DNA repair-related gene expression profiles.
Material/Methods
Mice were subjected to whole-body exposure with 60Co γ rays with a dose range of 0–8 Gy at a dose rate of 0.80 Gy/min. RNA was extracted from the peripheral blood of irradiated mice at 4, 8, 12, 24 and 48hrs post-irradiation. The mRNA transcriptional changes of 11 genes related to DNA damage and repair were detected using real-time quantitative polymerase chain reaction (RT-PCR).
Results
Of the 11 genes examined, CDKN1A (cyclin-dependent kinase inhibitor 1A or p21, Cip1) and ATM (ataxia telangiectasia mutated) expression levels were found to be heavily up- and down-regulated, respectively, with exposure dose increasing at different post-irradiation times. RAD50 (RAD50 homolog), PLK3 (polo-like kinase 3), GADD45A (growth arrest and DNA damage-inducible, alpha), DDB2 (damage-specific DNA-binding protein 2), BBC3 (BCL2-binding component 3) and IER5 (immediate early response 5) gene expression levels were found to undergo significant oscillating changes over a broad dose range of 2–8 Gy at post-exposure time points observed. Three of the genes were found not to change within the observed exposure dose and post-radiation time ranges.
Conclusions
The results of this study add to the biodosimetry with biomarker data pool and will be helpful for constructing appropriate gene expression biomarker systems to evaluate radiation exposure doses.
Keywords: irradiation, DNA repair-related gene expression, RT-PCR, biological dosimetry
Background
The nuclear power plant radiation accident of ‘3.11’ in Japan refocussed attention on the environmental and health effects of wide-spread use of nuclear power. Proper protective and emergency response measures should be available during use of nuclear technologies. Rapid and accurate evaluation of exposure dose plays an important role in early triage, diagnosis and medical treatment of the victims during emergency response efforts in nuclear accidents. In view of some of the serious shortcomings and limits presented by physical and chemical methods used in evaluation of radiation exposure dose [1], biodosimetry has emerged as one of the best techniques for use in individual exposure dose evaluation in many situations such as medical rescue of radiation accident victims, bio-effects research of radiation and radiation protection. Because gene expression is sensitive to environmental factors, the analysis of gene expression profiling of peripheral blood cells, particularly lymphocytes, has been used to assess the presence of certain diseases [2–8]. Accordingly, many radiobiologists have focussed on gene expression profiling analysis to find biomarkers that are suitable for assessment of individual exposure doses under different exposure conditions [9–13]. Considerable work has been done on radiation-induced gene alterations using cultured cell lines and peripheral blood cells irradiated in vitro or ex vivo[14–19]. Several genes, including GADD45, CDKN1A and BBC3, have been identified as radiation response expression genes at different exposure doses and post-irradiation times in human peripheral blood lymphocytes and tissue cells [20–22]. Recent reports have shown that gene expression signatures induced by IR are specific, durable and accurate in prediction of exposure doses in both mice and humans ]23–25].
Since there is a highly relevant among exposure doses, DNA damage degree and DNA repair-related gene expression level, in this study 11 DNA damage response genes related to DNA repair (ATM, BRCA1 and RAD50), cell cycle regulation (CDKN1A, GADD45A), and apoptosis (BBC3) (Table 1) were selected as targets to screen potential bio-markers for indicating exposure dose. The radiation-induced transcription level of 11 genes was investigated in white blood cells of TBI-treated BALB/c mice with a RT-PCR method.
Table 1.
Gene descriptions.
| Symbol | Accession | Description and Function |
|---|---|---|
| Beta-actin | NM_007393.3 | Used for normalization |
| CDKN1A | NM_001111099.1 | Cyclin-dependent kinase inhibitor 1A (p21, Cip1); plays a regulatory role in S phase DNA replication and DNA damage repair |
| BBC3 | NM_133234.2 | BCL2 binding component 3; essential mediator of p53-dependent and p53-independent apoptosis |
| DDB2 | NM_028119.4 | Damage-specific DNA-binding protein 2; activated by UV and X-ray irradiation; it recognizes pyrimidine dimers and increased p53 expression |
| GADD45A | NM_007836.1 | Growth arrest and DNA damage-inducible, alpha; the protein responds to environmental stresses by mediating activation of the p38/JNK pathway |
| IER5 | NM_010500.2 | Immediate early response 5; plays an important role in mediating the cellular response to mitogenic signals |
| PLK3 | NM_013807.2 | Polo-like kinase 3 (Drosophila); critical regulators of cell cycle progression, mitosis, cytokinesis and the DNA damage response |
| ATM | NM_007499.2 | Ataxia telangiectasia mutated; activated by double-strand breaks (DSB), acting as a DNA damage sensor |
| BRCA1 | NM_009764.3 | Breast cancer 1; maintains genomic stability, plays a role in transcription, DNA repair of DSBs, and recombination |
| XPC | NM_009531.2 | Xeroderma pigmentosum, complementation group; encodes a component of the nucleotide excision repair (NER) pathway |
| FDXR | NM_007997.1 | Ferredoxin reductase; encodes a mitochondrial flavoprotein that initiates electron transport for cytochromes P450 receiving |
| RAD50 | NM_009012.2 | Component of the MRN complex which plays a central role in DSB repair, DNA recombination, maintenance of telomere integrity and meiosis |
Material and Methods
Animals and exposure
Male BALB/c mice, 6–8 weeks old, provided by the Animal Center of the Second Military Medical University were divided into control (non-irradiated) and 4 exposure groups (5 mice in each group). The mice in the exposure groups were exposed to either 2, 4, 6 or 8 Gy doses of 60Co γ-rays with TBI at 0.80 Gy/min dose rate and room temperature (23±2°C). The 60Co γ-rays source (Shanghai Institute of Measurement and Testing Technology, SIMT China) provided a 98% uniform exposure field in 15×13 cm sides, which was measured with a graphite cavity ionization chamber. The mice were immobilized using a plastic restrainer during exposure. They were housed in a pathogen-free barrier facility (12-hour light/dark cycle) and were fed autoclaved standard rodent chow pre- and post-irradiation. All protocols were approved by the University Committee on Animal Research, and all experiments were carried out in accordance with related guidelines. All samples were run 3 times and a total of 375 mice including control group were used.
Blood sample collection and total RNA isolation
For each mouse approximately 0.5–0.7 ml of heparin-anticoagulated blood was collected from the orbital venous vein using polypropylene tubes at each post-irradiation time point.
Total RNA was extracted from whole blood using the Axygen Blood Total RNA Miniprep Kit, No. AP-MN-BL-RNA-250 (Axygen Scientific, Hangzhou, China) following the manufacturer’s recommendations. The red blood cells were removed using an AxyPrep special lysis buffer. Isolated white blood cells (WBCs) were lysed, and genomic DNA and protein were removed via precipitation. WBCs RNA was then isolated. The RNA was quantified using a NanoDrop-1000 spectrophotometer (Thermo Scientific, Waltham, MA), and the quality of the isolated RNA was assayed with agarose gel electrophoresis by analyzing 18S and 28S RNA.
Quantitative RT-PCR
Using the SYBR Prime Script RT Reagent Kit, No. DRR037A (Takara, Dalian, China), 1 μg of total RNA was reversely transcribed to cDNA according to the manufacturer’s instructions. The RT-PCR reactions were performed with the Rotor-Gene 6000 RT-PCR System using the SYBR Premix Ex Taq, No. DRR041A (Takara, Dalian, China) following the manufacturer’s recommendations. All samples were run 3 times. The RT-PCR cycling conditions were as follows: initial denaturation at 95°C for 10 min, followed by 35 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 30 s and primer extension at 72°C for 30 s. The final extension occurred at 72°C for 10 min. Relative fold inductions were calculated by the Ct method with averaged relative levels of beta-actin, which was used for normalization. The sequences of primers that were used for amplification are shown in Table 2. The primers were designed and synthesized by Invitrogen™ (Shanghai).
Table 2.
RT-PCR primers.
| Gene | Accession | Left primer | Right primer |
|---|---|---|---|
| Beta-actin | NM_007393.3 | AGGCTGTGCTGTCCCTGTATG | ACCCAAGAAGGAAGGCTGGAAA |
| CDKN1A | NM_001111099.1 | GTCCCACTTTGCCAGCAGAATAA | GGTCGGACATCACCAGGATTG |
| ATM | NM_007499.2 | GCACACGGATTGCTCAAGGA | GCCCATTCGGAATATGGATCAG |
| RAD50 | NM_009012.2 | CCAGGGACAGACTTGCCAAAC | TCCAAATCTTGGCTACCACAAACA |
| GADD45A | NM_007836.1 | CCTGCACTGTGTGCTGGTGA | CCACTGATCCATGTAGCGACTTTC |
| FDXR | NM_007997.1 | GCCCAGCTGGCTTCTACACA | AGGATGGTCAGGTGCCACA |
| XPC | NM_009531.2 | GTGGACCAAGGCACTGATGAAG | ACAGGCATGTCAGACGGTGAG |
| BRCA1 | NM_009764.3 | TCTGAAGACTGCTCGCAGAGTGATA | CCAGCACAGCTTCCAGGTGA |
| IER5 | NM_010500.2 | TTCAGACGCCGAGGGTACAAC | ATCCTGCCTTCGCTTCCAGA |
| PLK3 | NM_013807.2 | TCATCACGGATAACATGGAACTGAA | CACGTAGTTGGGAGTGCCACA |
| DDB2 | NM_028119.4 | AAGTTGGGCAAAGCCACCTG | GTGCCATGCCAAGGACGTAG |
| BBC3 | NM_133234.2 | GACCTCAACGCGCAGTACGA | GCTCCAGGATCCCTGGGTAA |
Statistical analysis
The results are listed as the means ± standard deviation (SD). Statistical analysis was performed with the GraphPad Prism 5.0 software package (GraphPad Software, USA). The statistical significance of differences between groups was analyzed by one-way analysis of variance with a post-hoc Tukey test. The significance level was set at P=0.05.
Results
Radiation responses of CDKN1A and ATM genes
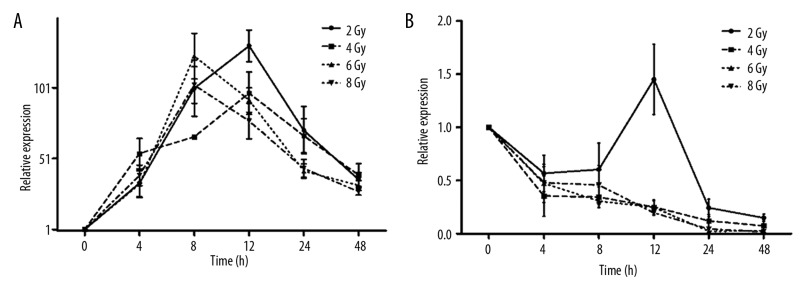
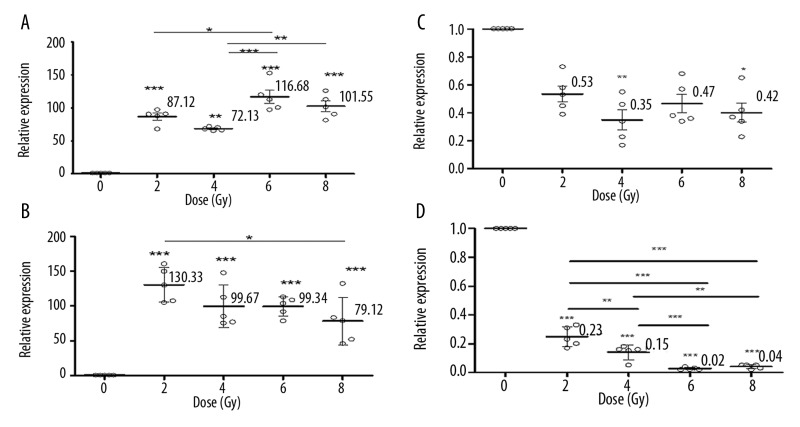
CDKN1A, which has a regulatory role in S phase DNA replication and is known to be activated by the tumor protein p53 (TP 53), had the strongest upregulation in expression level out of the 11 genes investigated in this study. As shown in Figure 1A, compared with the non-irradiated control, CDKN1A gene expression gradually increased to a maximum value of approximately 88-fold for 2 Gy (p<0.0001) and 130-fold for 4 Gy (p<0.0001) at 12 h post-irradiation, and approximately 116-fold for 6 Gy (p<0.0001) and 100-fold for 8 Gy (p<0.0001) at 8 h post-exposure. Then, CDKN1A gene expression gradually decreased but remained greater than 30-fold (p<0.05) higher than that of the control group at 48 h post-irradiation for all exposure doses. Figure 2A and B show the significant differences in CDKN1A gene expression induced by the different exposure doses. Significant differences can be seen by comparing the exposure groups of 2 and 6 Gy (p<0.05), 4 and 6 Gy (p<0.0001), and 4 and 8 Gy (p<0.001) at 8 h post-exposure (Figure 2A). At the 12 h post-irradiation time point, CDKN1A gene expression decreased along with an increase in exposure dose. The only significant difference was between 2 and 8 Gy.
Figure 1.
Temporal responses of CDKN1A (A) and ATM (B) gene expression at different exposure doses are plotted. Points denote the mean of responses in 5 different mice; error bars denote SD.
Figure 2.
The dose response of CDKN1A at 8 h (A), 12 h (B) and the dose response of ATM at 4 h (C) and 24 h (D) post-irradiation. The points denote the mean responses for 5 different mice; error bars denote SD. Asterisks indicate a significant difference in the relative values of expression among the control and irradiation groups and among the different dose groups at the indicated dose point.
ATM is a predominantly nuclear protein that is involved in the DNA damage repair signal transduction process. In this study, ATM gene expression decreased linearly with the exposure dose, with the exception of the 12 h post-irradiation time-point after a 2 Gy exposure (Figure 1B). Compared with non-irradiated controls, it could be determined from the time course of ATM gene expression (Figure 1B) that the gene was strongly downregulated in all exposure dose ranges at 4–48 h post-irradiation. A significant decrease was found for the 4 Gy (p<0.001) and 8 Gy (p<0.05) irradiation groups at 4 h post-irradiation (Figure 2C), and highly significant changes across the dose range of 2–8 Gy (p<0.0001) were found at 24 h post-irradiation (Figure 2D). Significant differences can also be seen by comparing the exposure groups of 2 and 4 Gy (p<0.001), 2 and 6 Gy (p<0.0001), 2 and 8 Gy (p<0.0001), 4 and 6 Gy (p<0.0001), and 4 and 8 Gy (p<0.001) at 24 h post-exposure (Figure 2D).
Radiation responses of the other tested genes
The genes BBC3, XPC, PLK3, RAD50, DDB2, FDXR, GADD45A, BRCA1 and IER5 are largely involved in DNA repair or cell cycle regulation associated with DNA repair. The results show that genes BRCA1, XPC and FDXR had no significant change in expression level post-irradiation across all observed dose ranges and post-irradiation time-points. The time- and dose-dependent responses of 6 other genes (BBC3, PLK3, DDB2, GADD45A, RAD50 and IER5) are shown in Figure 3.
Figure 3.
The dose responses of the expression levels of 6 genes after radiation exposure are plotted. The points denote the mean responses for 5 different mice; error bars denote SD.
The changes in expression levels of these genes were upregulated and downregulated in an oscillating pattern across the observed time-points within certain dose ranges. To determine which genes could potentially characterize exposure doses at different post-irradiation time-points, the results are charted in Figure 4. Because the change in CDKN1A expression is substantial, it is not included in Figure 4. The summary of relative expression levels of genes, which are significantly different from the control group, is shown in Table 3. These changes may serve as a useful biomarker for exposure dose evaluation.
Figure 4.
Relative values of IER5, GADD45A, PLK3, DDB2, BBC3 and ATM gene expression levels are plotted, and the dashed line indicates the non-irradiated control ratio. Data points denote the mean ±SD of 5 mice per treatment.
Table 3.
Summary of genes modulated by IR at different post-irradiation time points and exposure doses.
| 2 Gy | 4 Gy | 6 Gy | 8 Gy | |
|---|---|---|---|---|
| 4 h | ATM*(0.35), CDKN1A** (54.60) |
DDB2* (0.40), PLK3* (0.22) |
ATM* (0.42), BBC3* (5.72), CDKN1A* (39.13) |
|
| 8 h | ATM* (0.60), GADD45A* (1.53), IER5** (2.87), CDKN1A*** (86.68) |
ATM**(0.34), CDKN1A*** (68.37) |
ATM** (0.34), CDKN1A*** (68.37) |
ATM** (0.46), IER5** (2.84), BBC3* (2.40), CDKN1A*** (102.38) |
| 12 h | DDB2*** (0.44), CDKN1A*** (126.79) |
ATM** (0.25), DDB2*** (0.33), PLK3*** (1.9), IER5* (3.27), CDKN1A*** (96.485) |
ATM** (0.24), DDB2*** (0.27), CDKN1A*** (92.373) |
ATM** (0.20), DDB2*** (0.18), PLK3** (1.8), CDKN1A*** (77.58) |
| 24 h | ATM** (0.24), PLK3*** (0.55), BBC3* (2.21), CDKN1A*** (70.99), RAD50*** (0.28) |
ATM*** (0.12), PLK3*** (0.48), GADD45A** (2.01), BBC3*** (2.99), CDKN1A*** (67.11), RAD50*** (0.21) |
ATM*** (0.02), PLK3*** (0.25), GADD45A** (1.70), BBC3* (2.11), RAD50*** (0.09) |
ATM*** (0.04), PLK3*** (0.48), GADD45A* (1.38), BBC3** (2.45), CDKN1A* (40.75), RAD50*** (0.13) |
| 48 h | ATM*** (0.15), DDB2*** (0.30), PLK3** (0.48), CDKN1A*** (36.44), RAD50*** (0.40) |
ATM*** (0.07), DDB2*** (0.19), PLK3** (0.44), GADD45A** (2.25), BBC3** (5.42), CDKN1A*** (39.33), RAD50*** (0.08) |
ATM*** (0.03), DDB2*** (0.21), PLK3** (0.48), GADD45A* (1.89), BBC3** (4.78), CDKN1A*** (32.04), RAD50*** (0.11) |
ATM*** (0.01), DDB2*** (0.15), GADD45A* (1.57), IER5** (2.28), BBC3* (4.04), CDKN1A* (29.61), RAD50*** (0.07) |
The values in brackets represent the relative expression levels compared to the control group. Asterisks indicate a significant difference in expression values between the control and radiation treated group at the indicated dose and time point (*P<0.05; **P<0.001; ***P<0.0001).
Discussion
Gene expression analysis following ionizing radiation as a biomarker for individual exposure dose estimation and radiation effect prediction is becoming increasing attention [26–28]. As one of the most radiosensitive targets in mammalian cells, DNA damage degree induced by IR was largely dependent on type of radiation (LET), dose deposit in the cell, and dose rate [29]. An instinctive protection mechanism in living organisms can repair DNA damage induced by IR within certain exposure dose ranges through initiating repair-related gene expression and protein activity. In this process the quantity of repair-related gene expression and protein activating should be proportional to DNA damage degree and then be logically linked with exposure dose. Therefore the information associated with DNA damage repair-related gene expression and protein activity may be seen as a huge data mine from which to derive biological markers for exposure dose evaluation with biodosimetry.
In this study significant and meaningful exposure doses and observed post-radiation time ranges in diagnosis and treatment of acute radiation sickness were selected to obtain more complete gene expression profiles following different radiation doses and different post-irradiation times. To enhance accuracy and reliability of the results, radiosensitive mouse species and an accurate irradiation source with well-distributed exposure dose field were selected. Most of the target genes observed here were proved prior to this study as radiation response genes, and some of them were shown to have a well-defined dose-effect pattern in the changes of expression level when analysis was done with separated or cultured cells irradiated in vitro or ex vivo.
ATM, as an important protein in the cellular response to IR, plays a critical role in initiating the repair signaling pathway of DNA damage. IR could induce phosphorylation of serine 1981 of ATM protein, and phosphorylated ATM protein would pass the activating signal to downstream repair-related proteins. When DNA repair was finished, phosphorylated ATM protein would be dephosphorylated [30]. It has been reported that ATM protein level did not change during the radiation-induced repair process of DNA DSBs, but protein kinase activity of ATM might be changed following exposure doses when checking was done by a fluorescent foci observation [31–34]. In the present study, the change in transcriptional level of the ATM gene was first found to be downregulated in a dose-and post-irradiation time-dependent pattern, with the exception of the 2 Gy exposure dose at 12 h post-exposure, the reason for which requires further investigation. This finding implies that the information coming from ATM gene expression after radiation cannot be neglected as a biomarker in exposure dose indicating.
Strongly upregulated expression of the CDKN1A gene shown in this study slightly different from previous findings that showed only a several- or 10-fold increase in CDKN1A gene expression after radiation (from 4-fold to 10- or 50-fold or greater increase) [11,16,35–38]. It is supposed that the difference of testing mode, primary design of CDKN1A gene composition and application of the primaries might be responsible for the diversity of the results. For example, Mitsuhashi et al. [39] reported that multiple primary applications in CDKN1A gene composition showed higher induction of CDKN1A mRNA than single primary and control groups when leukocytes from multiple primary breast cancer patients were irradiated. The difference of mouse strain in experiment, exposure and treatment methods of the sample are also factors resulting in diversity of CDKN1A mRNA results.
The results of the present study show that peaked expression of CDKN1A gene appeared at 12 h post-irradiation for doses of 2 and 4 Gy and at 8 h post-irradiation for doses of 6 and 8 Gy, which is well in accord with the cell cycle arrest necessity for DNA repair after radiation. As an inhibitor of cell cycle process, radiation-induced DNA repair needs more CDKN1A protein expression. The heavier DNA damage caused by high dose the more CDKN1A protein needed to satisfy the demand of DNA repair, which accompanyed with a peak CDKN1A gene expression in the earlier phase of DNA repair. Combining our results with those of Dressman [25] who found only CDKN1A was in common between a PB signature of human TBI and the PB signatures of partial body irradiation exposure, suggesting that the information from CDKN1A gene expression can be a reliable and representative biomarker in estimating exposure dose within certain dose ranges.
DDB2, a damage-specific DNA-binding protein 2 that can be activated by UV and X-ray irradiation, had its gene expression level reduced to approximately 20% of the initial expression level with the radiation dose increases in this study. This result is not consistent with previous reports that showed the DDB2 gene was upregulated in both in vivo and in vitro experiments [16,25,35]. Different experimental conditions among studies might cause these differences. For example, some results come from just 1 TBI-treated patient and 6 hours to 4 days of observation time, and the study was performed by fractionated irradiation with a low dose and dose rate. In this study exposure dose was delivered once and dose rate was nearly 8-fold higher than mentioned above. Because sublethal damage repair and somewhat adaptive response exist during fractionated irradiation by low dose and dose rate, the DDB2 expression level increase was within the range of what one would expect. Recently, Stoyanova [40,41] reported that DDB2 could indirectly participate in nucleotide excision repair (NER) by regulating the cellular levels of p21Waf1/Cip1 (CDKN1A), and DDB2-deficient cells exhibited a significantly higher accumulation of p21Waf1/Cip1 (CDKN1A). They believed that DDB2 plays a critical role in attenuating the level of p21Waf1/Cip1 to allow apoptosis in response to DNA-damaging agents. Given their conclusions, our result that CDKN1A was highly upregulated and DDB2 was downregulated show a reasonable but unclear link when radiation response of 2 genes was evaluated.
GADD45A has previously been proposed as a potential dosimeter based on the linear dose response relationship observed in human cells that were irradiated in vitro[42], but the stress-response regulation of this gene was known to be complex. Decreasing expression of this gene was observed using WBC [14,36] and in many p53 wild-type cell lines when cells were irradiated in vitro; however, other reports have also shown that ionizing radiation did not induce GADD45A expression change in a subset of p53 wild-type cell lines [43,44]. In our study, GADD45A expression was upregulated approximately 2–3-fold only at the 48 h post-irradiation time-point within all exposure dose ranges, and no significant changes were found at other time-points. Different experimental subjects and exposure patterns might result in different results. For example, the gene expression and regulation patterns in TBI mice might be more complex compared to the cultured or isolated cells irradiated in vitro or ex vivo. The sensitivity of analytic methods could also result in differences in quantification of gene expression [45].
In hunting for biomarkers capable of indicating individual exposure dose, linear relationship with exposure dose and sensitivity of these markers are expected, but these conditions are hard to satisfy in reality. It is possible that selecting and gathering non-linear change genes together, which induced by different exposure doses and up- or downregulated, at different post-exposure times, form many biomarker groups which cantian many significantly up- or downregulated genes to distinguish and evaluate different threshold doses for medical triage and diagnosis purpose. For example, in Table 3, at 4 h post-irradiation, the gene profiles of ATM, XPC and CDKN1A, with statistically significant difference compared with unirradiated control group, can characterize a 4 Gy exposure dose, and the information coming from DDB2, XPC and PLK3 gene expression difference can characterize a 6 Gy exposure dose, and in the same way the gene expression difference information from ATM, BBC3, XPC and CDKN1A can characterize a 8 Gy exposure dose. In the situation where the gene expression profiles are the same in 2 different dose scales, such as 4 and 6Gy exposure groups at 8 h post-exposure in Table 3, further gene expression information screening is necessary until some genes are finally found with expression differences between the 2 different doses. Then this information on different genes can be selected and grouped based on the time elapsed after radiation exposure to identify and distinguish different exposure doses. Additionally, given that ATM and CDKN1A gene expression levels were significantly changed across the dose range and time course (0–48 h), it may be possible to use these 2 genes as biomarkers to distinguish people exposed to radiation from those who were not exposed, to reassure the “worried well” and to assign those with significant exposure to the appropriate medical care.
Conclusions
On the whole, although gene expression analysis is promising as a biomarker to indicate exposure dose, many problems remain to be solved before the method can be used to evaluate exposure in real-life settings. The present work represents part of the effort to establish this evaluation tool.
Footnotes
Source of support: Research was supported by the National Natural Scientific Foundation (30970874)
References
- 1.Al-Mohammed H, Mahyoub F, Moftah B. Comparative study on skin dose measurement using MOSFET and TLD for pediatric patients with acute lymphatic leukemia. Med Sci Monit. 2010;16(7):CR325–29. [PubMed] [Google Scholar]
- 2.Kowalski M, Bielecka-Kowalska A, Oszajca K, et al. Manganese superoxide dismutase (MnSOD) gene (Ala-9Val, Ile58Thr) polymorphism in patients with age-related macular degeneration (AMD) Med Sci Monit. 2010;16(4):CR190–96. [PubMed] [Google Scholar]
- 3.Wang Z, Liu H, Liu B, et al. Gene expression levels of CSNK1A1 and AAC-11, but not NME1, in tumor tissues as prognostic factors in NSCLC patients. Med Sci Monit. 2010;16(8):CR357–64. [PubMed] [Google Scholar]
- 4.Edwards CJ, Feldman JL, Beech J, et al. Molecular profile of peripheral blood mononuclear cells from patients with rheumatoid arthritis. Mol Med. 2007;13(1–2):40–58. doi: 10.2119/2006-000056.Edwards. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandel M, Achiron A. Gene expression studies in systemic lupus erythematosus. Lupus. 2006;15(7):451–56. doi: 10.1191/0961203306lu2332oa. [DOI] [PubMed] [Google Scholar]
- 6.Ramilo O, Allman W, Chung W, et al. Gene expression patterns in blood leukocytes discriminate patients with acute infections. Blood. 2007;109(5):2066–77. doi: 10.1182/blood-2006-02-002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg S, Elashoff MR, Beineke P, et al. Multicenter validation of the diagnostic accuracy of a blood-based gene expression test for assessing obstructive coronary artery disease in nondiabetic patients. Ann Intern Med. 2010;153(7):425–34. doi: 10.7326/0003-4819-153-7-201010050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas GP, Brown MA. Genomics of ankylosing spondylitis. Discov Med. 2010;10(52):263–71. [PubMed] [Google Scholar]
- 9.Paul S, Barker CA, Turner HC, et al. Prediction of in vivo radiation dose status in radiotherapy patients using ex vivo and in vivo gene expression signatures. Radiat Res. 2011;175(3):257–65. doi: 10.1667/RR2420.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brengues M, Paap B, Bittner M, et al. Biodosimetry on small blood volume using gene expression assay. Health Phys. 2010;98(2):179–85. doi: 10.1097/01.HP.0000346706.44253.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul S, Amundson SA. Development of gene expression signatures for practical radiation biodosimetry. Int J Radiat Oncol Biol Phys. 2008;71(4):1236–44. doi: 10.1016/j.ijrobp.2008.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turtoi A, Brown I, Oskamp D, Schneeweiss FH. Early gene expression in human lymphocytes after gamma-irradiation-a genetic pattern with potential for biodosimetry. Int J Radiat Biol. 2008;84(5):375–87. doi: 10.1080/09553000802029886. [DOI] [PubMed] [Google Scholar]
- 13.Turtoi A, Brown I, Schlager M, Schneeweiss FH. Gene expression profile of human lymphocytes exposed to (211)at alpha particles. Radiat Res. 2010;174(2):125–36. doi: 10.1667/RR1659.1. [DOI] [PubMed] [Google Scholar]
- 14.Blakely WF, Prasanna PG, Grace MB, Miller AC. Radiation exposure assessment using cytological and molecular biomarkers. Radiat Prot Dosimetry. 2001;97(1):17–23. doi: 10.1093/oxfordjournals.rpd.a006633. [DOI] [PubMed] [Google Scholar]
- 15.Amundson SA, Lee RA, Koch-Paiz CA, et al. Differential responses of stress genes to low dose-rate γ irradiation. Mol Cancer Res. 2003;1(6):445–52. [PubMed] [Google Scholar]
- 16.Amundson SA, Grace MB, McLeland CB, et al. Human in vivo radiation-induced biomarkers: gene expression changes in radiotherapy patients. Cancer Res. 2004;64(18):6368–71. doi: 10.1158/0008-5472.CAN-04-1883. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg Z, Schwietert CW, Lehnert B, et al. Effects of low-dose ionizing radiation on gene expression in human skin biopsies. Int J Radiat Oncology Biol Phys. 2004;58(2):567–74. doi: 10.1016/j.ijrobp.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 18.Khodarev NN, Park JO, Yu J, et al. Dose-dependent and independent temporal patterns of gene responses to ionizing radiation in normal and tumor cells and tumor xenografts. Proc Natl Acad Sci USA. 2001;98(22):12665–70. doi: 10.1073/pnas.211443698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khodarev NN, Kataoka Y, Murley JS, et al. Interaction of amifostine and ionizing radiation on transcriptional patterns of apoptotic genes expressed in human microvascular endothelial cells (HMEC) Int J Radiat Oncol Biol Phys. 2004;60(2):553–63. doi: 10.1016/j.ijrobp.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 20.Kang CM, Park KP, Song JE, et al. Possible biomarkers for ionizing radiation exposure in human peripheral blood lymphocytes. Radiat Res. 2003;159(3):312–19. doi: 10.1667/0033-7587(2003)159[0312:pbfire]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55(22):5187–90. [PubMed] [Google Scholar]
- 22.Mori M, Benotmane MA, Tirone I, et al. Transcriptional response to ionizing radiation in lymphocyte subsets. Cell Mol Life Sci. 2005;62(13):1489–501. doi: 10.1007/s00018-005-5086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meadows SK, Dressman HK, Daher P, Lucas J, Nevins JR, Chute JP, et al. Diagnosis of partial body radiation exposure in mice using peripheral blood gene expression profiles. PLoS One. 2010;5(7):e11535. doi: 10.1371/journal.pone.0011535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meadows SK, Dressman HK, Muramoto GG, et al. Gene expression signatures of radiation response are specific, durable and accurate in mice and humans. PLoS One. 2008;3(4):e1912. doi: 10.1371/journal.pone.0001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dressman HK, Muramoto GG, Chao NJ, et al. Gene expression signatures that predict radiation exposure in mice and humans. PLoS Med. 2007;4(4):e106. doi: 10.1371/journal.pmed.0040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filiano AN, Fathallah-Shaykh HM, Fiveash J, et al. Gene Expression Analysis in Radiotherapy Patients and C57BL/6 Mice as a Measure of Exposure to Ionizing Radiation. Radiat Res. 2011;176(1):49–61. doi: 10.1667/RR2419.1. [DOI] [PubMed] [Google Scholar]
- 27.Kabacik S, Mackay A, Tamber N, et al. Gene expression following ionizing radiation: identification of biomarkers for dose estimation and prediction of individual response. Int J Radiat Biol. 2011;87(2):115–29. doi: 10.3109/09553002.2010.519424. [DOI] [PubMed] [Google Scholar]
- 28.Paul S, Barker CA, Turner HC, et al. Prediction of in vivo radiation dose status in radiotherapy patients using ex vivo and in vivo gene expression signatures. Radiat Res. 2011;175(3):257–65. doi: 10.1667/RR2420.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson GA. Fundamental space radiobiology. Gravit Space Biol Bull. 2003;16(2):29–36. [PubMed] [Google Scholar]
- 30.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421(6922):499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki K, Okada H, Yamauchi M, et al. Qualitative and quantitative analysis of phosphorylated ATM foci induced by low-dose ionizing radiation. Radiat Res. 2006;165(5):499–504. doi: 10.1667/RR3542.1. [DOI] [PubMed] [Google Scholar]
- 32.Costes SV, Boissière A, Ravani S, et al. Imaging features that discriminate between foci induced by high- and low-LET radiation in human fibroblasts. Radiat Res. 2006;165(5):505–15. doi: 10.1667/RR3538.1. [DOI] [PubMed] [Google Scholar]
- 33.Costes SV, Chiolo I, Pluth JM, et al. Spatiotemporal characterization of ionizing radiation induced DNA damage foci and their relation to chromatin organization. Mutat Res. 2010;704(1–3):78–87. doi: 10.1016/j.mrrev.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canman CE, Lim DS, Cimprich KA, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281(5383):1677–79. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 35.Paul S, Amundson SA. Development of gene expression signatures for practical radiation biodosimetry. Int J Radiat Oncol Biol Phys. 2008;71(4):1236–44. doi: 10.1016/j.ijrobp.2008.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amundson SA, Do KT, Fornace AJ., Jr Induction of stress genes by low doses of gamma rays. Radiat Res. 1999;152(3):225–31. [PubMed] [Google Scholar]
- 37.Kis E, Szatmari T, Keszei M, et al. Microarray analysis of radiation response genes in primary human fibroblasts. Int J Radiat Oncol Biol Phys. 2006;66(5):1506–14. doi: 10.1016/j.ijrobp.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Warters RL, Packard AT, Kramer GF, et al. Differential gene expression in primary human skin keratinocytes and fibroblasts in response to ionizing radiation. Radiat Res. 2009;172(1):82–95. doi: 10.1667/RR1677.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitsuhashi M, Peel D, Ziogas A, Anton-Culver H. Enhanced Expression of Radiation-induced Leukocyte CDKN1A mRNA in Multiple Primary Breast Cancer Patients: Potential New Marker of Cancer Susceptibility. Biomark Insights. 2009;4:201–9. doi: 10.4137/bmi.s3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoyanova T, Roy N, Kopanja D, et al. DDB2 decides cell fate following DNA damage. Proc Natl Acad Sci USA. 2009;106(26):10690–95. doi: 10.1073/pnas.0812254106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoyanova T, Roy N, Kopanja D, et al. DDB2 (damaged-DNA binding protein 2) in nucleotide excision repair and DNA damage response. Cell Cycle. 2009;8(24):4067–71. doi: 10.4161/cc.8.24.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grace MB, McLeland CB, Blakely WF. Real-time quantitative RT-PCR assay of GADD45 gene expression changes as a biomarker for radiation biodosimetry. Int J Radiat Biol. 2002;78(11):1011–21. doi: 10.1080/09553000210158056. [DOI] [PubMed] [Google Scholar]
- 43.Zhan Q, Carrier F, Fornace AJ., Jr Induction of cellular p53 activity by DNA-damaging agents and growth arrest. Mol Cell Biol. 1993;13(7):4242–50. doi: 10.1128/mcb.13.7.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bae I, Smith ML, Sheikh MS, et al. An abnormality in the p53 pathway following gamma-irradiation in many wild-type p53 human melanoma lines. Cancer Res. 1996;56(4):840–47. [PubMed] [Google Scholar]
- 45.Snyder AR, Morgan WF. Gene expression profiling after irradiation: clues to understanding acute and persistent responses? Cancer Metastasis Rev. 2004;23(3–4):259–68. doi: 10.1023/B:CANC.0000031765.17886.fa. [DOI] [PubMed] [Google Scholar]