Summary
Background
Quality of life (QOL) has increasingly become a factor in management decisions in patients with chronic diseases. Chronic pancreatitis (CP) is a debilitating disorder that causes not only pain and endo/exocrine insufficiency but is also connected with some social issues. The aim of this study was to assess QOL in patients with chronic pancreatitis in correlation with the disease activity or the environmental/social factors that can influence their well-being.
Material/Methods
The study group comprised 43 patients with CP: M/F 37/6; mean age 47.9±8.6; range: 30–74 yrs. The control group consisted of 40 healthy volunteers of comparable demographics. Different degrees of CP activity were defined using the Cambridge classification. Pain intensity and frequency were assessed using a pain index. QOL was assessed using the Short-Form-36 questionnaire.
Results
Mean QOL scores in CP were lower compared to the control group in all SF-36 domains, particularly in general health perception, physical functioning, role-physical (p<0.001) and vitality (p<0.05). We observed correlation of QOL results and pain index in all domains, and number of the disease relapses and body weight in 5 out of 8 domains (p<0.001 and p<0.05, respectively). The worst QOL scores were obtained in retired patients, as well as in unemployed persons in almost all SF-36 domains (p<0.001).
Conclusions
Chronic pancreatitis significantly impairs patients’ quality of life. Severity of abdominal pain, low body weight, and loss of work were the factors most closely associated with poor health status perception.
Keywords: chronic pancreatitis, quality of life, SF-36
Background
Chronic pancreatitis (CP) as a chronic and progressive condition requires a specific approach for quality of life (QOL) assessment. As a chronic disease with debilitating symptoms, consequences of diabetes mellitus, malabsorption and weight loss and possibility of complications (including cancer), CP severely affects QOL [1–3].
Numerous studies have shown the heterogeneous character of the disease, with the interaction of physical, psychological and social factors [1,4,5].
As long as the pathophysiological background of pain in CP remains uncertain, the management of CP patients is based on a trial and error approach. Commonly used therapeutic methods of treatment remain insufficient because patients’ quality of life is highly impaired [1–3]. Chronic pancreatitis cannot be cured and may require many clinical interventions and frequent hospitalization. Quality of life is the priority outcome measure in chronic untreatable diseases. This also requires a high level of patient-doctor communication and compliance. Alcohol abuse and disease-related unemployment have negative impacts on coping with the disease. Chronic pancreatitis affects all aspects of patients’ lives: work, leisure, travel and relationships. It is not surprising that the multidimensional approach should be taken with these patients [1–3].
It is well documented that quality of life is an important variable and should be measured in CP patients. However, the available data in this field are not comparable because different QOL assessment methods were used [1,4–6].
The aim of the study was to assess QOL in patients with chronic pancreatitis and the correlation with the disease activity and selected environmental/social factors.
Material and Methods
The study group comprised 43 patients with CP: M/F 37/6; mean age 47.9±8.6; range: 30–74 yrs. Patients with chronic pancreatitis were recruited from those hospitalized in the Department of Digestive Tract Diseases, Medical University of Lodz, Poland.
The diagnosis of chronic pancreatitis was made by a combination of imaging, functional, pathologic and clinical findings. The degree of pancreatic damage was assessed using the Cambridge classification, where first degree (I) described less than 3 abnormal side branches of main duct; II – more than 3 abnormal side branches, III – abnormal main duct and branches and IV – plus 1 or more of: large cavities (>10 mm), gross gland enlargement (>2×), intraduct filling defects or calculi, duct obstruction, stricture or gross irregularity and contiguous organ invasion (7). In grades II and III there are 2 or more pathological signs according to computed tomography assessment: main duct enlarged (<4 mm), gland enlarged (<2×), cavities (<10mm), irregular ducts, duct wall echoes increased, irregular head contour [7].
Alcohol etiology was diagnosed when alcohol intake exceeded 80 g per day for at least 5 years.
QOL was assessed using 8 domains of the 36-item Short-Form Health Survey (SF-36), which is a short form measure of generic health status in the general population, designed for self-administration (8,9). SF-36 describes limitations in physical activity because of health problems (physical functioning -PF), limitations in social activities because of physical or emotional problems (social functioning – SF), limitations in usual role activities (role-physical – RP), presence of pain and limitations due to pain (bodily pain – BP), self-evaluation of personal health (general health perception – GH), psychological distress and well-being (mental health – MH), limitations in usual role activities because of emotional problems (role-emotional – RE), and energy and fatigue (vitality – VT). Scales range from 0–100, with higher scores indicating better functioning and well being [8,9].
The control group consisted of 40 healthy volunteers with matched demographic features. There is no Polish normative data using the SF-36 questionnaire. Pain assessment was performed according to Talley et al, who proposed a pain index (PI) – an additive model of pain frequency and intensity [5,10]. The frequency of abdominal pain attacks was described by 0–4 points: 0, once a year or less; 1, several times a year; 2, several times a month; 3, several times a week; and 4, daily. The intensity of abdominal pain was also described by 0–4 points from a UAS score: 0, no pain; 1, (1–25); 2, (26–50); 3, (51–75); and 4, (76–100), where 0 means no pain and 100 means the worst pain. The pain index was defined as the sum of points for frequency and intensity of abdominal pain [5,10].
The assessment interview and chart review recorded demographic data and disease history. The clinical and socio-demographic characteristics of the patients are shown in Table 1. The study was carried out in accordance with the Helsinki Declaration. The study protocol was approved by the local ethics committee of the Medical University of Lodz.
Table 1.
The sociodemographic characteristics of the study group.
| Features | Number of patients (%) |
|---|---|
| Etiology | Alcohol – 31 (72%) |
| Other – 12 (28%) | |
|
| |
| Complications | Diarrhea – 16 (37%) |
| Diabetes – 20 (46%) | |
|
| |
| Treatment | Surgical – 14 (32%) |
| Endoscopic – 8 (19%) | |
|
| |
| Smoking | Smokers – 38 (88%) |
| Non-smokers – 5 (12%) | |
|
| |
| Education | Primary – 13 (30%) |
| Technical – 8 (17%) | |
| Secondary – 19 (44%) | |
| University – 3 (7%) | |
|
| |
| Employment | Working – 12 (28%) |
| Unemployed – 9 (21%) | |
| Disease-related retired – 19 (44%) | |
| Retired due to age – 3 (7%) | |
|
| |
| Marital status | Single – 5 (12%) |
| Married – 31 (72%) | |
| Divorced – 6 (14%) | |
| Widowed – 1 (2%). | |
Statistical analysis was performed using the F-Snedecor test, the T test, and the Cochran-Cox test.
Results
Among patients studied, most (23 patients, 53%) had normal weight (BMI 15–34; mean 23.1 kg/m2); 2 patients were obese, 13 were overweight and 5 had BMI under 18.
Mean disease duration was 9 yrs (range 1–46), with 7.3 mean number of disease relapses (range 0–30).
The Pain Index mean result was 3.3 points, with range between 0 to 8 points. Mean pain intensity was 2 points, and mean frequency was 1.5 using PI. Frequency and intensity varied greatly, from 0 to maximum (4 points). However, most patients had the smallest intensity and frequency, what is also seen in PI points – 11 patients with 0 points.
Number of patients with CP scheduled for I degree according to Cambridge classification was 5 (12%); for II degree, 6 (14%); for III, 6 (14%); and for IV degree, 26 (60%).
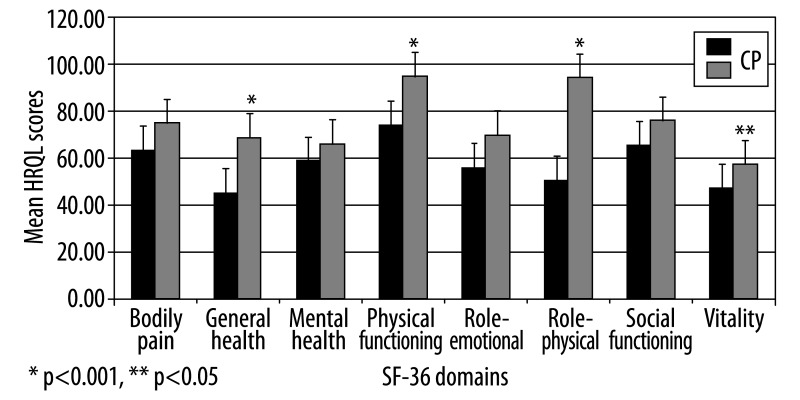
Mean QOL scores in CP were statistically lower compared to the control group in the following domains: physical functioning, role-physical, general health perception (p<0.001) and vitality (p<0.05) (74 vs. 95; 50 vs. 94; 45 vs. 69; 47 vs. 57; respectively) (Figure 1).
Figure 1.
QOL in CP patients compared to control group.
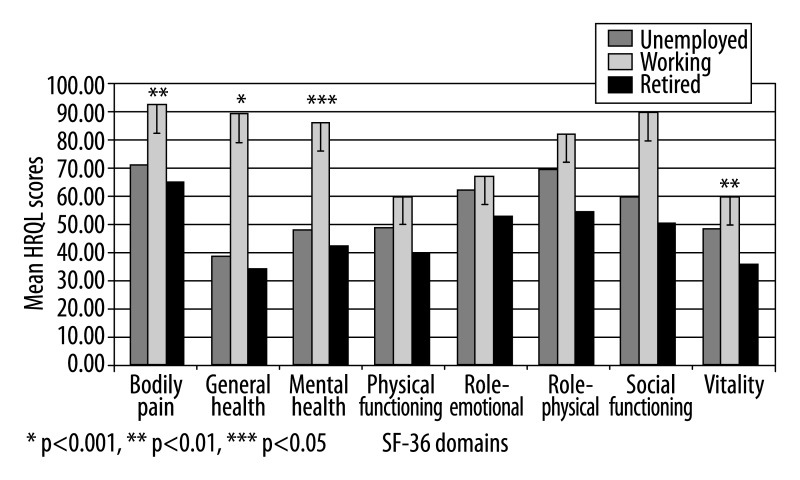
Mean QOL scores in unemployed patients were statistically lower compared to working patients (p<0.001; p<0.05) (Figure 2).
Figure 2.
Quality of life in CP patients in correlation to employment status.
There was the negative correlation between mean QOL scores and pain index results in all SF-36 domains (Table 2).
Table 2.
Association between SF-36 scores and the clinical variables in CP patients.
| Age (+) | Gender (female) (−) | Disease duration (−) | Disease relapses (−) | Pain (−) | Disease activity * (−) | Pain index (−) | BMI (+) | Diarrhoea (−) | |
|---|---|---|---|---|---|---|---|---|---|
| Physical functioning | p=.024 | p=.016 | p=.579 | p=.235 | p=.023 | p=.199 | p=.000 | p=.198 | p=.199 |
| Role-physical | p=.545 | p=.588 | p=.840 | p=.023 | p=.007 | p=.167 | p=.000 | p=.022 | p=.124 |
| Role-emotional | p=.862 | p=.508 | p=.496 | p=.012 | p=.044 | p=.566 | p=.001 | p=.004 | p=.066 |
| Vitality | p=.146 | p=.946 | p=.546 | p=.211 | p=.021 | p=.887 | p=.006 | p=.219 | p=.295 |
| Mental health | p=.152 | p=.332 | p=.483 | p=.145 | p=. 056 | p=.255 | p=.012 | p=.204 | p=.489 |
| Social functioning | p=.377 | p=.676 | p=.757 | p=.025 | p=.004 | p=.331 | p=.000 | p=.022 | p=.272 |
| Bodily pain | p=.347 | p=.781 | p=.532 | p=.014 | p=.001 | p=.206 | p=.000 | p=.004 | p=.094 |
| General health | p=.508 | p=.500 | p=.530 | p=.024 | p=.002 | p=.837 | p=.000 | p=.035 | p=.035 |
p<0.05 significant correlation in bold; (+) positive relationship; (−) negative relationship;
acc. to Cambridge classification.
The number of the disease relapses was associated with QOL impairment in 5 of 8 SF-36 domains: role-physical, role-emotional, social-functioning, bodily-pain and general health perception. The QOL scores in the same 5 domains were positively correlated with body weight (BMI) (p<0.001; p<0.05, respectively) (Table 2).
There was no statistical correlation between mean QOL scores and population demographic features (age, marital status, education level), CP etiology, smoking habits, disease duration and activity assessed using the Cambridge classification, history of surgical or endoscopic treatment, and coincidence of diabetes mellitus (Table 2).
Discussion
Our study confirmed that chronic pancreatitis has negative influences on patients’ quality of life. In addition, we have gained further insight into the relationship between disease status and QOL.
We used the SF-36 questionnaire, a generic instrument to assess QOL, validated in Poland [8,9]. The questionnaire has psychometric reliability, and content, convergent and discriminant validity and internal consistency when used in patients with many different disorders, including chronic pancreatitis [6,11,12].
Our study revealed the deterioration of QOL in CP in all SF-36 domains compared to healthy controls, particularly in physical functioning, role-physical, general health perception and vitality domains.
Similar findings were presented in the study by Wehler et al, who conducted QOL assessment in a 265 CP patients. All domains of SF-36 were reduced when compared to the general population. Decrements were most pronounced in role limitations caused by physical and emotional health problems and general health perceptions [6]. Similar results were obtained by Pezzilli et al., who described general health, role-physical and vitality as the most impaired domains [4].
The pain item was a major concern of the patients studied. We observed significant negative correlation between mean QOL scores and pain index in all SF-36 domains.
Our previous studies and most other similar studies have shown the significant pain-related impairment of QOL results, despite the fact that pain was measured in various ways [4,5,13]. Among the clinical factors examined by Pezzilli et al., only pain significantly decreased all 8 domains of SF-36 [4]. Similarly, in the study of Wehler et al, increasing severity of pancreatic pain significantly impaired QOL in all SF-36 domains [5].
It should be noted that Pezzilli et al. took into account the options pain/no pain, whereas in Wehler et al and in our study the pain index was used according to Talley et al. [4,5,10,14]. As there is a lack of good quantitative pain measurement in CP, we chose the pain index that provided a combined score representing pain intensity and frequency.
These results show how important the problem of pain is in CP and how often the management of these patients is not successful because the QOL is still impaired. There is not a direct relationship between abdominal pain and the presence of pathological structural changes of the pancreas. Since total pain relief is usually impossible, it is important to establish a solid doctor-patient communication. More effective individual therapies should be performed to control pain. Referral to a patient-based support group (e.g., National Pancreas Foundation) is helpful [15].
The structural pancreatic changes (according to the Cambridge classification) were not associated with QOL impairment. Similarly, other studies using SF-36 did not reveal any correlation between Cambridge stage and QOL scores [4,5]. Pezzilii et al. did not find any correlation in SF-36 scores and calcifications or pseudocysts and the presence of Wirsung duct dilatation [4,14]. When EORTC C30/PAN 26 (the specific instrument) was used, CP activity had influence on worse global health status, pain, nausea and vomiting, appetite loss, digestive symptoms, fear about future health, indigestion and taste changes items [13].
Disease duration did not influence QOL, similar to other studies with SF-36 [4]. However, studies using the CP-specific measure showed QOL impairment due to disease duration in a few domains [13]. The possible reason for these differences is that SF-36 as a generic instrument may not indicate specific differences in CP signs and symptoms [13]. On the other hand, the number of disease relapses was associated with QOL impairment in 5 of 8 SF-36 domains. To our best knowledge, the impact of CP relapses on QOL has not been studied previously.
Diabetes, which is a late consequence of CP, did not affect our QOL results, although other studies have shown its significant influence on QOL [14]. However, QOL in chronic pancreatitis is highly impaired due to the basic disease; it is possible that comorbidities such as diabetes do not add much to the final result.
Chronic diarrhea due to pancreatic exocrine insufficiency had an influence on the general health perception domain. Wehler et al. found that chronic diarrhea appeared to be an independent predictor of poor QOL results [5]. In our previous study using EORTC/PAN, diarrhea also had an impact on a few items: insomnia, altered bowel habit and diarrhea domains. In contrast, Pezzilli et al did not confirm these findings [13,14]. Therefore, early pancreatic enzymes replacement is recommended by numerous authors [15,16].
Like other studies using the SF-36 questionnaire or EORTC/PAN26, our study did not reveal any differences in QOL according to surgical/endoscopic procedures [4,13,14]. However, the aim of this study was not to assess the effect of treatment of CP. Future studies should have long follow-up periods and the time between disease onset and evaluation should be also taken into consideration. Previous studies have observed less improvement after treatment when disease duration is longer [17].
BMI score correlated positively with QOL role-physical, role-emotional, social-functioning, bodily-pain and general health perception domains. Similarly, low body weight independently contributed to the physical component score of the SF-36 and was the factor most closely associated with poor health status perception in the study by Wehler et al. [5].
The socioeconomic situation of the CP patients appeared to be unsatisfactory. Most (64% of patients) were under age 50 and the majority of them were retired or unemployed due to CP. Unemployment had a significant negative correlation with almost all SF-36 domains, with the exception of the mental health domain. Similarly, using EORTC QLQC30/PAN26, loss of work proved to have a negative influence on nausea and vomiting, insomnia and pancreatic pain domains [13].
Wehler et al demonstrated that unemployment and early disease-related retirement are independent and significant predictors of clinically important deterioration in most QOL domains [5]. In our population 44% of patients were retired due to CP, due to the requirements of the retirement system in Poland.
In a recent study by Gardner et al, out of a total of 111 CP patients only 37% were currently employed, 74% had their work lives altered by CP, 60% reported an effect on their social lives, and 46% reported an effect on their spouse/significant other relationship.
Respondents reported that they had not been treated with respect and dignity, and had been labeled as an alcoholic or a drug seeker.
The authors concluded that chronic pancreatitis had a profound impact on employment patterns and comprehensive efforts are needed to improve the health care experience of patients with this disease [18].
Conclusions
Our study shows that chronic pancreatitis is associated with deterioration in quality of life. Severity of pain, low body weight, disease relapses and unemployment are the main factors adversely affecting QOL. Consequently, controlling the pain remains the main therapeutic challenge. Our findings suggest that pain represents the most important challenge for clinicians in everyday practice. Since there are many different potential causes of pain in CP, no single therapy is effective in all patients [15]. Recently, the role of the central nervous system appears to be important in the development of pain. Pain treatment should not focus solely on the pancreas, but also address medications with effects on the underlying pain mechanisms [19]. Using alternative therapies could be promising; yoga has proved to be a tool improving the QOL in CP [20].
More individual effective therapies should be used to improve physical and emotional QOL in these patients; social factors have a particularly negative impact on QOL. Interactive, educational patient and family support groups for people with pancreatitis are needed [21].
The information given by quality of life assessment should be routinely included in the work-up of patients affected by chronic pancreatitis to select those patients with severely impaired physical and mental scores, and to plan an intensive program of medical and psychological follow-up [22].
Footnotes
Source of support: Grant of Medical University of Lodz no 502-03/1-002-01/502-14-043
References
- 1.Fitzimmons D, Kahl S, Butturini G, et al. Symptoms and quality of life in chronic pancreatitis assessed by structured interview and the EORTC QLQ-C30 and QLQ-PAN26. Am J Gastroenterol. 2005;100:918–26. doi: 10.1111/j.1572-0241.2005.40859.x. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann K, Mann O, Izbicki JR, Strate T. Chronic pancreatitis – a surgeons’ view. Med Sci Monit. 2008;14(11):RA198–205. [PubMed] [Google Scholar]
- 3.Madro A, Celiński K, Słomka M. The role of pancreatic stellate cells and cytokines in the development of chronic pancreatitis. Med Sci Monit. 2004;10(7):RA166–70. [PubMed] [Google Scholar]
- 4.Pezzilli R, Fantini L. Chronic pancreatitis: assessing the quality of life. JOP J Pancreas. 2005;6:406–9. [PubMed] [Google Scholar]
- 5.Wehler M, Nichterlein R, Fischer B, et al. Factors associated with health-related quality of life in chronic pancreatitis. Am J Gastroenterol. 2004;99:138–46. doi: 10.1111/j.1572-0241.2004.04005.x. [DOI] [PubMed] [Google Scholar]
- 6.Wehler M, Reulbach U, Nichterlein R, et al. Health-related quality of life in chronic pancreatitis: a psychometric assessment. Scand J Gastroenterol. 2003;38:1083–89. doi: 10.1080/00365520310005956. [DOI] [PubMed] [Google Scholar]
- 7.Sarner M, Cotton PB. Classification of pancreatitis. Gut. 1984;25:756–59. doi: 10.1136/gut.25.7.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ware JE, Snow KK, Kosinski M, et al. SF-36 health survey: Manual and interpretation guide. Boston, MA: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 9.Ware JE, Gandek B, Kosinski M, et al. The equivalence of SF-36 summary health scores estimated using standard and country-specific algorithms in 10 countries: results from the IA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51:1167. doi: 10.1016/s0895-4356(98)00108-5. [DOI] [PubMed] [Google Scholar]
- 10.Talley NJ, Haque M, Wyeth JW. Development of a new dyspepsia impact scale: The Nepean Dyspepsia Index. Aliment Pharmacol Ther. 1999;13:225–35. doi: 10.1046/j.1365-2036.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- 11.Andersson O, Ryden A, Ruth M, et al. Validation of the Swedish translation of LPR- HRQL Med Sci Monit. 2010;16(10):CR480–87. [PubMed] [Google Scholar]
- 12.Ceran F, Ozcan A. The relationship of the Functional Rating Index with disability, pain, and quality of life in patients with low back pain. Med Sci Monit. 2006;12(10):CR435–39. [PubMed] [Google Scholar]
- 13.Mokrowiecka A, Pinkowski D, Malecka-Panas E, Johnson CD. Clinical, emotional and social factors associated with quality of life in chronic pancreatitis. Pancreatology. 2010;10:39–46. doi: 10.1159/000225920. [DOI] [PubMed] [Google Scholar]
- 14.Pezzilli R, Morselli-Labate AM, Fantini L, et al. Assessment of the quality of life in chronic pancreatitis using Sf-12 and EORTC Qlq-C30 questionnaires. Dig Liver Dis. 2007;39:1077–86. doi: 10.1016/j.dld.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Chauhan S, Forsmark CE. Pain management in chrinic pancreatitis: a treatment algorithm. Best Pract Res Clin Gastroenterol. 2010;24:323–35. doi: 10.1016/j.bpg.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Dumasy V, Delhaye M, Cotton F, et al. Fat malabsorption screening in chronic pancreatitis. Am J Gastroenterol. 2004;99:1350–54. doi: 10.1111/j.1572-0241.2004.30661.x. [DOI] [PubMed] [Google Scholar]
- 17.Mariani A. The quality of life in chronic pancreatitis: the endoscopist’s point of view. JOP. 2006;11:117–19. [PubMed] [Google Scholar]
- 18.Gardner TB, Kennedy AT, Gelrud A, et al. Chronic pancreatitis and its effect on employment and health care experience: results of a prospective American multicenter study. Pancreas. 2010;39:498–501. doi: 10.1097/MPA.0b013e3181c5c693. [DOI] [PubMed] [Google Scholar]
- 19.Olesen SS, Brock C, Krarup AL, et al. Descending inhibitory pain modulation is impaired in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2010;8:724–30. doi: 10.1016/j.cgh.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Sareen S, Kumari V, Gajebasia KS, et al. Yoga: a tool for improving the quality of life in chronic pancreatitis. World J Gastroenterol. 2007;13:391–97. doi: 10.3748/wjg.v13.i3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shepp PH, Chase P, Rawls E. Pancreatitis Partners: a sharing and educational support group. Gastroenterol Nurs. 1999;22:155–57. doi: 10.1097/00001610-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Pezzilli R, Morselli Labate AM, Fantini L, et al. Quality of life and clinical indicators for chronic pancreatitis patients in a 2-year follow-up study. Pancreas. 2007;34:191–96. doi: 10.1097/mpa.0b013e31802e0301. [DOI] [PubMed] [Google Scholar]