Summary
Background
Gelclair is an oral lubricating gel used in the management of oral mucositis (OM). We evaluated its efficacy, tolerance and impact on oral cavity microbial colonization in patients with OM after allogeneic hematopoietic stem cells transplantation.
Material/Method
Gelclair was administered in a group of 22 patients with active OM. A control group of 15 patients used other rinsing solutions (chlorhexidine, benzydamine, salvia). Tests with oral cavity swabs for microbiology analysis were performed once a week.
Results
The characteristics of OM in both groups were comparable, and rinsing solutions had satisfactory tolerability. There was no difference in the median improvement of oral intake and OM-related pain relief, which was assessed mostly as “slight effect”. In the Gelclair group, the effect duration was longer (median 3 [0–5] vs. 1 [0–3] hours, p=0.001). There was significant increase of Enterococcus faecalis and Candida sp. colonization of the oral cavity over the course of the hospitalization and significantly reduced incidence of such colonization in patients with OM in the Gelclair group: 1/22 (5%) vs. 6/15 (40%), p=0.01. In vitro tests showed inhibited growth of Enterococcus faecalis and Candida sp. colonies within the area of the Gelclair application.
Conclusions
Gelclair may be individually helpful in the management of OM and pain in patients after allogeneic stem cells transplantation. Its use did not lead to worsened oral bacterial and yeast colonization and probably even helped to protect mucosa from Enterococcus and Candida sp. Further studies based on larger cohorts are needed.
Keywords: Gelclair, oral mucositis, pain, nursing, Enterococcus, Candida, microbiology
Background
Gelclair (Helsinn Healthcare SA, Switzerland) is a concentrated oral gel containing polyvinylpyrrolidone and sodium hyaluronate. It forms an adherent barrier and layer covering oral mucosa lesions, thus protecting sensitive nerve endings and lubricating the tissue. Several studies have suggested that it can help to manage pain and potentially can improve the ability to eat and drink in patients with oral mucositis (OM) after intensive chemotherapy or radiotherapy [1–5].
OM is a significant medical and nursing problem in high-dose chemotherapy and stem cells transplantation settings. This oral mucosa damage and injury is associated with pain and reduced oral intake. Since infections may also play a role in the pathophysiology of OM, antimicrobial mouthwashes such as chlorhexidine, benzydamine, povidone-iodine, and various local antibiotics/antimycotics are widely used in the nursing care and management of OM. However, there is little evidence supporting the use of these agents in the prophylaxis and treatment of this complication [6,7]. On the other hand, they are important in the treatment of oral bacterial and fungal infections [8,9]. Oral cavity cooling (cryotherapy) provided during high-dose melphalan bolus or short infusion administration contributes to local lower blood circulation and cytotoxic drug exposure and can significantly reduce OM in melphalan-containing protocols [10,11]. Effective analgesic therapy is necessary in patients suffering from this painful complication. Patient-controlled analgesia with morphine is a treatment of choice according to MASCC (Multinational Association of Supportive Care in Cancer), NCCN (National Comprehensive Cancer Network) and ESMO (European Society for Medical Oncology) recommendations [12–16].
Our observational study aimed to evaluate safety of Gelclair use with respect to the extent of bacterial and yeast colonization within the oral cavity, and to verify its clinical efficacy and tolerance in patients after allogeneic stem cells transplantation.
Material and Methods
We conducted a single-centre prospective and observational study in adult patients with oral mucositis developed after allogeneic hematopoietic stem cells transplantation (HSCT) in 2008–2009. The HSCT conditioning regimens were BuCY2 (busulphan total dose 16 mg/kg, cyclophosphamide total dose 120 mg/kg) or FLU/MEL (fludarabine total dose 120 mg/m2, melphalan 140 mg/m2). Patients were given the standard systemic antimycotic, antibacterial and antiviral prophylaxis (fluconazole, chinolons, acyclovir).
The patients signed informed consent. As Gelclair had already been implemented in the standard nursing care for several months at our institution, no Ethics Committee approval was necessary for this study. The study was non-sponsored.
The oral cavity nursing started on the first day of the conditioning chemotherapy administration and covered the whole inpatient stay. Prior to OM development, the patients were allowed to use chlorhexidine, benzydamine or salvia solutions for regular oral rinses. On the day of first signs of OM development (WHO criteria), the first 22 patients were consecutively assigned to the Gelclair treatment (Gelclair group) and afterwards the other 15 consecutive patients were assigned to carry on using the original oral rinses (Control group). After the patients recovered from OM (post-OM phase), all enrolled patients either carried on (Control group) or returned back (Gelclair group) to the standard oral care with benzydamine, chlorhexidine or salvia solutions. The oral rinses were recommended to be used at least 3 times a day and the Gelclair was used in concordance with the product brochure (Gelclair package insert, Helsinn Healthcare SA, Switzerland, 2006) and specific web pages instructions at www.gelclair.com.
The monitoring, assessment and definitions
OM was assessed daily using the WHO grading 0–4 (0 = absent; 1 = pain and erythema; 2 = ulcers, patient can swallow solid food; 3 = ulcers, patient cannot swallow solid food; 4 = mucositis to the extent that alimentation is not possible). The tolerability of oral rinses was evaluated daily by the patients, using the Visual Analog Scale (VAS) scoring: 1–5 (1 = tolerable without any problems, 2 = satisfactory, 3 = indifferent, 4 = unsatisfactory, 5 = intolerable). The OM pain reduction and food intake improvement were assessed daily by the patients using the VAS scoring: 1–5 (1 = excellent, 2 = good, 3 = slight effect, 4 = almost no effect, 5 = no effect at all).
Tests with oral cavity swabs for microbiology analysis were performed once a week in the morning — on the admission to the transplantation unit (pre-OM phase), during active OM (OM-phase) and after the OM resolution (post-OM phase). The smears comprised buccal, palatal and sublingual mucosa.
In vitro Gelclair inhibition test was performed using the Enterococcus faecalis, Candida albicans, Candida parapsilosis and Candida krusei strains suspensions inoculated separately onto Müller-Hinton agar and 3 drops of Gelclair were added on the inoculated area. The plate samples were incubated in stable temperature 37°C for 24 hour (48 hours in Candida krusei sample). All the microbiology samples were processed and cultivated under controlled laboratory conditions in the institutional Department of Microbiology.
Colonies of generally physiological oral bacteria (Streptococcus viridans or Neisseria spec. or Staphylococcus coagulase-negative) were considered potentially pathogenic because of the significant immunodeficiency of transplanted patients.
Statistics
Basic statistical univariate analyses were performed using statistical software (GraphPad InStat, GraphPad Software) with the Fisher’s exact test and Unpaired T test. The “p” values <0.05 were considered as statistically significant differences.
Results
A total of 22 patients were enrolled into OM treatment with Gelclair and 15 patients into the Control group using standard oral solutions with chlorhexidine (8/15, 53%), benzydamine (6/15, 40%) or salvia (1/15, 7%). Characteristics of the groups are shown in Table 1.
Table 1.
Characteristics of patients with oral mucositis (OM) after allogeneic stem cells transplantation.
| Gelclair treatment group | Control group | p= | |
|---|---|---|---|
| No. of patients | 22 | 15 | – |
|
| |||
| Age (years), median | 49.5 (22–68) | 39 (19–53) | 0.003 |
|
| |||
| Sex: males (%) | 11 (50%) | 8 (53%) | 1.0 |
|
| |||
| Diagnosis: | – | ||
| AML | 10 | 8 | |
| ALL | 3 | 5 | |
| CLL | 2 | 0 | |
| CML | 1 | 1 | |
| HL | 0 | 1 | |
| NHL | 1 | 0 | |
| MDS | 3 | 0 | |
| MM | 2 | 0 | |
|
| |||
| Chemotherapy conditioning: | |||
| BuCY2 | 15 | 13 | 0.261 |
| FLU/MEL | 7 | 2 | |
|
| |||
| OM duration (days), median | 10 (5–16) | 8 (2–19) | 0.489 |
|
| |||
| OM maximum grade WHO, median | 2 (2–4) | 2 (1–4) | 0.187 |
|
| |||
| No. of oral rinses per day | 3 (2–3) | 3 (2–6) | 0.062 |
|
| |||
| Systemic analgesic treatment with opioids | 15/22 (68%) | 7/15 (46%) | 0.030 |
AML – acute myeloid leukaemia; ALL – acute lymphoblastic leukaemia; CLL – chronic lymphocytic leukaemia; CML – chronic myeloid leukaemia; HL – Hodgkin lymphoma; NHL – non-hodgkin lymphoma; MDS – myelodysplasia; MM – multiple myeloma.
There was no difference in the median value of tolerability of the rinses in the Gelclair vs. Control group: 2 (1–5) vs. 2 (1–3), p=0.304. The individual tolerance in the Gelclair group in details was: 1 – tolerable without any problems in 9%, 2 – satisfactory in 73%, 3 – indifferent in 9%, 4 – unsatisfactory in 4.5% and 5 – intolerable in 4.5% patients. The individual tolerance in the Control group was: 1 – tolerable without any problems in 33%, 2 – satisfactory in 40% and 3 – indifferent in 27% patients.
Regarding the improvement of oral intake and OM pain relief after oral rinsing, there was no difference in the median value of improvement intensity observed between the Gelclair and the Control groups: 3 (2–4) vs. 3 (2–4) and 3 (2–5) vs. 3 (1–4) VAS score, p=0.381 and 0.190 (3 = slight effect). The median value of duration of pain relief was significantly longer in the Gelclair group: 3 (0–5) vs. 1 (0–3) hours, p=0.001. Significantly more patients on systemic analgesic opioid treatment were in the Gelclair group: 15/22 (68%) vs. 7/15 (46%), p=0.03. The analgesic medication administered in the Gelclair group was: buprenorphine 35 ug/hour transdermal patch in 13/22 (59%), buprenorphine 52.5 ug/hour transdermal patch in 1/22 (4.5%) and tramadol 4.1 mg/hour I.V. in 1/22 (4.5%) patients. The analgesic treatment administered in the Control group was: buprenorphine 35 ug/hour transdermal patch in 5/15 (33%), buprenorphine 52.5 ug/hour transdermal patch in 2/15 (13%) patients.
Oral microbiology swabs
In the whole group of 37 patients, bacterial or yeast pathogens were detected in 6 (16%) patients in oral cavity swabs during the pre-OM phase, and in 18 (49%) in the post-OM phase (p=0.0024). During the OM phase, significantly fewer pathogens were found in the Gelclair compared to the Control group: 1/22 (5%) vs. 6/15 (40%), p=0.01. After the OM resolution and Gelclair use termination (the post-OM phase), there was a significant increase of pathogen colonization observed in the Gelclair group in comparison with the previous condition in this group: 12/22 (55%) vs. 1/22 (5%), p=0.0006. Negative microbial swab results were obtained in the Gelclair group only during the OM phase and in 2 patients later on. No difference was observed in patients using either chlorhexidine or benzydamine solutions. For more details see Table 2.
Table 2.
Oral cavity microbiology swab results in patients within pre-, post-, and during oral mucositis (OM) phase.
| Gelclair treatment group (n=22) | Control group (n=15) | p= | |
|---|---|---|---|
| Pre-OM phase with standard oral solutions used in both groups | Negative: 0 Potentially pathogenic: 18/22 (82%) Pathogenic: 4/22 (18%) E. faecalis 4/4 C. glabrata 1/4 |
Negative: 0 Potentially pathogenic: 13/15 (87%) Pathogenic: 2/15 (13%) E. faecalis 2/2 |
1.00 |
| OM-phase with Gelclair (Gelclair group) or standard oral solution (Control group) | Negative: 13/22 (59%) Potentially pathogenic: 8/22 (36%) Pathogenic: 1/22 (5%) E. faecalis 1/1 |
Negative: 0 Potentially pathogenic: 9/15 (60%) Pathogenic: 6/15 (40%) E. faecalis 5/6 E. faecium 1/6 |
0.011 |
| Post-OM phase with standard oral solutions used in both groups | Negative: 2/22 (9%) Potentially pathogenic: 8/22 (36%) Pathogenic: 12/22 (55%) E. faecalis 8/12 E. faecium 1/12 VRE 1/12 C. glabrata 2/12 C.inconspicua 1/12 |
Negative: 0 Potentially pathogenic: 9/15 (60%) Pathogenic: 6/15 (40%) E. faecalis 4/6 E. faecium 1/6 K. pneumoniae 1/6 C. krusei 1/6 |
0.50 |
Negative – no microbial species detected ever; potentially pathogenic – colonies of Streptococcus viridans or Neisseria spec. or Staphylococcus coagulase-negativ; pathogenic – any other microbial colonies. C. – Candida; E. – Enterococcus; OM – oral mucositis; VRE – vancocin resistant enterococcus.
In vitro Gelclair inhibition test
There was evident growth inhibition of the Enterococcus faecalis and Candida colonies within the area of Gelclair application (Figures 1 and 2).
Figure 1.
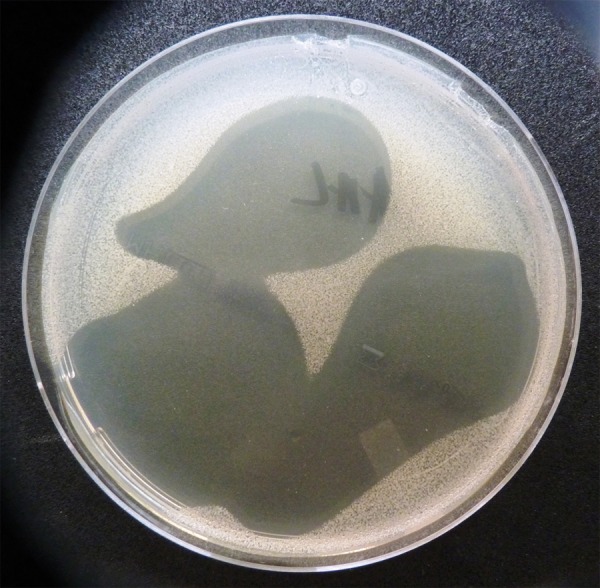
The inhibition of growth of Candida albicans colonies on the Müller-Hinton agar in the area of Gelclair application (the clear zone).
Figure 2.
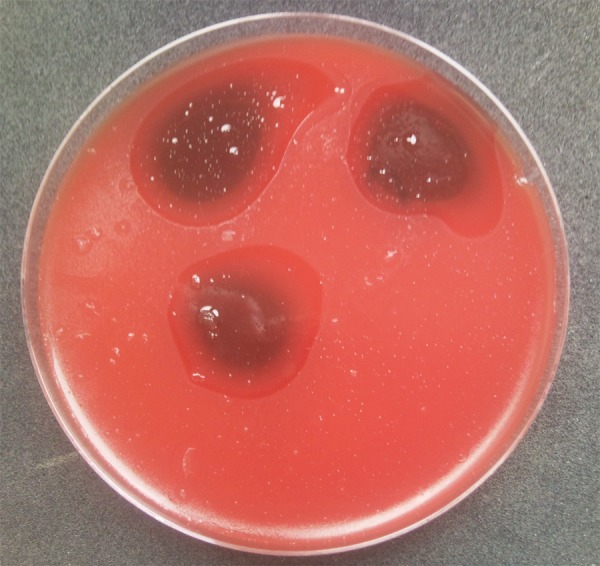
The inhibition of growth of Enterococcus faecalis colonies on the Müller-Hinton agar in the area of Gelclair application (the clear zone).
Discussion
Gelclair oral gel containing polyvinylpyrrolidone and sodium hyaluronate has been used in the management of oral mucositis, stomatitis and various ulcerative oral conditions with some positive results, suggesting that it can help to reduce pain and improve the ability to eat and drink [2–4]. In a small group of patients with radiotherapy-induced OM, there was also short-term pain relief observed initially after the use of Gelclair; however, there was no improvement in capacity of oral intake [1]. To date there has been limited experience with the use of Gelclair in allogeneic stem cells transplantation patients and there is no available information about Gelclair’s impact on oral cavity microbial colonization. Based on the fact mentioned above, we decided to conduct this study.
Gelclair was administered in the group of 22 patients in order to treat an active oral mucositis since the first symptoms appeared until OM was resolved. Fifteen other patients were in the control group and carried on with the standard oral care with solutions containing chlorhexidine, benzydamine or salvia. As our patients were allowed to freely select one of the 3 solutions (according to their individual preference and taste), there was in general a good tolerability of these solutions and none of control group patients considered them as “unsatisfactory” or “intolerable”, which happened in the Gelclair group, where one of the patients even refused to carry on using the gel due to individual intolerance. The tolerability of Gelclair, however, was in general rather good in the majority of cases. Thus, the results of oral rinse tolerability must be considered in a larger context.
As for the OM pain relief and oral intake improvement, those issues were assessed as “light” in both study groups, with somewhat longer duration in the Gelclair group (median duration of 3 (0–5) vs. 1 (0–3) hours, p=0.001). It is necessary to mention the possible impact of systemic analgesic medication administered in order to reduce the intensity OM pain in the majority of patients. However, to get the most reliable data possible, the patients were individually and specifically asked to focus only on actual local analgesic effect of the rinses applied.
The characteristics of OM in both study groups were comparable. The median value for duration of the complication in both study groups was statistically comparable, as well as the median of OM maximal intensity. It is impossible to objectively compare differences of OM in patients after the FLU/MEL or BuCY2 conditioning regimens because of the small numbers of patients and selection bias – patients without OM were not enrolled into the study.
Although there were differences in age and diagnoses variety between the study groups, in our opinion this had no significant impact on the study results.
Very interesting and somewhat surprising were the results of microbial tests with oral cavity swabs, as there has not been any information yet on antimicrobial effect of Gelclair available, and Gelclair, in fact, does not contain any specific antimicrobial agent. Firstly, we observed a significant increase of pathogens colonization (predominantly Enterococcus faecalis and Candida sp.) of the oral cavity over the course of the hospitalization. Secondly, there was a significantly reduced incidence of such pathogen colonization, and even microbial negativity, in patients with active OM (OM-phase) in the Gelclair group. The difference observed was remarkable and significant compared to the Control group colonization, and compared to the post-OM phase within the group of patients with original Gelclair treatment (that means significant increase of pathogens in the post-OM phase when Gelclair use was discontinued). Based on these clinically positive results, we decided to test the possible inhibitory effect of Gelclair in vitro. The tests performed on Müller-Hinton agar showed inhibited growth of Enterococcus faecalis and Candida sp. colonies within the area of Gelclair application. These results, however, should be considered only as informative, because the methodology used for the testing was not based on a standardized protocol and had no specific certification or validation. We did not use latex agglutination assay for detection of circulating Candida antigen [17].
Conclusions
The results of this observation suggest that Gelclair may be individually helpful in the management of OM and pains in some patients after allogeneic stem cells transplantation. The use of Gelclair did not lead to worsened local bacterial and yeast colonization in the oral cavity and probably even helped to protect mucosa from Enterococcus and Candida colonization. Gelclair use appears to be safe in patients after allogeneic stem cells transplantation. Further observations and analysis based on larger cohorts of patients are needed.
Acknowledgements
Cervena Jarmila, Dolejsova Lucie, Karasova Lenka, Kibitzova Petra, Krivankova Irena, Lastovkova Alena, Novotna Romana, Rerichova Michaela, Ruttnerova Marta, Schröderova Ruzena, Vohrnova Dana, Zikova Jindra (nurses, University Hospital Plzen, Haemato-Oncological Dept., Transplant unit) – the nursing and care for the patients.
Prim. MU Dr. Tamara Bergerova, MU Dr. Jitka Audesova and MU Dr. Helena Janouskovcova (University Hospital Plzen, Microbiology Dept.) – microbiological analysis of the samples.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Source of support: Departmental sources
References
- 1.Barber C, Powell R, Ellis A, et al. Comparing pain control and ability to eat and drink with standard therapy vs Gelclair: a preliminary, double centre, randomised controlled trial on patients with radiotherapy-induced oral mucositis. Support Care Cancer. 2007;15:427–40. doi: 10.1007/s00520-006-0171-1. [DOI] [PubMed] [Google Scholar]
- 2.Bonassi L, Cotroneo G, Nastasi G, et al. Treatment with Gelclair in patients suffering grade III–IV oral mucositis: efficacy and impact on quality of life (QOL) Anna Oncol. 2003;14:58. [Google Scholar]
- 3.Flook C, Calman F, Mant M, et al. Gelclair vs benzydamine in randomise controlled study in patients with oral mucositis due to radical radiotherapy. Support Care Cancer. 2005;13:443–444. [Google Scholar]
- 4.Innocenti M, Moscatelli G, Lopez S. Efficacy of Gelclair in reducing pain in palliative care patients with oral lesions: preliminary finding from an open pilot study. J Pain Symptom Manag. 2002;24:456–57. doi: 10.1016/s0885-3924(02)00524-9. [DOI] [PubMed] [Google Scholar]
- 5.Buchsel PC. Polyvinylpyrrolidone-sodium hyaluronate gel (Gelclair): a bioadherent oral gel for the treatment of oral mucositis and other painful oral lesions. Expert Opin Drug Metab Toxicol. 2008;4:1449–54. doi: 10.1517/17425255.4.11.1449. [DOI] [PubMed] [Google Scholar]
- 6.Donnelly P, Bellm L, Epstein J, et al. Antimicrobial therapy to prevent or treat oral mucositis. Lancet Infect Dis. 2003;7:405–12. doi: 10.1016/s1473-3099(03)00668-6. [DOI] [PubMed] [Google Scholar]
- 7.Karthaus M, Rosental C, Ganser A. Prophylaxis and treatment of chemo- and radiotherapy-induced oral mucositis – are there new strategies? Bone Marrow Transplant. 1999;24:1095–108. doi: 10.1038/sj.bmt.1702024. [DOI] [PubMed] [Google Scholar]
- 8.Ferreti GA, Raybould TP, Brown AT, et al. Chlorhexidine prophylaxis for chemotherapy- and radiotherapy-induced stomatitis: a randomised double-blind trial. Oral Surg Oral Med Oral Pathol. 1990;69:331–38. doi: 10.1016/0030-4220(90)90295-4. [DOI] [PubMed] [Google Scholar]
- 9.Weisdorf DJ, Bostrom B, Raether D, et al. Oropharyngeal mucositis complicating bone marrow transplantation: prognostic factors and the effect of chlorhexidine mouth rinse. Bone Marrow Transplant. 1989;4:89–95. [PubMed] [Google Scholar]
- 10.Aisa Y, Mori T, Kudo M, et al. Oral cryotherapy for the prevention of high-dose melphalan-induced stomatitis in allogeneic hematopoietic stem cell transplant recipients. Support Care Cancer. 2005;13:266–69. doi: 10.1007/s00520-004-0726-y. [DOI] [PubMed] [Google Scholar]
- 11.Svanberg A, Ohrn K, Birgegard G. Oral cryotherapy reduces mucositis and improves nutrition - a randomised controlled trial. J Clin Nurs. 2010;19:2146–51. doi: 10.1111/j.1365-2702.2010.03255.x. [DOI] [PubMed] [Google Scholar]
- 12.Rubenstein E, Peterson D, Schubert M, et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer. 2004;100:2026–46. doi: 10.1002/cncr.20163. [DOI] [PubMed] [Google Scholar]
- 13.Keefe D. Mucositis guidelines: what have they achieved, and where to from here. Support Care Cancer. 2006;14:489–91. doi: 10.1007/s00520-006-0056-3. [DOI] [PubMed] [Google Scholar]
- 14.Keefe DM, Schubert MM, Elting LS, et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109(5):820–31. doi: 10.1002/cncr.22484. [DOI] [PubMed] [Google Scholar]
- 15.Bensinger W, Schubert M, Ang KK, et al. Task Force Report. Prevention and management of mucositis in cancer care. J Natl Compr Canc Netw. 2008;6:1–21. [PubMed] [Google Scholar]
- 16.Peterson D, Bensadoun RJ, Roila F. Management of oral and gastrointestinal mucositis: ESMO Clinical Recommendations. Ann Oncol. 2009;20:174–77. doi: 10.1093/annonc/mdp165. [DOI] [PubMed] [Google Scholar]
- 17.Misaki H, Iwasaki H, Ueda T. A comparison of the specificity and sensitivity of two Candida antigen assay systems for the diagnosis of deep candidiasis in patients with hematologic diseases. Med Sci Monit. 2003;9(2):MT1–7. [PubMed] [Google Scholar]


