Summary
Background
In this prospective, randomized, placebo-controlled, double-blinded clinical trial we tested the hypothesis that preemptive analgesia with bupivacaine applied in the area of the surgical incision in patients undergoing mastectomy for breast cancer would reduce post-operative acute pain and would reduce the amount of analgesics used during surgery and in the post-operative period.
Material/Methods
Participants were assigned into 1 of 2 groups – with bupivacaine applied in the area of surgical incision or with placebo. We assessed the intraoperative consumption of fentanyl, the postoperative consumption of morphine delivered using a PCA method, and the subjective pain intensity according to VAS score reported by patients in the early post-operative period.
Results
Out of 121 consecutive cases qualified for mastectomy, 112 women were allocated randomly to 1 of 2 groups – group A (bupivacaine) and group B (placebo). The final study group comprised 106 breast cancer cases. Between the groups, a statistically significant difference was observed with respect to: lower fentanyl consumption during surgery (p=0.011), lower morphine (delivered by means of a PCA) consumption between the 4–12th postoperative hours (p=0.02) and significantly lower pain intensity assessed according to VAS score at the 4th and 12th hours after surgery (p=0.004 and p=0.02 respectively) for the group A patients.
Conclusions
Preemptive analgesia application in the form of infiltration of the area of planned surgical incisions with bupivacaine in breast cancer patients undergoing mastectomy decreases post-operative pain sensation, limits the amount of fentanyl used during surgery, and reduces the demand for opiates in the hours soon after surgery.
Keywords: preemptive analgesia, breast cancer, bupivacaine, mastectomy
Background
Mastectomy is a surgical option in treatment of breast cancer that leads to acute post-surgical pain of patients. In 20–30% of patients it may further evolve into post-mastectomy chronic pain syndrome (PMPS) [1,2]. Tissue damage resulting from surgery causes, in the first phase, a nociceptive stimulation reaching the CNS, and, in the second phase, a transient inflammatory reaction [3,5]. Preemptive analgesia (PA) is one of the methods of pain management used in the perioperative period. The PA strategy originated in early 1980s [6]. Application of analgesic agents in the area of the surgical incision reduces the number of signals generated by pain receptors (nociceptors), preventing central hypersensitivity of the CNS and consequently reduces the incidence of disproportionate pain sensation in the perioperative period.
PA has been shown to have a beneficial analgesic effect in a large number of surgical procedures including laparoscopy, hernia surgeries, amputations of extremities, operations of the spinal cord, and in orthopedics [7–10]. Application of the PA strategy was also the subject of study in the field of breast cancer surgery; however, the obtained results were indecisive with respect to the role of preemptive analgesia in lumpectomy and mastectomy [11–15]. As only a small number of randomized clinical trials have been reported, and they differed in the applied methodology and lacked reliable (clinically verified) evidence proving PA efficacy, we decided to perform the present study.
We tested the hypothesis that preemptive analgesia with bupivacaine applied in the area of the surgical incision in patients undergoing mastectomy for carcinoma of the breast would reduce post-operative acute pain and would reduce the amount of analgesics used during surgery and in the post-operative period.
Material and Methods
The study was designed as a prospective, randomized, placebo-controlled, double-blind clinical trial. All study procedures and data collection was performed in the Department of Surgical Oncology, Medical University of Gdansk during the period July 2009–March 2010. The present prospective single-centre randomized clinical trial was performed according to the guidelines of the Ethics Examining Committee of Human Research at the Medical University of Gdansk, Poland (approval no. 195/2009).
Study participants
We recruited 121 women from 7 oncological out-patient clinics in the Pomeranian region of Poland with a histopathologically confirmed breast carcinoma (clinical stage I, II, IIIA) and anesthesia risk I-III according to the ASA (American Society of Anesthesiologists) scale, who were referred to the Department of Surgical Oncology and were considered candidates for inclusion in the present study. Patients were qualified for radical modified mastectomy by 5 independent oncologic surgery specialists. Patients were excluded from the study if any of the following criteria were met: 1) patient declined to give a written informed consent, 2) patient reported to be allergic to bupivacaine or any other local analgesic agent, 3) patient reported to be allergic to any of the drugs used in the analgesia protocol, 4) patient was surgically treated for breast cancer prior to enrollment in the study, 5) there was a history of treatment for a chronic pain condition, 5) there was a history of a psychiatric disorder, and 7) patient’s weight was less then 50kg.
Working group
With the aim of improving accuracy of the primary and secondary results, an introductory informational conference was organized prior to the start of the study. All the members of the working group participated in the meeting, including oncological surgeons, anesthesiology team, quality-of-life team, nursing staff, the person responsible for patient randomization, the person responsible for statistical analysis and the project coordinator. During the gathering all the members were provided with detailed instructions regarding allocated tasks. Patient assignment to groups A (preemptive) and B (placebo) was performed by 1 person alone who had no contact with the rest of the working team or with the patients. The project coordinator was made responsible for supervision of the compliance with the algorithms of preoperative, operative and postoperative management; for keeping records of the investigation results; for elaboration of the raw data and their submission for statistical analysis; and for continual contact with the trial participants. Before the study started, a simulation of all the subsequent steps of the project was performed twice with all the persons involved in the project. Only 1 interim analysis was performed in October 2009. In view of the obtained results, the decision was made to continue recruiting and to go on with the study.
Randomization
The method of sequence generation was a random-number table (randomization ratio 1:1). All patients had an equal probability of assignment to the groups. Simple randomization method without blocking, stratification or minimalization was used. A computer random-number table was drawn up by the statistician and given to the person responsible for preparation of an unlabeled drug preparation and who did not participate in any of the therapeutic processes. The allocation sequence was generated by the statistician, while the project coordinator was responsible for enrollment of participants, and the person who prepared the unlabeled drug based on the random-number list assigned consequent participants to the trial groups. All of the personnel and participants who participated in the study were blinded to treatment assignment for the duration of the study. Only the study statistician and the person who prepared the unlabeled drug preparation saw the unblended data, but none of them had any contact with the study participants. The person who prepared the unlabeled preparation was responsible for proper preparation of the drug/placebo, its delivery, and compliance with the rules of blinding. In addition, in case of necessity of uncovering the content of the preparation in case of an adverse event, the person was obliged to provide details regarding its composition. The code was revealed to the researchers once recruitment and data collection were completed.
Study procedures
All patients received a detailed physical examination and had mammography and breast ultrasound performed. After the patient was qualified for mastectomy, information regarding the study was provided. If the patient fulfilled all of the above criteria and provided written informed consent, the planned line of incision was marked on the skin with a skin-marker. Each patient was informed that participation in the study was voluntary, and that they could withdraw from the study at any time without needing to provide an explanation.
Depending on random allocation to the specific group, on the day of surgery 1 of the preparations (40ml) was prepared: for group A, 100mg bupivacainum hydrochloricum (Polfa, Poland) dissolved in 0.9% NaCl solution; and for group B (control), 0.9% NaCl. Information regarding composition of the preparation was stored in a special, private table (containing patients’ names and the allocated type of preparation) kept by the person responsible for its preparation, who was an independent person separated from the rest of the research team. The syringes containing the preparation were labeled with patient’s name, date of preparation and a tag “for subcutaneous administration” and stored in a special container in which they were delivered to the operating theatre.
Each patient received standard oral premedication with 7.5 mg midazolam (Dormicum, Roche, Poland) 45 minutes prior to anesthesia. Induction of anesthesia was performed using propofol 2 mg/kg body weight (Fresenius Kabi, Germany), fentanyl 2 μg/kg body weight (WZF Polfa, Poland) and rocuronium 0.6mg/kg body weight (Organon, Holland). After intubation, the preparation was injected subcutaneously along the intended line of incision, then 15 minutes later the operation began. For anesthesia maintenance, a respiratory gas mixture composed of 40% oxygen, 60% nitrous oxide and 1–2 vol% of sevoflurane (Baxter, Poland) was used. In the case of intensification of pain sensation demonstrated as increased heart rate and/or arterial blood pressure, an anesthesiologist administered repeatable boluses of fentanyl 2 μg/kg body weight. Patients received the first dose of 2.5 g metamizole (Polpharma, Poland) in a 30-minute intravenous drip infusion 30 minutes before end of the operation.
Once the operation was finished and the patient was awake, she was supplied with a patient-controlled analgesia (PCA; Graseby 3300 Pump, Smith Medical International, UK) loaded with the initial dose of 20 mg morphine (WZF Polfa, Poland). Initial PCA set-up was: drug concentration - 1mg/ml morphine; bolus 1mg (for patients <65 kg body weight) and 2mg (for patients >65 kg body weight), lockout period - 5 minutes, total dose limit 4 hours.
Post-operative period
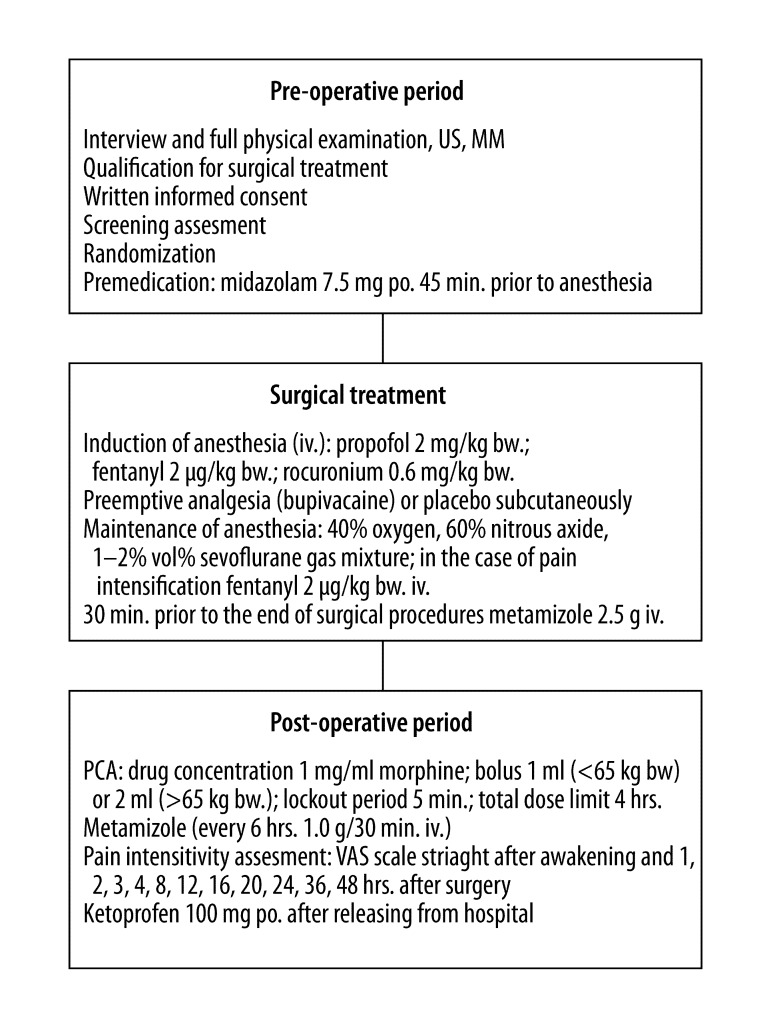
Metamizole (1 g) administered every 6 hours in a 30-minute intravenous drip infusion and a 24-hr PCA were used in pain management. Total dose of morphine used during the first 24 hrs was recorded in a print-out. On the day of discharge from the hospital the patient was instructed to take oral ketoprofen 100 mg (Lek Pharmaceuticals, Slovenia) in the event of pain. The trial algorithm is summarized in Figure 1. Patients were referred to the out-patient clinic at the Department of Surgical Oncology, Medical University of Gdansk for the standard post-operative follow-up.
Figure 1.
Trial algorithm. US – Ultrasound Imaging; MM – Mammography; po. – per os; iv. – intravenous; bw. – body weight; VAS – Visual Analogue Scale; PCA – Patient Controlled Analgesia; hrs – hours.
Primary outcome measures
In the post-operative period the insensitivity of pain was measured using VAS (Visual Analog Scale) ranging from 0–10, where 0 = no pain and 10 = worst pain. The patient was assessed immediately after waking from anesthesia and at 1, 2, 3, 4, 8, 12, 16, 20, 24, 36 and 48 hours after surgery. For assessment of pain insensitivity each patient received a slide with the VAS scale. On the first day after surgery pain intensity was assessed by nursing staff. After discharge from the hospital, additional information was collected by phone.
Secondary outcome measures
Secondary outcome measures included analysis of numerical values of pain intensity according to the VAS scale, summed up in the following time ranges: 0–4 hrs, 4–12 hrs, 12–24 hrs, 24–48 hrs, 0–12 hrs and 0–48 hrs. Also, a comparison between the group of patients reporting pain (VAS=1–10) vs. the group of patients who did not report pain (VAS=0) and a comparison between the group of patients with no or only slight pain sensation (VAS=0–1) vs. the group of patients with stronger pain (VAS >1) were performed. The quality of multimodal analgesia provided during surgical treatment was assessed in view of fentanyl consumption. In addition, the time of the first morphine dose delivered by PCA, total morphine consumption and the number of attempts to launch PCA during lockout were measured. Amounts of morphine consumed in the following time ranges were analyzed: 0–1 hrs (from the time of the end of the surgery until the end of the first post-operative hour), 0–4 hrs, 0–12 hrs, 1–2 hrs, and 4–12 hrs.
Taking into account multiple observations and training of assessors, at a meeting scheduled halfway through the project the clinical end-point committee decided to refine procedures related to providing information to the patients. In particular, the committee decided to provide more detailed information on the VAS scale and principles of PCA pump application (for elderly patients specifically) not only on the day prior to scheduled surgery but also on the day of surgery and immediately after surgery.
Statistical methods
All data analysis was carried out according to a pre-established analysis plan. Altman’s nomogram was used for simple size measurement. We believed that the distinction in VAS score of pain would be significant if there were at least 1 point of difference between patients who received bupivacaine as PA before mastectomy vs. the control group (variability estimated from interim analysis, SD=1.8). Thus, assuming α=0.05 and power of the study at 0.80, a total sample size of 100 patients would be required (50 in each group). To compensate for patients who dropped out, we had planned to enroll 110 patients.
The normality of distribution of the initial data was assessed using Kolmogorov-Smirnov and Shapiro-Wilk W tests. The data that followed a normal distribution pattern were analyzed using T testing for equality of means. Equality of variances was estimated using Levene’s test. The data that did not follow normal distribution were analyzed using the nonparametric Mann-Whitney U test. Pearson goodness-of-fit chi-square test was used to analyze associations between independent variables. P<0.05 was chosen as the cut-off point for significance. Statistical analysis was performed using SPSS v. 13.0 (SPSS Inc, USA) and Statistica v. 8.0 (StatSoft Inc, USA).
Results
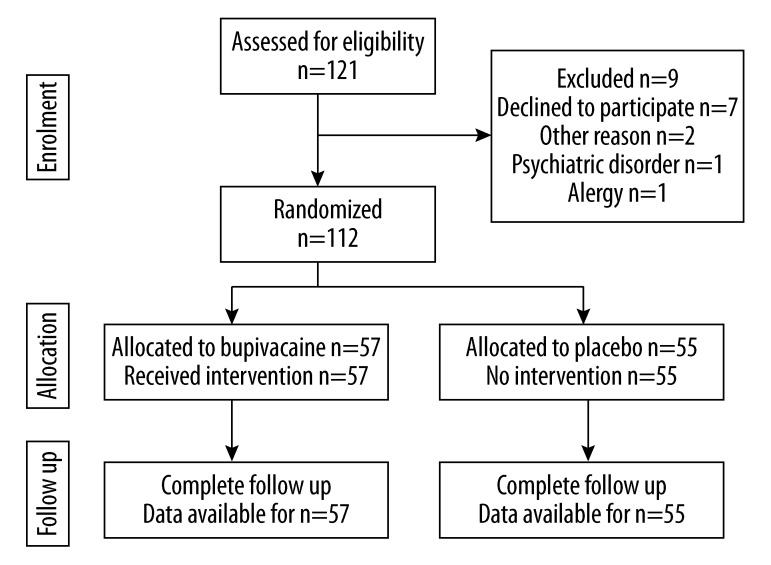
A total of 121 women, admitted during the period from July 2009 till March 2010 to the Department of Oncological Surgery, Medical University of Gdansk, Poland for surgical treatment of breast carcinoma, were evaluated before enrollment to the study; 112 patients were qualified to the study; and 9 were excluded from participation. Seven of the excluded cases did not give an informed consent, 1 was found to be allergic to metamizole and 1 had a history of psychiatric disorder. The 112 patients enrolled to the study were subject to randomization into 2 groups – group A (n=57) treated with bupivacaine along the line of the planned surgical incisions prior to main surgery, and group B treated with placebo (n=55). After the surgery, 3 patients were excluded from group A – the first because the advanced stage of the disease necessitated modification of the surgery due to neoplastic infiltration of the axillary vein; the second due to difficulties in anesthesia and objections of the anesthesiologist regarding metamizole administration; and the third due to necessity of re-operation on the second day after surgery due to post-operative bleeding. Similarly, after the surgery, 3 patients were excluded from group B (placebo) – the first because of the advanced stage of the disease with neoplastic infiltration of the pectoralis major muscle; and the reaming 2 cases due to objections of the anesthesiologist regarding metamizole administration because of difficulties during anesthesia. Eventually, 106 patients were subjected to intention-to-treat, analysis and follow-up – these included 54 women in group A (bupivacaine) and 52 women in group B (placebo). The follow-up was completed in March 2010. The flow diagram of the trial is presented in Figure 2. None of the patients participating in the study developed any adverse effect related to the study protocol. Clinico-pathological characteristics of the study participants are summarized in Table 1. Statistically significant lower intraoperative fentanyl consumption was observed in group A (bupivacaine) vs. group B (placebo); mean fentanyl consumption was 0.38 and 0.43, respectively (p=0.011). In group A (bupivacaine) lower morphine (administered using PCA) consumption between the 4th and 12th hour after surgery was observed vs. consumption in group B (placebo) – 1.24 mg vs. 2.35mg, respectively (p=0.02). For group A (bupivacaine) lower morphine consumption (however, not reaching the level of statistical significance) was also observed for the other time intervals (during the first, the first 4, and the first 12 hours after surgery and between the 1st and 4th hour after surgery) mean values 0.72 mg vs. 1.23 mg; 3.3 mg vs. 4.15; 3.68 mg vs. 5.31 mg and 3.72 mg vs. 4.31, respectively, p>0.05) (Table 2). No significant differences between groups A and B were observed with respect to the time to the first dose of morphine administered using PCA in the post-operative period (mean 123 min vs. 175 min), total amount of morphine administered using PCA (mean 5.49 mg vs. 6.67mg), number of attempts to launch PCA during lockout (mean 2.2 vs. 1.2), and number of morphine doses administered using PCA (mean 3.1 vs. 3.9).
Figure 2.
CONSORT diagram showing flow of participants through the trial.
Table 1.
Baseline clinico-pathological data (n=106).
| Group A (bupivacaine) (n=54) | Group B (placebo) (n=52) | ||
|---|---|---|---|
| Age (years)* | 58.8 (30.6–81.7) | 60.1 (24.8–83) | |
| Body weight (kg)* | 70 (50–105) | 71 (50–110) | |
| BMI (kg/m2)* | 27.0 (19–39) | 26.7 (18.4–39.1) | |
| ASA | I | 9 | 9 |
| II | 33 | 30 | |
| III | 12 | 13 | |
| cTNM | I | 13 | 11 |
| II | 26 | 24 | |
| IIIA | 15 | 17 | |
| pTNM | I | 14 | 16 |
| II | 23 | 21 | |
| IIIA | 17 | 15 | |
| Duration of operation (min)* | 75 (32–168) | 80 (37–185) | |
| Duration of anesthesia (min)* | 110 (65–190) | 103.5 (65–210) | |
| Theatre time (min)* | 135 (80–240) | 135 (50–225) | |
| Hospital stay (day)* | 1.0 (1–5) | 2.0 (1–6) | |
Values are median (range);
BMI – body mass index; ASA – anesthesia risk according to American Society of Anesthesiologists; cTNM – clinical classification of malignant tumors according to UICC(Sobin and Wittekind, 2002) [28]; pTNM – histopathological classification of malignant tumors according to UICC (Sobin and Wittekind, 2002) [28].
Table 2.
Summary of the results (primary and secondary endpoints) (n=106).
| Group A (bupivacaine)(n=54) | Group B (placebo)(n=52) | p value | ||||
|---|---|---|---|---|---|---|
| Median | Range | Median | Range | |||
| Primary endpoints | ||||||
| VAS | 0 (immediately after awaking) | 0 | 0–7 | 1 | 0–9 | 0.077 |
| 1st post-operative hour | 2 | 0–6 | 3 | 0–8 | 0.126 | |
| 2nd post-operative hour | 1 | 0–6 | 2 | 0–9 | 0.09 | |
| 3rd post-operative hour | 0 | 0–8 | 1 | 0–6 | 0.105 | |
| 4th post-operative hour | 0 | 0–6 | 1 | 0–6 | 0.004 | |
| 8th post-operative hour | 0 | 0–8 | 0 | 0–8 | 0.193 | |
| 12th post-operative hour | 0 | 0–4 | 0 | 0–9 | 0.02 | |
| 16th post-operative hour | 0 | 0–5 | 0 | 0–6 | 0.088 | |
| 20th post-operative hour | 0 | 0–5 | 0 | 0–4 | 0.299 | |
| 24th post-operative hour | 0 | 0–8 | 1 | 0–4 | 0.352 | |
| 36th post-operative hour | 0 | 0–8 | 0 | 0–4 | 0.095 | |
| 48th post-operative hour | 0 | 0–7 | 0 | 0–6 | 0.499 | |
| Secondary endpoints | ||||||
| VAS | 0–4th post-operative hours | 5 | 0–33 | 9.5 | 0–30 | 0.016 |
| 4–12th post-operative hours | 1 | 0–15 | 2 | 0–16 | 0.011 | |
| 12–24th post-operative hours | 1 | 0–18 | 3 | 0–15 | 0.106 | |
| 24–48th post-operative hours | 3 | 0–23 | 5 | 0–20 | 0.997 | |
| 0–12th post-operative hours | 5.5 | 0–42 | 11 | 0–37 | 0.027 | |
| 0–48th post-operative hours | 9 | 0–61 | 13 | 0–50 | 0.076 | |
| Intraoperative fentanyl consumption [mg] | 0.4 | 0.2–0.7 | 0.4 | 0.25–0.9 | 0.011 | |
| PCA-MF consumption during 1st post-operative hour | 0 | 0–4 | 0 | 0–14 | 0.479 | |
| PCA-MF consumption during first 4 post-operative hours | 2 | 0–24 | 2 | 0–24 | 0.517 | |
| PCA-MF consumption during first 12 post-operative hours | 2 | 0–26 | 4 | 0–24 | 0.099 | |
| PCA-MF consumption from 1st till 4th post-operative hour | 2 | 0–24 | 2 | 0–26 | 0.519 | |
| PCA-MF consumption from 4th till 12th post-operative hour | 0 | 0–12 | 2 | 0–9 | 0.02 | |
MF – morphine; VAS – visual analogue scale; PCA – bad demand – number of attempts to launch PCA during lockout; PCA – patient controlled analgesia.
Additional analysis was performed on the group of patients under the age of 60 years (n=59), including bupivacaine-treated patients (n=32, former group A) and patients receiving placebo (n=27, former group B). Statistically significant differences were observed with respect to mean time to first dose of morphine delivered using PCA method (70 min vs. 130 min; p=0.008), and the total number of morphine doses was lower for bupivacaine-receiving patients (2.5 vs. 4.3; p=0.049).
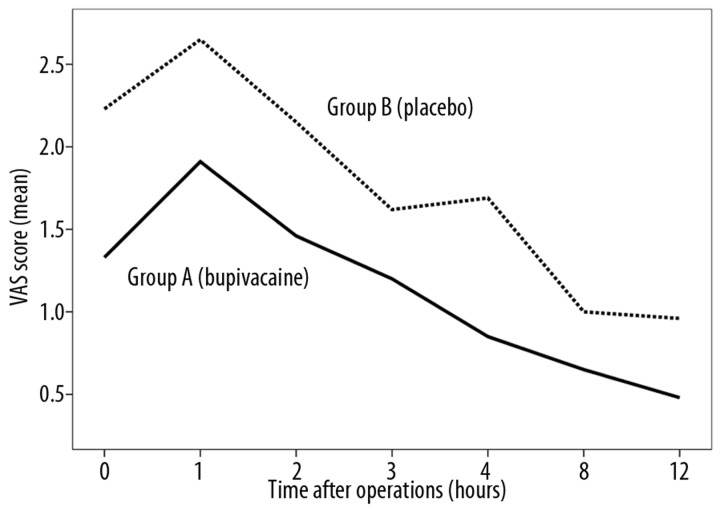
Significantly lower pain sensation assessed using the VAS scale was observed in group A (bupivacaine) as compared to group B (placebo) at the 4th postoperative hour (mean VAS score 0.85 vs. 1.69; p=0.004) and at the 12th postoperative hour (mean VAS score: 0.48 vs. 0.96; p=0.02) (Table 2). No statistically significant differences in subjective assessment of pain intensity were observed for the 2 groups at other time intervals – immediately after waking after surgery, and at 1,2,3,8,16,24,36 and 48 hours after surgery (p>0.05). Nonetheless, in group B (placebo) the mean VAS score values were found to be higher at these time intervals (Figure 3).
Figure 3.
Diagram of mean VAS score in postooperative period in patients in group A (bupivacaine) and group B (placebo).
Analysis of numerical values of pain intensity according to VAS scale summed up in the following time ranges – 0–4 hrs, 4–12 hrs, 12–24 hrs, 12–24 hrs, 24–48 hrs, 0–12 hrs and 0–48 hrs – showed lower pain intensity reported by patients from group A (bupivacaine) vs. group B (placebo) (Table 2). Statistical significance was observed for 2 time intervals – the first 4 hours after surgery and the first 12 hours after surgery.
Any pain (defined as VAS score 1–10) was reported less frequently by the patients from group A (bupivacaine) vs. group B (placebo); this association was noted at the 3rd, 4th and 12th postoperative hours. No pain (VAS=0) or just slight pain sensation (VAS=1) was reported more often by group A patients vs. group B patients, but the association was statistically significant only at the 4th postoperative hour (Table 3).
Table 3.
Comparison of the groups of patients with no pain (VAS=0) vs. any pain (VAS=1–10) and no pain or only slight pain sensation (VAS=0 or VAS=1) vs. strong pain (VAS=2–10) between group A (bupivacaine) and group B (placebo).
| Time | Group A (bupivacaine)(n=54) | Group B (placebo)(n=52) | p value | ||
|---|---|---|---|---|---|
| VAS=0 | VAS=1–10 | VAS=0 | VAS=1–10 | ||
| 0 (immediately after awaking) | 32 | 22 | 25 | 27 | 0.248 |
| 1st post-operative hour | 19 | 35 | 15 | 37 | 0.485 |
| 2nd post-operative hour | 25 | 29 | 17 | 35 | 0.152 |
| 3rd post-operative hour | 30 | 24 | 19 | 33 | 0.05 |
| 4th post-operative hour | 35 | 19 | 19 | 33 | 0.004 |
| 8th post-operative hour | 37 | 17 | 31 | 21 | 0.339 |
| 12th post-operative hour | 41 | 13 | 27 | 25 | 0.01 |
| 16th post-operative hour | 42 | 12 | 33 | 19 | 0.105 |
| 20th post-operative hour | 33 | 21 | 26 | 26 | 0.25 |
| 24th post-operative hour | 30 | 24 | 21 | 31 | 0.118 |
| VAS=0 or VAS =1 | VAS=2–10 | VAS=0 or VAS=1 | VAS=2–10 | ||
| 0 (immediately after awaking) | 35 | 19 | 28 | 24 | 0.25 |
| 1st post-operative hour | 25 | 29 | 20 | 32 | 0.415 |
| 2nd post-operative hour | 31 | 23 | 25 | 27 | 0.336 |
| 3rd post-operative hour | 38 | 16 | 32 | 20 | 0.337 |
| 4th post-operative hour | 41 | 13 | 29 | 23 | 0.028 |
| 8th post-operative hour | 46 | 8 | 37 | 15 | 0.08 |
| 12th post-operative hour | 46 | 8 | 41 | 11 | 0.395 |
| 16th post-operative hour | 46 | 8 | 40 | 12 | 0.277 |
| 20th post-operative hour | 43 | 11 | 37 | 15 | 0.311 |
| 24th post-operative hour | 37 | 17 | 33 | 19 | 0.583 |
VAS – Visual Analogue Scale.
Discussion
Pain is inevitably associated with any surgical procedure, having first the form of acute and later chronic disturbance. Pain sensation depends on the degree of surgical trauma, previous pain experiences of the patient, the patient’s general medical condition and environmental and individual conditionings [3]. Every patient requires an individual approach to pain management. Multimodal analgesia (MA) is one of the possible treatment options, and is based on simultaneous administration of a few analgesic agents what allows for making the most of their synergistic effects and at the same time ensures better efficacy when compared with monotherapy. Opioids are one of the MA elements used in pain management in the post-operative period. Their use is associated with a number of adverse effects such as drowsiness, nausea and vomiting that, in the post-operative period, may be particularly inconvenient; therefore various techniques of local anesthesia are more and more frequently included in the MA protocols since local anesthesia is associated with considerably fewer adverse effects. The application of long-acting analgesics for infiltration of surgical incisions, plexus nerves and intercostal nerves, as well as for intrapleural analgesia and spinal anesthesia, allows for several hours of pain control in a large group of patients. Nonetheless, despite the application of even better therapeutic methods in pain management in the post-operative period, pain sensation is still reported by more then half of patients [16].
At the beginning of the 1980s it was suggested that stimulation associated with surgical trauma modifies response of the nervous system, leading to a decrease of the threshold of excitability of the nerve endings located in the spinal cord [5,16–18]. Preemptive analgesia (PA) is one of the methods preventing the occurrence of the above-mentioned hypersensitivity to pain stimuli. In the last 20 years a number of studies have been published on the application of PA strategy in surgery. In the surgeries performed in the areas of the body that have segmental nerves only (eg, the extremities), PA is effective. Conversely, in abdominal surgery the structures have both segmental nerves and visceral nerves and therefore PA is ineffective [8]. A small number of reports have been published in the area of breast surgery, evaluating application of epidural (morphine, bupivacaine, fentanyl) and intravenous (ketamine, non-steroidal anti-inflammatory drugs [NSAIDs], opiates) drugs as well as local infiltration of the area of the surgical incision by anesthetic agents (bupivacaine, lidocaine) [11–13,19–21]. In a 2002 meta-analysis of 16 clinical studies, no beneficial analgesic effect of infiltration of surgical incisions with a local anesthetic agent (bupivacaine, ropivacaine or lignocaine) was observed for patients undergoing hernia repair surgery, appendectomy, tonsillectomy and breast biopsy [7,22]. As regards mastectomy, in the same meta-analysis and in a few subsequent studies on PA application, no benefits were correlated with the application of local anesthetic agents to the area of surgical incision prior to surgery [23].
Another weighty issue associated with pain management and, in particular, selection of adequate analgesic strategy, is the proper methodology for assessment of pain intensity and the possibility of its comparison between the patients. For the evaluation of pain intensity, subjective methods can be used in which patients themselves assess the strength of pain, while some more objective methods are based, for instance, on assessment of the amount of analgesic agents used by the patient [7,9]. The Visual Analogue Scale (VAS) has a graphical character, and is among the most widely used subjective methods [24]. On a 10cm-long horizontal line the patient indicates a point that corresponds to the amount of pain felt, ranging across a continuum from none (VAS=0) to an extreme amount of pain (VAS=10). In the case of local anesthetic agents such as bupivacaine or ropivacaine, pain evaluation is particularly important during the period of drug action (ie, the first 12 hours after their administration). In the present study we have shown that patients preemptively receiving bupivacaine along the line of the planned surgical incisions reported less pain intensity according to VAS scale vs. patients receiving placebo. The observed good effect provides evidence supporting the hypothesis that decreasing the number of stimuli reaching the central nervous system from the nociceptors achieved through administration of local anesthetic agents limits the development of peripheral and central sensitization [16]. The obtained results support the hypothesis that PA is an efficient anesthetic approach in the surgery of organs nerved segmentally. Good results of PA application were previously observed in hand surgery [8,25]. The results of the present study are in contrast to the previously reported studies assessing PA application in patients undergoing mastectomy or in those who had breast biopsy performed. Previously no beneficial effect of preemptive infiltration of the planned surgical incisions with local anesthetics was observed. It seems that the observed discrepancy results from dissimilar study methodology and low number of analyzed cases, other analgesics used for anesthesia (ropivacaine), and from the fact that the previously published studies for the most part did not comply with the conditions of a double-blind randomized study [12,14].
The amount of anesthetic agents used during surgery and/or in the post-operative period can be used to refine the assessment of pain intensity. In the present study it was the responsibility of the anesthesiologist to decide on the quantity of fentanyl administered during surgery. The decision was based on clinical symptoms presented by the patient that might suggest pain distress, such as increase in HR and/or RR. The amount of fentanyl used in the group of patients undergoing mastectomy with preemptive infiltration of the planned surgical incisions with bupivacaine was lower as compared to the amount used in the control group. This further supports the hypothesis that local anesthetics limit peripheral and central sensitization. Our observations serve as decisive arguments supporting the introduction of PA into everyday clinical practice of pain management in patients undergoing mastectomy. In the available literature no previous study was found that showed a difference in the amount of analgesic agents used during surgery when pre-emptive anesthesia of surgical incision lines was performed. Nevertheless, Dohda et al reported that use of gabapentin in preemptive analgesia allowed significant reduction in the amount of fentanyl administered during surgery [26].
The analysis of the amount of morphine delivered by means of a PCA in the post-operative period was yet another step in the pain assessment performed in our study on the group of patients undergoing mastectomy. In the hours soon after surgery the amount of morphine used was lower in the group of patients who had PA prior to mastectomy as compared to the control group. However, if the entire post-operative period was analyzed, no statistically significant differences in morphine (PCA) use were observed between the analyzed groups.
In the available literature no study using a similar methodological approach (ie, assessing the total amount of morphine in the post-operative period in patients receiving PA prior to main surgery) was found. Vallejo et al found that usually fentanyl and not morphine was used in the post-operative period, and that patients undergoing segmental mastectomy and not receiving a prior local anesthesia with bupivacaine had higher amounts of fentanyl use when compared to those receiving PA [11].
Not all patients are eligible for infiltration with a local anesthetic agent prior to mastectomy. The criteria of exclusion from PA are known allergies to local anesthetic agents and past history of a chronic pain disorder or a psychiatric disease. In the present study no adverse event related to the usage of local anesthetics occurred. On the other hand, following the suggestion of an anesthesiologist, some of the patients did not receive metamizole. In most of the cases the rationale for such a modification of the study protocol derived from the complicated course of anesthesia and concern for possible adverse effects related to administration of metamizole. All of those situations were carefully described in the study protocol. The other problem connected with application of PA methodology in clinical practice is lack of patient compliance, which obstructs efficient pain management and appropriate assessment of the therapy efficiency.
The encouraging results obtained in the course of our study on a group of patients undergoing mastectomy due to breast cancer – decreased pain intensity, less fentanyl uptake during surgery and lower amount of morphine used in the early post-operative hours in patients undergoing PA – may become the starting point for further studies on the application of local anesthesia to the area of planned surgical incisions in other surgical procedures, not only in breast surgery (for instance, breast-conserving surgery), but also in other organs nerved segmentally. In patients undergoing mastectomy, the infiltration of the skin in the area of the planned surgical incision with a local anesthetic agent not only improves quality of life and reduces time to recovery, but also may allow for shorter hospitalization of the patient and therefore reduced costs of treatment. It appears that bupivacaine application in breast cancer patients does not extend duration of surgery and it does not modify the surgical procedure. The time needed for the drug to start acting may be used to carry out compulsory preparations of the patient for the surgery. Lower pain intensity in the early hours after the surgery allows for earlier mobilization of the patient, which might reduce the number of embolic and thrombotic complications. No difference in the duration of the post-operative period for patients undergoing breast amputation due to cancer was observed in our study because our center, unlike most European centers, has a “short-stay” policy in which after surgery the stay in the hospital is limited to the necessary minimum [27]. Application of bupivacaine prior to surgery might be of help in deciding on early discharge from the hospital in case other contraindications were not present in this particular group of patients.
Conclusions
PA application in the form of infiltration of the area of planned surgical incisions with bupivacaine in patients undergoing mastectomy because of breast cancer decreases post-operative pain sensation, limits the amount of fentanyl used during surgery, and reduces the demand for opiates in the hours soon after surgery. The beneficial effect of bupivacaine on the level of pain reported by patients in the post-operative period contributed to eventual inclusion of this method in the standard procedures of treatment for patients undergoing mastectomy for breast cancer in our Department. Furthermore, the combination of local anesthesia administered prior to main surgery with PCA-administered drugs in the post-operative period constitutes an efficient pain control strategy. Our results should persuade clinicians to try similar procedures in other surgical treatments.
Acknowledgments
We thank the staff of the Department of Surgical Oncology, Medical University of Gdansk, and the clinical trial team for their collaboration, and the patients who participated in the study. Beata Lipska MD, PhD helped in translation of the manuscript.
Abbreviations
- PCA
Patient Controlled Analgesia
- PMPS
Post-Mastectomy Chronic Pain Syndrome
- PA
Preemptive Analgesia
- ASA
American Society of Anesthesiologists
- VAS
Visual Analog Scale
- MA
Multimodal Analgesia
- NSAID
Non-Steroidal Anti-Inflammatory Drugs
- US
Ultrasound Imaging
- MM
Mammography
- cTNM-UICC
clinical classification of malignant tumors according to UICC
- pTNM-UICC
pathological classification of malignant tumors according to UICC
- MF
Morphine
Footnotes
Conflict of interest
This study did not receive any financial support. ClinicalTrials.gov registration number: ISRCTN33898123.
Source of support: Departmental sources
References
- 1.Lakdja F, Dixmérias F, Bussières E, et al. Preemptive analgesia on postmastectomy pain syndrome with ibuprofen-arginine. Bull Cancer. 1997;84:259–63. [PubMed] [Google Scholar]
- 2.Reuben SS, Makari-Judson G, et al. Evaluation of efficacy of the perioperative administration of venlafaxine XR in the prevention of postmastectomy pain syndrome. J Pain Symptom Manage. 2004;27:133–39. doi: 10.1016/j.jpainsymman.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Kelly DJ, Ahmad M, Brull SJ. Preemptive analgesia I: Physiological pathways and pharmacological modalities. Can J Anesth. 2001;48:1000–10. doi: 10.1007/BF03016591. [DOI] [PubMed] [Google Scholar]
- 4.Kelly DJ, Ahmad M, Brull SJ. Preemptive analgesia II: Recent advances and current trends. Can J Anesth. 2001;48:1091–101. doi: 10.1007/BF03020375. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman E, Epstein JB, Gorsky M, et al. Preemptive analgesia and local anesthesia as a supplement to general anesthesia: a review. Anesth Prog. 2005;52:29–38. doi: 10.2344/0003-3006(2005)52[29:PAALAA]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wall PD. The prevention of postoperative pain. Pain. 1988;33:289–90. doi: 10.1016/0304-3959(88)90286-2. [DOI] [PubMed] [Google Scholar]
- 7.Møiniche S, Kehlet H, Dahl JB. A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: The role of timing of analgesia. Anesthesiology. 2002;96:725–41. doi: 10.1097/00000542-200203000-00032. [DOI] [PubMed] [Google Scholar]
- 8.Aida S, Baba H, Yakakura T, et al. The Effectivness of Preemtive Analgesia Varies According to the Type of Surgery: A Randomized, Double-Blind Study. Anesth Analg. 1999;89:711–16. doi: 10.1097/00000539-199909000-00034. [DOI] [PubMed] [Google Scholar]
- 9.Ong CK, Lirk P, Seymour RA, et al. The efficacy of preemptive analgesia for acute postoperative pain management: A meta-analysis. Anesth Analg. 2005;100:757–73. doi: 10.1213/01.ANE.0000144428.98767.0E. [DOI] [PubMed] [Google Scholar]
- 10.Reuben SS, Buvanendran A. Preventing the development of chronic pain after orthopaedic surgery with preventive multimodal analgesic techniques. J Bone Joint Surg Am – Series A. 2007;89:1343–58. doi: 10.2106/JBJS.F.00906. [DOI] [PubMed] [Google Scholar]
- 11.Vallejo MC, Phelps AL, Sah N, et al. Preemptive Analgesia With Bupivacaine for Segmental Mastectomy. Reg Anesth Pain Med. 2006;31:227–32. doi: 10.1016/j.rapm.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Rica MAI, Norlia A, Rohaizak M, et al. Preemptive ropivacaine local anaesthetic infiltration versus postoperative ropivacaine wound infiltration in mastectomy: Postoperative pain and drain outputs. Asian J Surg. 2007;30:34–39. doi: 10.1016/S1015-9584(09)60125-1. [DOI] [PubMed] [Google Scholar]
- 13.Adam F, Libier M, Oszustowicz T, et al. Preoperative small-dose ketamine has no preemptive analgesic effect in patients undergoing total mastectomy. Anesth Analg. 1999;89:444–47. doi: 10.1097/00000539-199908000-00036. [DOI] [PubMed] [Google Scholar]
- 14.Sidiropoulou T, Buonomo O, Fabbi E, et al. A prospective comparison of continuous wound infiltration with ropivacaine versus single-injection paravertebral block after modified radical mastectomy. Anesth Analg. 2008;106:997–1001. doi: 10.1213/ane.0b013e31816152da. [DOI] [PubMed] [Google Scholar]
- 15.Shen XF, Wang FZ, Xu SQ, et al. Comparison of the analgesic efficacy of preemptive and preventive tramadol after lumpectomy. Pharmacol Rep. 2008;60:415–21. [PubMed] [Google Scholar]
- 16.Gottschalk A, Smith DS. New concepts in acute pain therapy: Preemptive analgesia. Am Fam Physician. 2001;63:1979–86. [PubMed] [Google Scholar]
- 17.Grape S, Tramèr MR. Do we need preemptive analgesia for the treatment of postoperative pain? Best Pract Res Clin Anaesthesiol. 2007;21:51–63. doi: 10.1016/j.bpa.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Pogatzki-Zahn EM, Zahn PK. From preemptive to preventive analgesia. Cur Opin Anaesthesiol. 2006;19:551–55. doi: 10.1097/01.aco.0000245283.45529.f9. [DOI] [PubMed] [Google Scholar]
- 19.Pettersson N, Perbeck L, Hahn RG. Efficacy of subcutaneous and topical local anaesthesia for pain relief after resection of malignant breast tumours. Eur J Surg. 2001;167:825–30. doi: 10.1080/11024150152717652. [DOI] [PubMed] [Google Scholar]
- 20.Riest G, Peters j, Weiss M, et al. Does perioperative administration of rofecoxib improve analgesia after spine, breast and orthopedic surgery? Eur J Anaesthesiol. 2006;23:219–26. doi: 10.1017/S026502150500222X. [DOI] [PubMed] [Google Scholar]
- 21.Wang F, Shen XF, Xu SQ, et al. Preemptive combined preventive delivery of flurbiprofen axetil produced effective analgesia after lumpectomy. Acute Pain. 2008;10:65–71. [Google Scholar]
- 22.Inanoglu K, Akkurt BCO, Turhanoglu S, et al. Intravenous ketamine and local bupivacaine infiltration are effective as part of a multimodal regime for reducing post-tonsillectomy pain. Med Sci Monit. 2009;15(10):CR539–43. [PubMed] [Google Scholar]
- 23.Baudry G, Steghens A, Laplaza D, et al. Ropivacaine infiltration during breast cancer surgery: Postoperative acute and chronic pain effect. Ann Fr Anesth Reanim. 2008;27:979–86. doi: 10.1016/j.annfar.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Myles PS, Fanzach F, Troedel S, et al. The Pain Visual Scale: Is it Linear or Nonlinear? Anesth Analg. 1999;89:1517–20. doi: 10.1097/00000539-199912000-00038. [DOI] [PubMed] [Google Scholar]
- 25.Rømsing J, Møiniche S, Østergaard D, et al. Local infiltration with NSAIDs for postoperative analgesia: Evidence for a peripheral analgesic action. Acta Anaesthesiol Scand. 2000;44:672–83. doi: 10.1034/j.1399-6576.2000.440607.x. [DOI] [PubMed] [Google Scholar]
- 26.Dohda NM, Rady A, El Azab SR. Preoperative use of gabapentin decreases the anesthetic and analgetic requirements in patients undergoing radical mastectomy. Egypt J Anesth. 2010;26:287–91. [Google Scholar]
- 27.De Kok M, Weijden T, Voogd A, et al. Implementation of short-stay programme after breast cancer surgery. Br J Surg. 2010;97:189–94. doi: 10.1002/bjs.6812. [DOI] [PubMed] [Google Scholar]
- 28.Sobin LH, Wittekind Ch. TNM Classification of Malignant Tumours. 6th edition. John Wiley & Sons; New Jersey: 2002. [Google Scholar]