Summary
Background
Hemorrhagic shock (HS) followed by resuscitation can induce the production of several inflammatory mediators and lead to multiple organ dysfunction. The molecular mechanism of biologic responses to rosiglitazone has an anti-inflammatory effect. The present study was designed to investigate the effects of rosiglitazone on physiopathology and inflammatory mediators after HS in rats.
Material/Methods
HS was induced in rats by withdrawing 60% of the total blood volume from a femoral artery catheter, immediately followed by intravenous injection of 0.3 mg/kg rosiglitazone. Mean arterial pressure (MAP) and heart rate (HR) were monitored continuously for 12 h. Levels of biochemical parameters, including GOT, GPT, BUN, Cre, LDH, CPK, and lactate were measured at 30 min before induction of HS and 0, 1, 3, 6, 9, and 12 h after HS, while an equal volume of normal saline was replaced as fluid resuscitation. Inflammatory mediators, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1), were measured in serum at 1 and 12 h after HS. The kidneys, liver, lungs, and small intestine were removed for histological assessment by hematoxylin and eosin stained at 48 h after HS.
Results
HS significantly increased blood GOT, GPT, BUN, Cre, LDH, CPK, lactate, glucose, TNF-α, IL-6 and MCP-1 levels, induced tachycardia, and decreased mean arterial pressure (MAP) in rats. Treatment with rosiglitazone improved survival rate, decreased the markers of organ injury, and suppressed the release of TNF-α, IL-6, and MCP-1 after HS in rats.
Conclusions
Treatment with rosiglitazone suppresses the release of serum TNF-α, IL-6 and MCP-1, and ameliorates HS-induced organ damage in rats.
Keywords: hemorrhagic shock, rosiglitazone, cytokine, chemokine, rats
Background
Hemorrhagic shock (HS) is one of the most frequent types of shock [1]. HS can lead to hemodynamic instability, decrease in oxygen delivery, and induce tissue hypoperfusion, along with causing cellular hypoxia, organ damage, and death [1,2]. After HS, nuclear factor-κB (NF-κB) is activated. NF-κB signaling is the activation of inflammatory genes that cause the expression of several cytokines and adhesion molecules [1]. Overwhelming production of inflammatory cytokines such as tumor necrosis factor-α (TNF-α) or interleukin-6 (IL-6) or chemokine (MCP-1) can lead to multiple organ dysfunction and death after HS [1–3].
Peroxisome proliferator-activated receptors (PPAR) are ligand-activated transcription factors belonging to the nuclear hormone receptor superfamily, and activation of these receptors can regulate inflammatory responses [4]. PPAR-γ can repress gene transcription by negatively interfering with signal-transduction pathways, such as the NF-κB signaling pathway, and decreases the expression of inflammatory mediators such as TNF-α, IL-6 and MCP-1 production [5,6]. Our previous study found rosiglitazone suppresses the release of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) production, and decreases the levels of markers of organ injury in endotoxic shock in rats [7]. HS can produce the same inflammatory effect as endotoxic shock after LPS [8]. For these reasons, rosiglitazone administration may provide protection against the effects of HS. In the present study, we examined the effects of rosiglitazone on HS-induced inflammatory mediators (TNF-α, IL-6, and MCP-1) and organ damage (liver, kidney, lung, and small intestine) in rats.
Material and Methods
Preparation of animals
Thirty-two male Wistar-Kyoto rats weighing 260–300 grams were purchased from the National Animal Center (Taipei, Taiwan). They were housed in the university Animal Center in a controlled environment at a temperature of 22±1°C with a 12-h light/dark cycle. Food and water were provided ad libitum. The Animal Care and Use Committee of Tzu Chi University approved the experimental protocol. The animals were anesthetized with ether inhalation for about 15 min. During the period of anaesthesia, a polyethylene catheter (PE-50) was inserted into the femoral artery to collect blood samples and was connected to a pressure transducer (Gould Instruments, Cleveland, OH, USA) to record arterial pressure (AP) and heart rate (HR) on a polygraph recorder (Power Lab, AD Instruments, Mountain View, CA, USA). Another PE-50 catheter was inserted into the femoral vein for intravenous administration of drugs or fluid. The operation was completed within 15 min, leaving a small wound (less than 0.5 cm2). After the operation, the animals were placed in a conscious rat metabolic cage (Shingshieying Instruments, Hualien, Taiwan). Rats awakened soon after the operation and HS was induced 24 h later, with the rats in a conscious state [9–11].
Hemorrhagic shock
HS was induced by drawing blood from the femoral arterial catheter into a 10 ml syringe. An infusion pump controlled the withdrawal rate to mimic a typical bleeding event. The blood volume withdrawn was 60% of the total blood volume (6 ml/100 gm BW). The duration of blood withdrawal was 30 min. Within these 30 min blood was withdrawn and the HS-procedure was carried out, followed by resuscitation with 0.5 ml normal saline at 0, 1, 3, 6, 9, and 12 h after HS. After blood withdrawal, the animals were continuously observed for 48 h and sacrificed later for pathology [9–11].
Experimental design
The animals were randomly divided into 4 groups. Rats in the Rosiglitazone group (n=8), were not subjected to HS and were given 0.3 mg/kg rosiglitazone (GlaxoSmithKline Taiwan Ltd, Taipei, Taiwan) in 0.5 ml normal saline intravenously over over a period of 10 min [12]. Rats in the HS group (n=8), HS was induced and the rats immediately received an intravenous drip of 0.5 ml normal saline over over a period of 10 min. In the Rosiglitazone + HS group (n=8), HS was induced and the rats immediately received 0.3 mg/kg rosiglitazone intravenously over over a period of 10 min. In the Control group (n=8), rats were not subjected to HS and immediately received an intravenous drip of 0.5 ml normal saline over over a period of 10 min.
Blood sample analysis
Arterial blood samples were obtained for baseline values before heparinization. Heparin (2 IU/gm BW) in 1 ml normal saline was injected via the catheter into rats over a period of 20 min [7–9]. Arterial blood samples (0.5 ml) were collected for measurement of glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), blood urea nitrogen (BUN), creatinine (Cre), lactic dehydrogenase (LDH), creatine phosphokinase (CPK), lactate, and glucose at 30 min before induction of HS and at 0, 1, 3, 6, 9, and 12 h after HS while an equal volume of 0.5 ml normal saline was used. Blood samples were immediately centrifuged at 3000 g for 10 min. The serum was decanted and separated into 2 parts; 1 part was stored at 4°C within 1 h after collection for biochemical analysis. Serum levels of GOT, GPT, BUN, Cre, LDH, CPK, lactate, and glucose were measured with an autoanalyzer (COBAS Integra C111, Roche Diagnostics, Basel, Switzerland) to obtain various biochemical data. The other part of the serum collected at 1, and 12 h after HS was stored at −80°C for later measurement of TNF-α, IL-6, and MCP-1 concentrations [9–11].
TNF-α, IL-6, and MCP-1 Measurement by ELISA
After 1 h and 12 h of the induction of HS, TNF-α, IL-6, and MCP-1 concentrations in the blood samples were measured separately by antibody enzyme-linked immunosorbent assay (ELISA) with commercial antibody pairs, recombinant standards, and a biotin-streptavidin-peroxidase detection system (Endogen, Rockford, IL, USA) as described previously [9–11]. Blood samples were collected in serum-separated tubes. All reagents, samples, and working standards were brought to room temperature and prepared according to the manufacturer’s directions. Reactions were quantified by optical density using an automated ELISA reader (Sunrise, Tecan Co., Grödingen, Austria) at 450/540 nm wavelength.
Histological examination
The liver, kidney, lung and small intestine were removed immediately after sacrifice at 48 h after induction of HS. Examples of the particular type of these tissues specimens were fixed overnight in 4% buffered formaldehyde, processed using standard methods, and stained for hematoxylin and eosin (H & E). One observer, who was blinded to the group assignment, performed the tissue analysis. The severity of liver injury observed in the tissue sections was scored as follows: 0, no evidence or minimal evidence of injury; 1, mild injury consisting of cytoplasmic vacuolation and focal nuclear pyknosis; 2, moderate to severe injury with extensive nuclear pyknosis, cytoplasmic hypereosinophilia, and loss of intercellular borders; and 3, severe necrosis with disintegration of the hepatic cords, hemorrhage, and neutrophil infiltration [9,10]. The severity of renal tubular injury was scored by estimating the percentage of tubules in the cortex or the outer medulla that showed epithelial necrosis or had luminal necrotic debris, tubular dilation, and hemorrhage: 0, none; 1, <5%; 2, 5% to 25%; 3, 25% to 75%; and 4, >75% [9–11]. Lung injury was scored as follows: 0, no evidence; 1, mild injury; 2, moderate injury; 3, severe injury with lung oedema, interstitial inflammatory cell infiltration, and hemorrhage [9–11]. The severity of small intestine injury was scored from 0 to 3 as follows: 0, normal (no damage); 1, mild (focal epithelial edema and necrosis); 2, moderate (diffuse swelling or necrosis of the villi); 3, severe (diffuse necrosis of the villi with evidence of neutrophil infiltration in the submucosa and/or hemorrhage) [9–11]. All evaluations were made on 5 fields per section and 5 sections per organ.
Statistical analysis
Data are expressed as mean ±SD. Statistical comparisons between different groups at corresponding time points were made by repeated measures of 2-way ANOVA followed by a post hoc test (Bonferroni’s method). Survival analysis was performed using the Kaplan-Meier estimator and comparisons among the mortality rates of the groups were made with the log rank test. Histological scores were analyzed by the Kruskal-Wallis test followed by the Dunn’s test. A p value less than 0.05 was considered statistically significant.
Results
Survival rate, mean arterial pressure (MAP) and heart rate (HR)
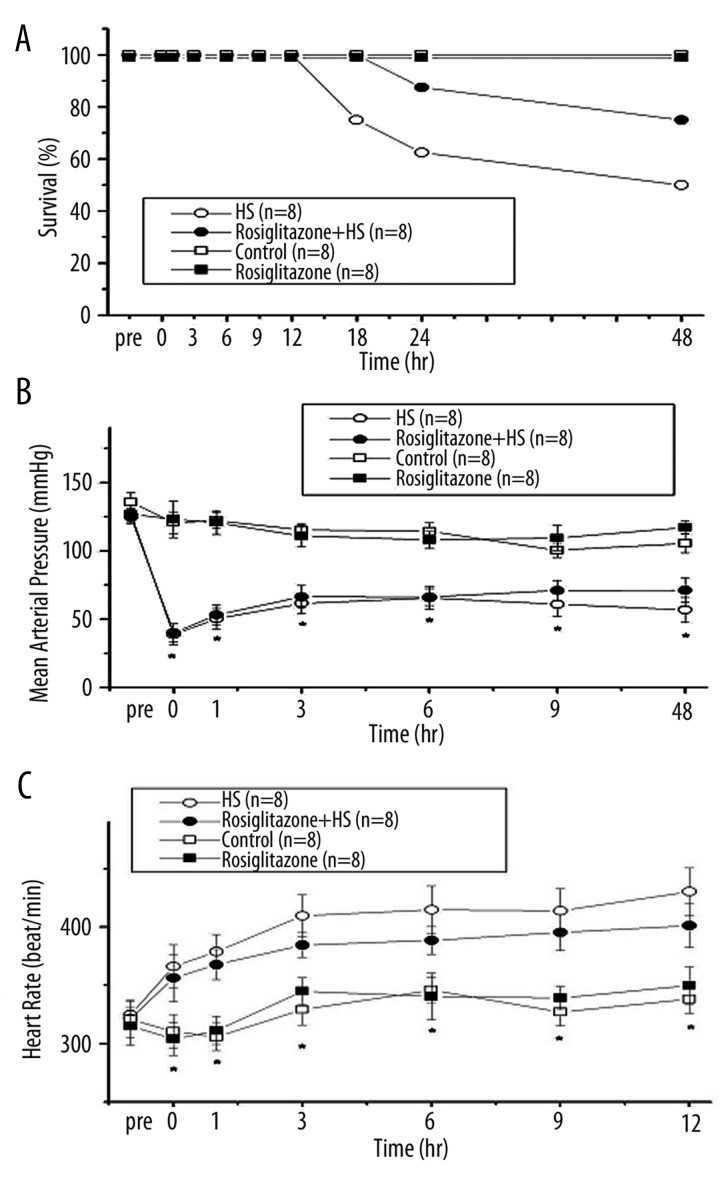
All rats were alive at 12 h after induction of HS. However, in the Rosiglitazone + HS group the rats were dead by 18 h after induction of HS, and in the HS group the rats were dead by 24 h after induction of HS. The survival rate at 48 h after induction of HS was 50% in the HS group, 100% in the Control group, 100% in the Rosiglitazone group, and 75% in the Rosiglitazone + HS group (Figure 1A). The mortality rate between groups in the Rosiglitazone + HS group was significantly lower than in the HS groups (log rank test; p=0.021). Mean arterial pressure (MAP) decreased rapidly after withdrawal of 60% of total blood volume from the femoral arterial catheter in rats. MAP stayed relatively low during 12 h after induction of HS (Figure 1B). Compared with the HS group, treatment with rosiglitazone did not affect the MAP after HS (Figure 1B). The heart rate (HR) was significantly increased during HS (Figure 1C). Compared with the HS group, HR in the rosiglitazone + HS group did not change after HS (Figure 1C).
Figure 1.
Survival curves (A), mean artery pressure (MAP) (B) and heart rate (HR) (C) after hemorrhagic shock in rats. *P<0.05 for the HS group compared with the Control group. #P<0.05 for the Rosiglitazone + HS group compared with the HS group.
Glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT)
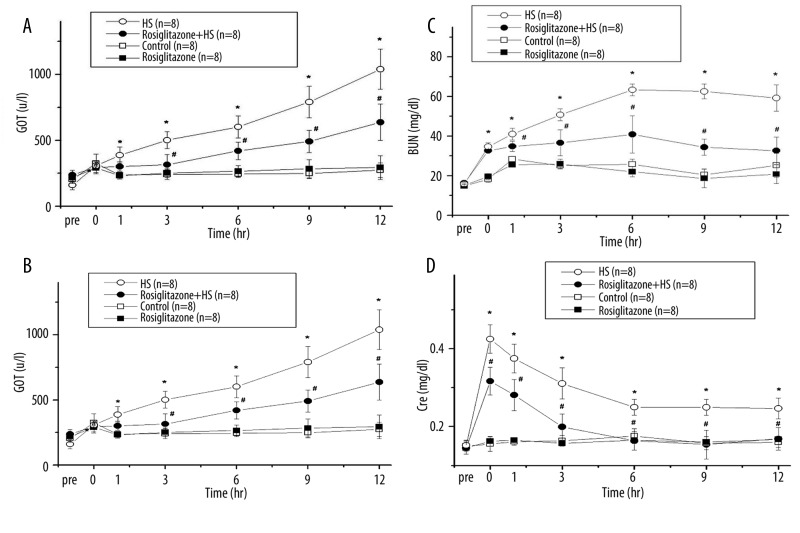
GOT was gradually increased after induction of HS (Figure 2A). Compared with the HS group, treatment with rosiglitazone decreased GOT at 3, 6, 9, and 12 h after induction of HS (#P<0.05; Figure 2A). Serum GPT increased at 0, 1, 3, 6, 9, and 12 h after HS compared with the Control group (*P < 0.05; Figure 2B). Compared with the HS group, treatment with rosiglitazone decreased the GPT at 3, 6, 9, and 12 h (#P<0.05; Figure 2B). The GOT and GPT were no statistically different between the Rosiglitazone group and the Control group.
Figure 2.
Change in serum glutamic oxaloacetic transaminase (GOT) (A), glutamic pyruvic transaminase (GPT) (B), blood urea nitrogen (BUN) (C) and creatinine (Cre) (D) after hemorrhagic shock in rats. *P<0.05 for the HS group compared with the Control group. #P<0.05 for the Rosiglitazone + HS group compared with the HS group.
Blood urea nitrogen (BUN) and creatinine (Cre)
HS increased blood BUN to a peak at 6 h (Figure 2C). Compared with the HS group, treatment with rosiglitazone decreased the BUN at 1, 3, 6, 9, and 12 h (#P<0.05; Figure 2C). Serum Cre increased rapidly after induction of HS. The values of serum Cre increased at 0, 1, 3, 6, 9, and 12 h after HS, compared with the Control group (*P<0.05; Figure 2D). Compared with the HS group, treatment with rosiglitazone decreased the serum Cre at 0, 1, 3, 6, 9, and 12 h after HS (#P<0.05; Figure 2D). The BUN and Cre were no statistically different between the Rosiglitazone group and the Control group.
Lactic dehydrogenase (LDH) and creatine phosphokinase (CPK)
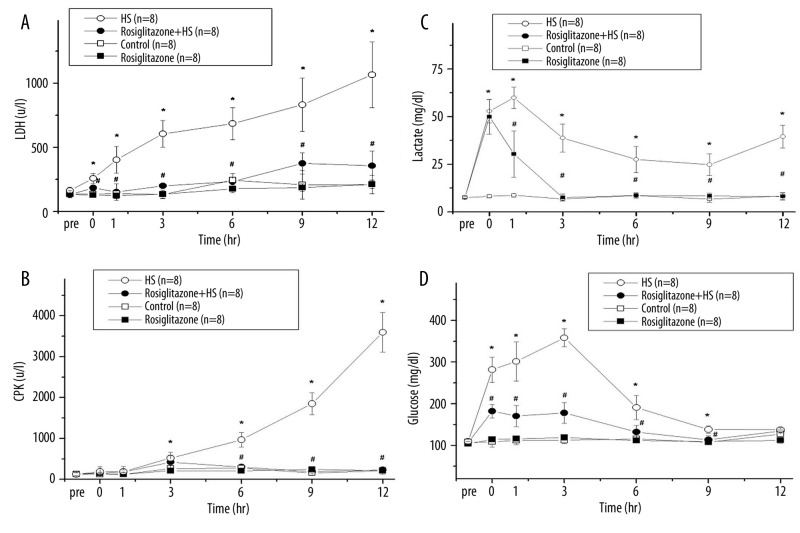
LDH was gradually increased after induction of HS (Figure 3A). Compared with the HS group, treatment with rosiglitazone decreased the LDH at 0, 1, 3, 6, 9, and 12 h after induction of HS (#P<0.05; Figure 3A). Blood CPK increased gradually after induction of HS (Figure 3B). Compared with the HS group, treatment with rosiglitazone decreased the CPK at 6, 9, and 12 h after induction of HS (#P<0.05; Figure 3B). The LDH and CPK were no statistically different between the Rosiglitazone group and the Control group.
Figure 3.
Change in serum lactic dehydrogenase (LDH) (A), creatine phosphokinase (CPK) (B), lactate (C) and glucose (D) after hemorrhagic shock in rats. *P<0.05 for the HS group compared with the Control group. #P<0.05 for the Rosiglitazone + HS group compared with the HS group.
Lactate and glucose
Serum lactate increased at 0, 1, 3, 6, 9, and 12 h after HS, compared with the Control group (*P<0.05; Figure 3C). Compared with the HS group, treatment with rosiglitazone decreased the serum lactate at 1, 3, 6, 9, and 12 h after HS (#P<0.05; Figure 3C). Blood glucose increased to a peak at 3 hr (Figure 3D) and returned to baseline at 12 hr after induction of HS. Compared with the HS group, the Rosiglitazone + HS group demonstrated decreased glucose at 0, 1, 3, 6 and 9 h (#P<0.05; Figure 3D). The lactate and glucose were not statistically different between the Rosiglitazone group and the Control group.
Tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1)
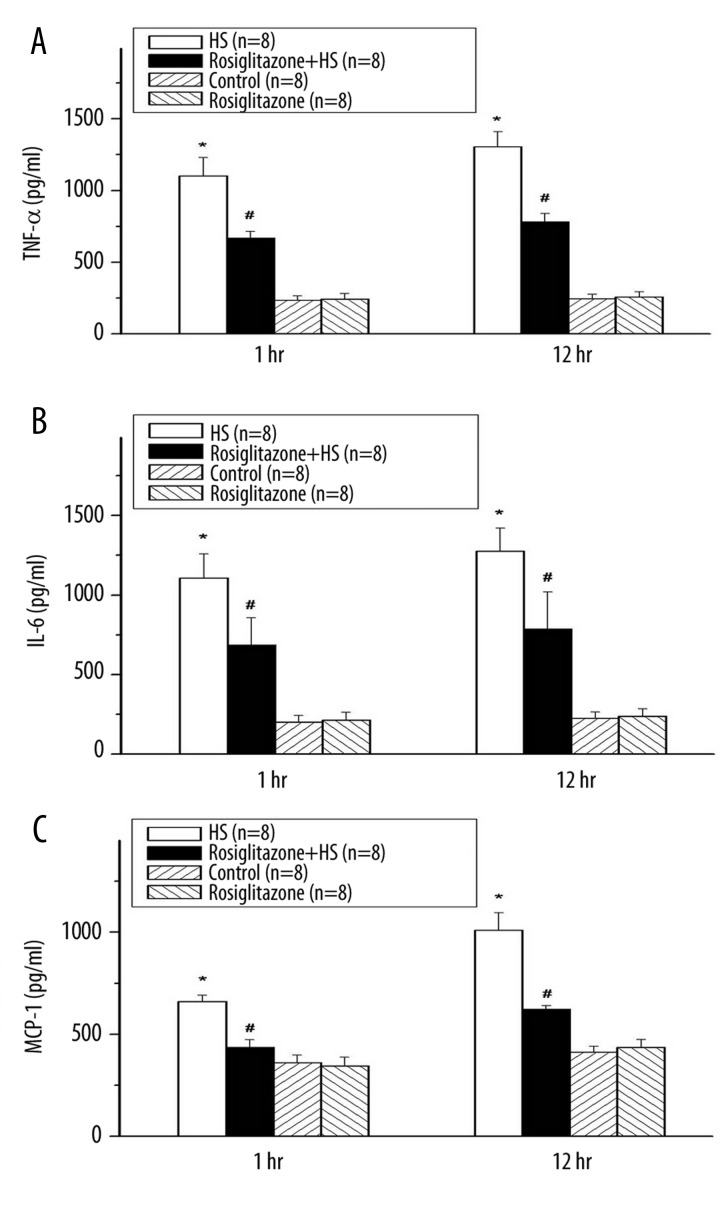
HS greatly elevated the serum TNF-α compared with the Control group at 1 and 12 h after induction of HS (*P<0.05; Figure 4A). Treatment of rosiglitazone significantly decreased the serum TNF-α at 1 and 12 h after induction of HS (#P<0.05; Figure 4A). HS increased the serum IL-6, compared with the Control group at 1 and 12 h after induction of HS (*P<0.05; Figure 4B). Rosiglitazone decreased the serum IL-6, compared with the HS group at 1 and 12 h after induction of HS (#P<0.05; Figure 4B). HS increased the serum MCP-1 compared with the Control group (*P<0.05; Figure 4C). Treatment with rosiglitazone decreased the serum MCP-1 compared with the HS group after induction of HS (#P<0.05; Figure 4C). The TNF-α, IL-6 and MCP-1 were not statistically different between the Rosiglitazone group and the Control group.
Figure 4.
Change in serum tumor necrosis factor-α (TNF-α) (A), interleukin-6 (IL-6) (B) and monocyte chemoattractant protein-1 (MCP-1) (C) after hemorrhagic shock in rats. *P<0.05 for the HS group compared with the Control group. #P<0.05 for the Rosiglitazone + HS group compared with the HS group.
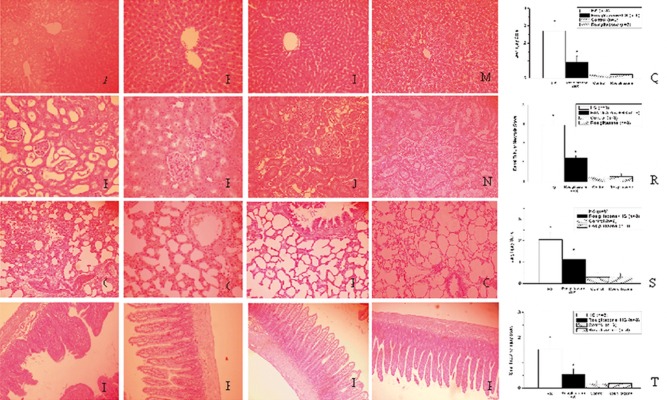
Histopathology of liver, kidney, lung, and small intestine
After the induction of HS at 48 h, only 4 rats were alive in the HS group, 6 rats were alive in the Rosiglitazone + HS group, and all rats were alive in the Control group. These rats were sacrificed and arranged for pathology study. H & E-stained tissue sections from the liver (Figure 5I), kidney (Figure 5J), lung (Figure 5K), and small intestine (Figure 5L) of the Control group, and the liver (Figure 5M), kidney (Figure 5N), lung (Figure 5O), and small intestine (Figure 5P) of the Rosiglitazone group are shown in Figure 5. Compared with the Control group, histopathologic analysis of H & E-stained tissue sections from the liver, kidney, lung, and small intestine after HS revealed hepatic cells necrosis and hemorrhage in the liver (Figure 5A), tubular cells swelling, nuclear loss, tubular dilatation, brush border loss and hemorrhage in the kidney (Figure 5B), pulmonary edema, hemorrhage and interstitial cell infiltration in the lung (Figure 5C), and loss of epithelial and mucosal architecture, and destruction of the vili in the small intestine (Figure 5D). Compared with the HS group, treatment with rosiglitazone abolished the histopathologic changes in the liver, kidney, lung and small intestine (Figure 5E–H). Compared with the HS group, the injury scores of the liver, kidney, lung, and small intestine were significantly decreased in the Rosiglitazone + HS group (#P<0.05; Figure 5Q–T).
Figure 5.
Histopathologic changes in the liver, kidney, lung and small intestine after hemorrhagic shock in rats. Histologic sections from the HS group (A–D), Rosiglitazone + HS group (E–H), Control group (I–L), and Rosiglitazone group (M–P), stained with hematoxylin and eosin (liver, kidney, lung: magnification ×200; small intestine: magnification ×100). Photomicrographs A, E, I and M are liver sections; B, F, J and N are kidney sections; C, G, K and O are lung sections; and D, H, L and P are small intestine sections. Histopathologic injury score in liver (Q), kidney (R), lung (S) and small intestine (T) after hemorrhagic shock in rats. *P<0.05 for the HS group compared with the Control group. #P<0.05 for the Rosiglitazone + HS group compared with the HS group.
Discussion
We found that the treatment of test rats with rosiglitazone ameliorated severe HS-induced organ damage (liver, kidney, lung, and small intestine) by decreasing serum TNF-α, IL-6, MCP-1 levels in rats.
HS is a leading cause of death in trauma patients worldwide [2]. Treatment with PPAR-γ agonist decreases mucosal injury and protects against intestinal ischemia/reperfusion injury in rats [13,14]. The results of our study show that rosiglitazone preserved intestinal epithelial and mucosal architecture, along with reducing inflammatory cell infiltration in the small intestine after HS. Among the organs damaged by HS, the lung is especially vulnerable to neutrophil accumulation, which can result in acute respiratory distress syndrome [15]. Treatment with ciglitazone ameliorated lung injury after HS in rats [16]. Our results show that treatment of rats with rosiglitazone reduced HS-induced lung injury after HS. PPAR-γ agonist protects renal tissues from different models of acute renal failure [17]. PPAR-γ antagonist GW9662 (2-chloro-5-nitrobenzanilide) augments the degree of liver injury associated with hemorrhage in the anaesthetized rat [18]. PPAR-gamma agonists modulate the course of the inflammatory reaction. The administration of rosiglitazone decreased the intensity of the inflammatory process by decreasing alpha-amylase, lipase, aminotransferases, and bilirubin in the course of sodium taurocholate-induced experimental acute pancreatitis [19]. Our results show that treatment of rats with rosiglitazone reduced the HS-induced increase in serum GOT, GPT, BUN, and Cre levels and also alleviated injury to the liver and kidney after HS. The PPAR-γ agonist 15d-PGJ2 abolished the renal dysfunction, and reduced the liver, lung and intestinal injury caused by hemorrhagic shock in rats [20]. Our results also demonstrate that treatment with rosiglitazone ameliorated severe HS-induced organ damage (liver, kidney, lung, and small intestine) in rats. Hyperlactatemia is a common complication during HS, and serum lactate has been shown to be a predictor of HS outcome [21]. In present study, we found that hyperlactatemia was observed after severe HS in rats. The increase in serum lactate generally originates from both increased lactate production and decreased hepatic or renal clearance [22]. We found that rosiglitazone decreased HS-induced liver and kidney damage, decreased lactic acid production, and increased the survival of rats after HS.
HS leads to significant loss of intravascular volume, and resuscitation can induce several inflammatory pathways such as leukocyte activation and sequestration to organs [1,2]. Endothelial cells exhibit morphologic damage, including cellular swelling, loss of adherence to the basement membrane, and adherence of activated leukocytes after HS [2]. Endothelial cell damage, accumulation of leukocytes, disseminated intravascular coagulation, and microcirculatory dysfunction finally lead to programmed cell death and necrosis of parenchymal cells, with the development of multiple organ damage in HS [1]. PPAR-γ agonist activation inhibits endothelial inflammation by suppressing inflammatory gene expression and therefore improves endothelial dysfunction [4]. These actions show rosiglitazone has vascular protection/repair properties through the mobilization and recruitement of endothelial progenitor cells to sites of endothelial injury [23].
During hemorrhage, NF-κB is involved in apoptosis and the inflammatory cascade, such as TNF-α and IL-6 [1,2]. TNF-α and IL-6 have been shown to peak early after HS [9–11]. Treatment with monoclonal antibodies to TNF-α can reduce HS-induced acute lung injury [24]. Moreover, administration of recombinant IL-6 blunts lung mRNA levels of the pro-inflammatory cytokine TNF-α production after HS in swine [25]. Regulation of the innate immunity system through NF-κB is emerging as a critical function of PPAR-γ activation [4]. Ciglitazone treatment lowered plasma IL-6 levels after HS [16]. Our results note treatment with rosiglitazone ameliorated severe HS-induced serum TNF-α and IL-6 production in rats.
Chemokines play a major role in selectively recruiting monocytes, neutrophils, and lymphocytes, as well as in inducing chemotaxis through the activation of G-protein-coupled receptors [26]. MCP-1 is one of the key chemokines that regulate migration and infiltration of monocytes/macrophages [27]. A marked increase in serum MCP-1 production was observed in HS [28]. Toll-like receptor-4 regulates significant increase in systemic MCP-1 levels, as well as in Kupffer cell MCP-1 production following hemorrhage [3]. Ciglitazone treatment decreased plasma MCP-1 levels after HS [16]. In this study, treatment with rosiglitazone also reduced HS-induced serum MCP-1 production in rats.
Stress-induced hyperglycemia is exceedingly common in critically ill patients, particularly those with HS [29]. HS induces TNF-α and IL-6 production [1]. Increased release of pro-inflammatory mediators such as TNF-α and IL-6 may play an important role in stress-induced hyperglycemia [30]. Hyperglycemia appears detrimental for the outcome of critically ill patients, because maintenance of normoglycemia with intensive insulin therapy has been shown to improve survival and reduce morbidity in prolonged critically ill patients [31]. Our result noted rosiglitazone attenuates serum TNF-α and IL-6 production and decreased HS-induced hyperglycemia in rats. Because the hypotension persisted and tissues take prolonged hypoperfusion during the experiment, the continuously high biochemical enzyme and cytokine levels, as well as high tissue injury even 48 h after HS, were noted.
Limitations of the present study include the fact that it is an animal study, and rosiglitazone has also been associated with an increased risk of myocardial infarction or/and cardiovascular mortality in humans [32–34]. In May 2007, safety concerns were raised that rosiglitazone was associated with a 43% higher risk of myocardial infarction and a 64% higher risk of cardiovascular death in humans [35]. The US Food and Drug Administration (FDA) released a safety alert concerning a possible increased risk of ischemic cardiovascular events in patients prescribed rosiglitazone. The European Medicines Agency (EMEA) recommended in September 2010 that the drug be suspended from the European market by November of that year. More data are needed to clarify the effects of rosiglitazone on ischemic heart disease events.
Conclusions
HS leads to activation of the immune system and overwhelming production of inflammatory cytokines, which can lead to multiple organ dysfunction and death [1–3]. Blocking such inflammatory cytokines can have beneficial effects after HS [1,36]. A previous study noted insulin attenuates the inflammatory response by decreasing the inflammatory cytokines, and thus restoring systemic homeostasis, which has been shown to be critical for organ function and survival in trauma patients [37]. In this study, treatment with rosiglitazone suppressed the release of TNF-α, IL-6, MCP-1 and decreased the levels of markers of organ injury after induction of HS in rats.
Footnotes
Source of support: This work was supported by grants from the Tzu Chi University (TCIRP 95008-03Y3), Tzu Chi College of Technology (TCCT-981A16), and National Science Council (NSC 96-2314-B-303-004-MY2)
References
- 1.Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36:691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Angele MK, Schneider CP, Chaudry IH. Bench-to-bedside review: latest results in hemorrhagic shock. Crit Care. 2008;12:218. doi: 10.1186/cc6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frink M, Hsieh YC, Thobe BM, et al. TLR4 regulates Kupffer cell chemokine production, systemic inflammation and lung neutrophil infiltration following trauma-hemorrhage. Mol Immunol. 2007;44:2625–30. doi: 10.1016/j.molimm.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Duan SZ, Usher MG, Mortensen RM. Peroxisome proliferator-activated receptor-gamma-mediated effects in the vasculature. Circ Res. 2008;102:283–94. doi: 10.1161/CIRCRESAHA.107.164384. [DOI] [PubMed] [Google Scholar]
- 5.Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 6.Mohanty P, Aljada A, Ghanim H, et al. Evidence for a potent antiinflammatory effect of rosiglitazone. J Clin Endocrinol Metab. 2004;89:2728–35. doi: 10.1210/jc.2003-032103. [DOI] [PubMed] [Google Scholar]
- 7.Wu WT, Lee CC, Lee CJ, et al. Rosiglitazone ameliorates endotoxin induced organ damage in conscious rats. Biol Res Nurs. 2011;13(1):38–43. doi: 10.1177/1099800409353358. [DOI] [PubMed] [Google Scholar]
- 8.Haveman JW, Muller Kobold AC, Tervaert JW, et al. The central role of monocytes in the pathogenesis of sepsis: consequences for immunomonitoring and treatment. Neth J Med. 1999;55:132–41. doi: 10.1016/s0300-2977(98)00156-9. [DOI] [PubMed] [Google Scholar]
- 9.Lee CC, Lee RP, Subeq YM, et al. Fluvastatin attenuates severe hemorrhagic shock-induced organ damage in rats. Resuscitation. 2009;80:372–78. doi: 10.1016/j.resuscitation.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Lee CJ, Lee RP, Subeq YM, et al. Propofol protects hemorrhagic shock-induced organ damage in conscious spontaneously hypertension rats. Biol Res Nurs. 2009;11:152–62. doi: 10.1177/1099800409334750. [DOI] [PubMed] [Google Scholar]
- 11.Wu WT, Lin NT, Subeq YM, et al. Erythropoietin protects severe hemorrhagic shock-induced organ damage in conscious rats. Injury. 2010;41:818–24. doi: 10.1016/j.injury.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Cuzzocrea S, Pisano B, Dugo L, et al. Rosiglitazone and 15-deoxy-Delta12,14-prostaglandin J2, ligands of the peroxisome proliferator-activated receptor-gamma (PPAR-gamma), reduce ischaemia/reperfusion injury of the gut. Br J Pharmacol. 2003;140:366–76. doi: 10.1038/sj.bjp.0705419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiest R, Rath HC. Gastrointestinal disorders of the critically ill. Bacterial translocation in the gut. Best Pract Res Clin Gastroenterol. 2003;17:397–425. doi: 10.1016/s1521-6918(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 14.Baregamian N, Mourot JM, Ballard AR, et al. PPAR-gamma agonist protects against intestinal injury during necrotizing enterocolitis. Biochem Biophys Res Commun. 2009;379:423–27. doi: 10.1016/j.bbrc.2008.11.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senthil M, Watkins A, Barlos D, et al. Intravenous injection of trauma-hemorrhagic shock mesenteric lymph causes lung injury that is dependent upon activation of the inducible nitric oxide synthase pathway. Ann Surg. 2007;246:822–30. doi: 10.1097/SLA.0b013e3180caa3af. [DOI] [PubMed] [Google Scholar]
- 16.Chima RS, Hake PW, Piraino G, et al. Ciglitazone ameliorates lung inflammation by modulating the inhibitor kappaB protein kinase/nuclear factor-kappaB pathway after hemorrhagic shock. Crit Care Med. 2008;36:2849–57. doi: 10.1097/ccm.0b013e318187810e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao Z, Ong AC. Peroxisome proliferator-activated receptor gamma agonists in kidney disease – future promise, present fears. Nephron Clin Pract. 2009;112:c230–41. doi: 10.1159/000224789. [DOI] [PubMed] [Google Scholar]
- 18.Collin M, Abdelrahman M, Thiemermann C. Endogenous ligands of PPAR-gamma reduce the liver injury in haemorrhagic shock. Eur J Pharmacol. 2004;486:233–35. doi: 10.1016/j.ejphar.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 19.Celiński K, Madro A, Prozorow-Król B, et al. Rosiglitazone, a peroxisome proliferator-activated receptor gamma (PPARgamma)-specific agonist, as a modulator in experimental acute pancreatitis. Med Sci Monit. 2009;15(1):BR21–29. [PubMed] [Google Scholar]
- 20.Abdelrahman M, Collin M, Thiemermann C. The peroxisome proliferator-activated receptor-gamma ligand 15-deoxyDelta12,14 prostaglandin J2 reduces the organ injury in hemorrhagic shock. Shock. 2004;22:555–61. doi: 10.1097/01.shk.0000144132.13900.24. [DOI] [PubMed] [Google Scholar]
- 21.Rixen D, Siegel JH. Bench-to-bedside review: oxygen debt and its metabolic correlates as quantifiers of the severity of hemorrhagic and post-traumatic shock. Crit Care. 2005;9:441–53. doi: 10.1186/cc3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luft FC. Lactic acidosis update for critical care clinicians. J Am Soc Nephrol. 2001;12(Suppl 17):S15–19. [PubMed] [Google Scholar]
- 23.Besler C, Doerries C, Giannotti G, et al. Pharmacological approaches to improve endothelial repair mechanisms. Expert Rev Cardiovasc Ther. 2008;6:1071–82. doi: 10.1586/14779072.6.8.1071. [DOI] [PubMed] [Google Scholar]
- 24.Bahrami S, Yao YM, Leichtfried G, et al. Significance of TNF in hemorrhage-related hemodynamic alterations, organ injury, and mortality in rats. Am J Physiol. 1997;272( 5 Pt 2):H2219–26. doi: 10.1152/ajpheart.1997.272.5.H2219. [DOI] [PubMed] [Google Scholar]
- 25.Brundage SI, Zautke NA, Holcomb JB, et al. Interleukin-6 infusion blunts proinflammatory cytokine production without causing systematic toxicity in a swine model of uncontrolled hemorrhagic shock. J Trauma. 2004;57:970–77. doi: 10.1097/01.ta.0000141970.68269.ac. [DOI] [PubMed] [Google Scholar]
- 26.Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28:1950–59. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- 27.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–26. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh CH, Frink M, Hsieh YC, et al. The role of MIP-1 alpha in the development of systemic inflammatory response and organ injury following trauma hemorrhage. J Immunol. 2008;181:2806–12. doi: 10.4049/jimmunol.181.4.2806. [DOI] [PubMed] [Google Scholar]
- 29.Mizock BA. Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab. 2001;15:533–51. doi: 10.1053/beem.2001.0168. [DOI] [PubMed] [Google Scholar]
- 30.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17:107–24. doi: 10.1016/s0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- 31.Fahy BG, Sheehy AM, Coursin DB. Glucose control in the intensive care unit. Crit Care Med. 2009;37:1769–76. doi: 10.1097/CCM.0b013e3181a19ceb. [DOI] [PubMed] [Google Scholar]
- 32.Ajjan RA, Grant PJ. The cardiovascular safety of rosiglitazone. Expert Opin Drug Saf. 2008;7:367–76. doi: 10.1517/14740338.7.4.367. [DOI] [PubMed] [Google Scholar]
- 33.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–18. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 34.Nissen SE, Wolski K. Rosiglitazone Revisited: An Updated Meta-analysis of Risk for Myocardial Infarction and Cardiovascular Mortality. Arch Intern Med. 2010 doi: 10.1001/archinternmed.2010.207. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 36.Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury. 2007;38:1336–45. doi: 10.1016/j.injury.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Jeschke MG, Klein D, Herndon DN. Insulin treatment improves the systemic inflammatory reaction to severe trauma. Ann Surg. 2004;239:553–60. doi: 10.1097/01.sla.0000118569.10289.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]