Summary
Background
Serum CA-125 has been used as a biomarker of gynecological tumors. In this study, we investigated the CA-125 levels in cervical and vaginal secretions from Chinese patients with endometrial polyps, hyperplasia and carcinoma in comparison with those in endometrium and serum.
Material/Methods
An electro-chemiluminescent immunoassay was utilized to determine the levels of CA-125 in 51 healthy Chinese women and 97 patients with polyps, hyperplasia or endometrial cancer. An immunohistochemistry method was used to detect endometrial CA-125 expression in 242 subjects.
Results
Our study demonstrated that serum CA-125 levels were much lower than those in cervical and vaginal secretions in healthy and diseased women. The levels of CA-125 in serum, and cervical and vaginal secretions were significantly increased in complex hyperplasia and endometrial cancer. The increase of CA-125 content in serum, cervical and vaginal secretions was lesser significant in grade 3 cancer than that in grade 1 and 2 cancer. Generally, serum CA-125 levels correlated with those in cervical and vaginal secretions and CA-125 content in cervical secretion correlated with that in vaginal secretion. There was only a weak CA-125 expression in normal endometrium and simple endometrial hyperplasia. There was a significant difference in CA-125 expression among patients with pathological grade 1, 2 and 3 of endometrial carcinoma.
Conclusions
Endometrial CA-125 expression together with its levels in the serum and cervical and vaginal secretions can be used as a potential biomarker in the diagnosis of precancerous diseases and endometrial carcinoma
Keywords: CA-125, endometrial cancer, hyperplasia, clinical diagnosis, Chinese women
Background
The cancer antigen 125 (CA-125) is a transmembranaire glycoprotein with a high molecular weight of >200 kd [1]. CA-125 derives its numeric designation from its monoclonal antibody OC-125, which was generated by immunization of BALB/c mice with the OVCA43 cell line isolated from ascites fluid of a patient with serous papillary cystadenocarcinoma of the ovary in 1981 [2]. It is a mucin with a carbohydrate content estimated to be 24–28%. CA-125 is encoded by the gene MUC16 which is rich of tandem repeats [3,4]. This glycoprotein is expressed normally in fetal coelomic epithelium, by epithelial ovarian tumors, normal and pathologic tissues of Müllerian duct origin, and mesothelial cells [5,6].
Although serum CA-125 levels have been used as a valuable tumor marker for the diagnosis of epithelial ovarian cancer and the detection of recurrence after primary treatment, its role is controversial in endometrial cancer (EC) as a useful tumor marker [5,7,8]. A number of studies have reported that serum CA-125 levels were important for preoperative diagnosis and prediction of disease recurrence, and their elevation correlated with advanced-stage EC [6,9–15]. Nevertheless, the inconsistency between serum CA-125 levels and the extent of disease or prognosis still exists in EC because most related studies had some limitations such as a small number of patients and various histological types [13,16–19]. In several reports, the serum CA-125 levels seem to be increased in the majority of patients with advanced disease but only in about 10–20% of patients with stage I EC [6]. However, no such correlation between CA125 levels and extent of disease has been observed in other reports [16,20]. There are few studies on the detection of endometrial CA-125 expression as a tumor marker of EC [21–24]. Large-scale and long-term studies are required to evaluate the role of serum CA-125 levels for predicting prognosis in EC because most of patients have early-stage diseases cured by surgery alone.
There have been only few studies on the detection of CA-125 in cervical and vaginal secretions in healthy women and women with endometrial diseases. de Bruijn et al. [25] first observed very high levels of CA 125 in cervical mucus from women with regular menstrual cycles and this was confirmed by Martinez et al. [26]. We have recently reported that CA125 was detected in cervical and vaginal secretion of healthy Chinese women using the automatic electro-chemiluminescent immunoassay [27]. In this study, we further investigated the CA-125 levels in cervical and vaginal secretions from Chinese patients with endometrial polyps, hyperplasia and carcinoma in comparison with those in endometrium and serum.
Material and Methods
Subjects
A total of 254 subjects were recruited into this study from January 2005 to December 2009 and they were classified into six groups, namely proliferative phase endometrium (n=24), secretory phase endometrium (n=29), non-functional endometrial polyps (n=24), simple hyperplasia (n=24), complex hyperplasia (n=26), and endometrial carcinoma (n=126). Among 148 women from these subjects, their blood and cervical and vaginal secretions were collected for determination of CA-125. All subjects had no prior treatment history before sample collection. The diagnosis of these diseases was based on the criteria of International Classification of Diseases (ICD) version 11. Formalin-fixed hematoxylin & eosin-stained 6-μm slides from the endometrial tissue of all subjects were performed and revised by two senior pathologists in order to verify the diagnosis. This study was approved by the Ethics Committee of Xiaolan Hospital affiliated to Southern Medical University, Zhongshan, China. All participants had a written consent.
Immuonoassay for CA-125 determination
Electro-chemiluminescent immunoassay was used to determine the CA-125 levels in serum and cervical and vaginal secretions using an automatic Elecsys 2010 analyzer (Roche Diagnostics, Indianapolis, IN). The limit of detection was determined by analyzing 10 replicates of the zero calibrator and 4 replicates of the lowest nonzero calibrator (15 U/ml). The limit of detection for CA-125 in human serum, and cervical and vaginal secretions was determined to be 0.50 U/ml. The precision and accuracy were within 12% in terms of coefficients of variation (CVs) for the low and high controls (35 and 114 U/ml, respectively).
Immunohistochemistry for endometrial CA-125 determination
Endometrial CA-125 was detected by immunohistochemical technique (EliVision plus) using a commercially available kit (Maixin Bio, Fuzhou, China). Four-μm unstained slides were prepared from each case for immunohistochemical staining for anti-CA125 antigens with clone OV 125 diluted 1:2. Immunohistochemistry was performed by deparaffinization of slides in xylene, absolute ethyl alcohol, and degradated alcohols 96% and 70%. Immunoperoxidase stains were performed using a modified labeled streptavidin technique and run on an automated system using a commercially available chromogen. All sections were counterstained with Mayer’s hematoxylin. Sections of ovarian serous carcinoma were used as positive controls.
Two parameters in each case were evaluated. The percentage of tissue area stained in 10–12 high power fields and its intensity scored as 0, 1, 2, and 3. Cancer tissue with staining of more than 50% of the area examined and an intensity of 2 and 3 was considered as positive (score 1). Pathologic grading of the tumor cells was done according to the 1988 FIGO grading system. The tumor was graded based on the percentage of solid non-squamous growth as follows: grade 1, ≤5% solid growth; grade 2, 6–50% solid growth; grade 3, >50% solid growth. The overall grade of architecturally grade 1 or grade 2 tumors was increased by one if there was notable nuclear atypia. Mixed mesodermal sarcomas were graded according to the glandular element.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). Differences among groups of subjects were compared by the χ2 or Fisher’s exact test. Correlation analysis was performed to determine the relationship between the CA-125 levels measured in different biological matrices. A P value of <0.05 was considered significant.
Results
CA-125 levels in serum, cervical and vaginal secretion, and endometrium in healthy Chinese women
In proliferative and secretory phases of healthy Chinese women, the CA-125 contents in cervical and vaginal secretions were 483.56, 114.79, and 500.65 and 124.00 U/ml, respectively (Table 1). The cervical secretion contained 4.0-fold higher CA-125 than vaginal secretion. The content of CA-125 in the serum was 6.8- and 28.8-fold lower than those in the vaginal and cervical secretions, respectively. There was insignificant difference in levels of CA-125 in serum, and cervical and vaginal secretions between the proliferative and secretory phase. In normal proliferative and secretory phases of menstrual cycle, most Chinese women expressed endometrial CA-125 (score 1), but none of them expressed CA-125 at levels of score 2 or 3.
Table 1.
CA-125 contents (U/ml) in serum, and cervical and vaginal secretions in healthy women and women with endometrial diseases.
| Group | Number of subjects | CA-125 (U/ml) | ||
|---|---|---|---|---|
| Serum | Cervical secretion | Vaginal secretion | ||
| Normal | ||||
| Proliferative phase | 24 | 16.80±2.99 | 483.56±58.21a | 114.70±19.86a,b |
| Secretory phase | 29 | 19.16±3.56 | 500.65±61.05a | 124.00±25.63a,b |
| Non-functional polyps | 24 | 22.18±5.03 | 796.03±141.14a | 178.90±34.07a,b |
| Hyperplasia | ||||
| Simple hyperplasia | 24 | 18.98±2.76 | 518.12±70.28a | 122.76±25.48a,b |
| Complex hyperplasia | 21 | 36.41±14.34c | 1220.39±684.88a,c | 497.76±379.15a,b,c |
| Endometrial cancer | ||||
| Grade 1 | 10 | 37.12±16.74d | 950.56±58.73a,d | 300.78±196.42a,b,d,e |
| Grade 2 | 9 | 43.12±13.58d | 1806.16±927.97a,d,e | 892.30±365.04a,b,d |
| Grade 3 | 7 | 26.77±3.64e | 852.73±85.75a,e | 271.90±75.86a,b,d,e |
| Subtotal | 26 | |||
| Total | 148 | |||
P<0.05, serum vs. cervical or vaginal secretion;
P<0.05, cervical vs. vaginal secretion;
P<0.05, complex vs. simple hyperplasia, normal or polyps;
P<0.05, cancer vs. normal or simple hyperplasia;
P<0.05, grade II vs. III or grade I vs. II.
A correlation analysis indicated that there was a significant correlation between CA-125 levels in the serum and cervical and vaginal secretions in the proliferative phase (P=0.004 and 0.003, respectively), but there was no correlation between CA-125 levels in cervical and vaginal secretions. However, there was a correlation between CA-125 levels in cervical and vaginal secretions in the secretory phase (P=0.0007), while the correlation between serum CA-125 levels and cervical and vaginal secretions disappeared.
CA-125 levels in serum, cervical and vaginal secretion, and endometrium in Chinese women with endometrial polyps
In Chinese women with non-functional polyps, the levels of CA-125 in serum, and cervical and vaginal secretions were slightly higher than those in healthy women, but did not achieve statistical significance (Table 1). In 24 Chinese women with non-functional polyps, 16 and 6 expressed CA-125 to score 2 and 3, respectively (Table 2). There was a significant difference in endometrial CA-125 expression level between non-functional endometrial polyps and normal endometrium (χ2=46.492, P<0.0001, see Table 3).
Table 2.
Endometrial expression of CA-125 in 242 subjects.
| Group | Number of subjects | CA-125 staining score | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| Normal | |||||
| Proliferative phase | 24 | 0 | 22 (91.67) | 2 (8.33) | 0 (0) |
| Secretory phase | 21 | 0 | 19 (90.48) | 2 (9.52) | 0 (0) |
| Non-functional polyps | 24 | 0 | 1 (8.33) | 16 (66.67) | 6 (25.00) |
| Hyperplasia | |||||
| Simple hyperplasia | 24 | 0 | 20 (83.33) | 4 (16.67) | 0 (0) |
| Complex hyperplasia | 26 | 0 | 0 (0) | 16 (61.54) | 10 (38.46) |
| Endometrial cancer | |||||
| Grade 1 | 63 | 0 | 0 (0) | 33 (52.38) | 30 (47.62) |
| Grade 2 | 42 | 0 | 0 (0) | 14 (33.33) | 28 (66.67) |
| Grade 3 | 18 | 0 | 0 (0) | 16 (88.89) | 2 (11.11) |
| Subtotal | 123 | 0 | 0 (0) | 63 (51.22) | 60 (48.78) |
| Total | 242 | 0 | 63 (26.03) | 103 (42.56) | 76 (31.41) |
Data in the brackets are the percentage values.
Table 3.
Statistical analysis results (p values) of the difference in endometrial CA-125 staining in different groups of subjects.
| Group | Normal | Polyps | Simple hyperplasia | Complex hyperplasia | Grade 1 endometrial cancer | Grade 2 endometrial cancer | Grade 3 endometrial cancer |
|---|---|---|---|---|---|---|---|
| Normal | |||||||
| Polyps | <0.0001 (χ2, 46.492) | ||||||
| Simple hyperplasia | 0.4354 (F, 2.050) | <0.0001 (χ2=27.932) | |||||
| Complex hyperplasia | <0.0001 (χ2=57.212) | 0.2317 (χ2=2.925) | <0.0001 (χ2=37.182) | ||||
| Grade 1 endometrial cancer | <0.0001 (χ2=93.322) | 0.0181 (χ2=8.029) | <0.0001 (χ2=69.142) | 0.4878 (F=0.6875) | |||
| Grade 2 endometrial cancer | <0.0001 (χ2=74.542) | 0.002 (χ2=12.382) | <0.0001 (χ2=52.562) | 0.0270 (F=0.3125) | 0.0717 (F=2.200) | ||
| Grade 3 endometrial cancer | <0.0001 (χ2=47.322) | 0.2011 (χ2=3.208) | <0.0001 (χ2=28.932) | 0.0833 (F=5.000) | 0.0058 (F=0.1375) | 0.0001 (F=0.0625) |
F – Fisher’s exact test; χ2 – chi-square test. For Fisher’s exact test, the value in the brackets is odds ratio, and the value in the brackets is χ2 value for χ2 test.
In non-functional polyps, there were significant correlations between serum CA-125 levels and cervical and vaginal secretions (P<0.05). In addition, the levels of CA-125 in cervical secretions correlated with that in vaginal secretion.
CA-125 levels in serum, cervical and vaginal secretion, and endometrium in Chinese women with endometrial hyperplasia
The expression profile of CA-125 in serum, and cervical and vaginal secretions in women with simple hyperplasia was similar to that in healthy women (Table 1). However, the contents of CA-125 in serum, and cervical and vaginal secretions from women with complex hyperplasia were significantly higher than the corresponding values in healthy women or women with simple hyperplasia (P<0.05).
In simple hyperplasia, there were significant correlations between serum CA-125 levels and cervical and vaginal secretions (P<0.05). In addition, the levels of CA-125 in cervical secretions correlated with that in vaginal secretion. In complex hyperplasia, there were significant correlations between serum CA-125 levels and cervical secretion (P=0.016), but not with vaginal secretion (P=0.169). In addition, the levels of CA-125 in cervical secretions significantly correlated with that in vaginal secretion (P=0.0006).
The endometrial expression of CA-125 in 24 cases of simple hyperplasia was comparable to that of normal proliferative or secretory phase endometrium (P=0.4354 by Fisher’s exact test). However, 16 and 10 out of 26 cases of complex hyperplasia showed score 2 and 3 endometrial staining of CA-125, respectively. There was a significant difference in endometrial CA-125 expression levels between complex endometrial hyperplasia and simple hyperplasia or normal endometrium (χ2=37.182, P<0.0001; χ2=57.212, P<0.001, respectively). There was a significant difference in endometrial CA-125 expression levels between simple endometrial hyperplasia and polyps (χ2=27.932, P<0.0001), but there was no significant difference between in endometrial CA-125 expression levels between complex endometrial hyperplasia and polyps (χ2=2.925, P=0.2317),
CA-125 levels in serum, cervical and vaginal secretion, and endometrium in Chinese women with endometrial cancer
In 26 Chinese women with confirmed endometrial cancer, grade 1 and 2 cancer had significantly higher CA-125 levels in serum, cervical and vaginal secretions compared to healthy women and women with simple hyperplasia (Table 1). Grade 1 and 2 endometrial cancer demonstrated an expression profile of CA-125 in serum, and cervical and vaginal secretions similar to that of complex hyperplasia. Grade 3 endometrial cancer showed slightly higher levels of CA-125 than healthy women and women with simple hyperplasia, but significantly lower levels of CA-125 compared to grade 1 and 2 cancer.
When all cases of endometrial cancer were pooled, there were significant correlations between serum CA-125 levels and cervical and vaginal secretions (P<0.05). In addition, the levels of CA-125 in cervical secretions correlated with that in vaginal secretion. However, when the patients were grouped based on pathological grade, such correlations disappeared. In addition, there was no significant correlation between serum CA-125 levels and endometrial expression of CA-125.
In 123 cases of endometrial cancer, 63, 42 and 18 were pathological grade 1, 2, and 3, respectively (Table 2). Overall, In 63 cases of grade 1 endometrial cancer, 33 and 30 showed score 2 and 3 staining of CA-125, respectively (Figure 1). This figure was 14 and 28 in 42 cases of grade 2 endometrial cancer. In 18 cases of grade 3 endometrial carcinoma, 16 and 2 displayed score 2 and 3 staining of CA-125, respectively. Overall, 63 (51.22%) and 60 (48.78%) subjects of 123 cases showed score 2 and 3 staining of endometrial CA-125, respectively. There was a significant difference in endometrial CA-125 expression among patients with pathological grade 1, 2 and 3 of endometrial carcinoma (χ2=15.632, P=0.0004). The endometrial expression of CA-125 in each grade of cancer was significantly different from that of normal endometrium. There was a significant difference in CA-125 expression between grade 1 and 3 endometrial carcinoma (P=0.0058 by Fisher’s exact test) whereas there was no significant difference in CA-125 expression between grade 1 and 2 endometrial carcinoma (P=0.0717 by Fisher’s exact test). In addition, there was a significant difference in CA-125 expression between grade 2 and 3 endometrial carcinoma (P=0.0001 by Fisher’s exact test).
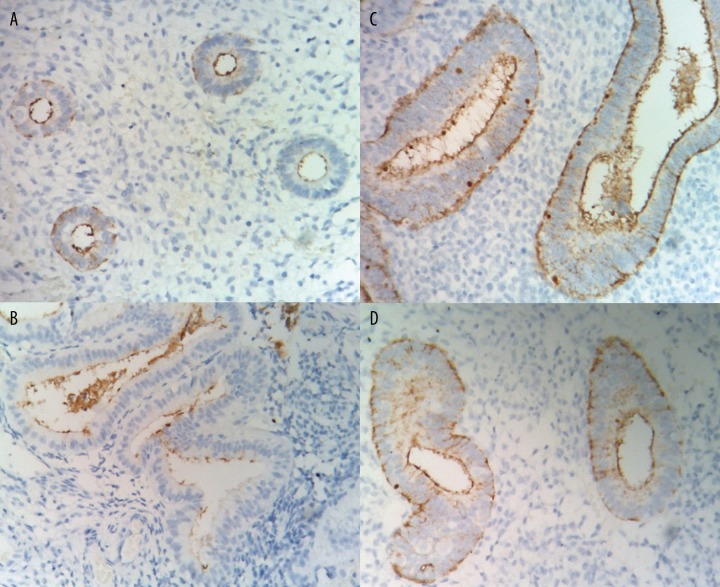
Figure 1A–D.
Endometrial staining of CA-125 in different groups of subjects (magnification × 400). (A) proliferative phase endometrium; (B) secretory phase endometrium; (C) non-functional endometrial polyps; (D) simple hyperplasia.
We further compared the difference in endometrial expression of CA-125 between endometrial cancer, polyps and hyperplasia and the results are listed in Table 3. There was a significant difference in endometrial CA-125 expression between cancer and polyps (χ2=29.646; P<0.0001). There was a significant difference in endometrial CA-125 expression levels between grade 1 and 2 cancer and polyps (χ2=8.029, P=0.0181; χ2=12.382, P=0.002, respectively), but the difference between grade 2 cancer and polyps was insignificant (χ2=3.208; P=0.2011).
There was a significant difference in endometrial CA-125 expression between cancer and simple hyperplasia (χ2=137.66; P<0.0001). There was a significant difference in endometrial CA-125 expression levels between grade 1, 2, or 3 cancer and simple hyperplasia (χ2=69.142, P<0.0001; χ2=52.562, P<0.0001; χ2=28.932, P<0.001, respectively).
There was a significant difference in endometrial CA-125 expression between cancer and complex hyperplasia (χ2=16.603; P=0.0009). There was a significant difference in endometrial CA-125 expression levels between grade 2 cancer and complex hyperplasia (P=0.027 by Fisher’s exact test). However, there was no significant difference in endometrial CA-125 expression levels between grade 1 or 3 cancer and complex hyperplasia (P=0.4878 and 0.0833 by Fisher’s exact test, respectively).
Discussion
A sensitive immunoradiometric assay for CA-125 was developed in 1983 [28], and several second-generation immunochemical methods are now available for accurate determination of this important tumor marker. To improve on the sensitivity and specificity of the first-generation assays, a double-determinant monoclonal antibody-based format was developed using 2 monoclonal antibodies with specificities against the antigenic domains OC125 and M11 in the large CA-125 protein. Presently, serum CA-125 is widely used as a biochemical marker for the diagnosis of epithelial ovarian cancer, which is the fifth most common cancer of women in the United States. However, the assay for serum CA-125 is not useful for screening for ovarian cancer in asymptomatic women. Serum CA-125 concentrations exceed 35 U/ml in 80% of patients with nonmucinous epithelial ovarian cancer, and the concentrations correlate well with the progression or regression of ovarian cancer [6,9–15].
We have found that cervical secretion contained 4.0-fold higher CA-125 than vaginal secretion in healthy women and the content of CA-125 in the serum was 6.8- to 28.8-fold lower than those in the vaginal and cervical secretions. It appears that cervical and vaginal secretions are better matrix than the serum for the monitoring of CA-125 content. Epithelial cells in the cervix may produce more CA-125 than those in the vagina. Since CA-125 is synthesized in epithelial cells of Müllerian duct origin and then leaked into the circulation and secretions in the genital duct, it is not a surprise that the levels of CA-125 in the serum and cervical and vaginal secretions correlate, as observed in this study.
Positive staining for CA-125 is known to be present in normal endometrial tissue and especially during the secretory phase [29]. Consistent with this is our observation of weak staining of CA-125 in normal proliferative and secretory phase endometrium. Slightly more cells in secretory phase endometrium were stained positively compared to proliferative phase endometrium.
Non-functional endometrial polyps are not considered to be precancerous lesions but a recent meta-analysis indicates that both symptomatic vaginal bleeding and postmenopausal status in women with endometrial polyps are associated with an increased risk of endometrial malignancy [30]. Multiple molecular mechanisms have been proposed to play a role in the development of endometrial polyps. These include monoclonal endometrial hyperplasia, overexpression of endometrial aromatase, and gene mutations [31,32]. In this study, we cannot find significantly increased CA-125 levels in the serum and cervical and vaginal secretions, but found that 66.67% and 25.00% of the subjects with polyps showed score 2 and 3 endometrial staining of CA-125. This reflects that the MUC16 gene has been significantly up-regulated in endometrial polyps. The mechanism for this is unknown.
The widely used World Health Organization system classifies endometrial hyperplasia into four levels according to glandular crowing and nuclear appearance: simple, complex, simple atypical, and complex atypical hyperplasia. Simple hyperplasia refers to diffuse and variably sized glands with a normal ratio of glands to stroma; complex hyperplasia consists of architecturally irregular glands and an increased gland-to-stroma ratio. When there is nuclear enlargement with chromatin evenly dispersed or clumped, it is called simple or complex atypical hyperplasia. In this study, simple hyperplasia did not show significantly elevated CA-125 in the serum and cervical and vaginal secretions and endometrial expression of CA-125, while complex hyperplasia displayed significantly increased CA-125 in the serum and cervical and vaginal secretions and endometrial expression of CA-125. As such, CA-125 in the serum, cervical and vaginal secretions, and endometrium may be used to monitor the progression of hyperplasia. Long-term risk among women with simple or complex hyperplasia is less than 5%, but the risk among women with atypical hyperplasia is about 30% [33]. The results showed that there was a significant difference in endometrial CA-125 levels between normal subjects and patients with complex hyperplasia. This implicates that endometrial CA-125 expression determination may be used as a screening approach to identifying high-risk subpopulation with a potential to progress to carcinoma.
With a lifetime risk of 2–3% among women, EC is the most common gynecologic tumors in developed countries. Approximately 75–80% of EC cases have the International Federation of Gynecology and Obstetrics (FIGO) stages I–II disease confined to the uterine corpus due to early symptoms including vaginal bleeding [34]. The most common basis for determining the risk of recurrent disease has been classification of endometrial cancers into two subtypes based on light microscopic appearance, clinical behavior, and epidemiology [35]. Type I EC accounts for the majority (70–80%) of cases and is associated with unopposed estrogen exposure and are often preceded by premalignant disease (e.g. endometrial hyperplasia). Type I is associated with a low-stage, low-grade, endometrioid histology, while type II is characterized by a high-stage, high-grade and non-endometrioid histology (usually papillary serous or clear cell) [35]. Hormonal risk factors have not been identified and there is no observed premalignant phase for type II EC. Type I occurs predominantly in pre- and perimenopausal women but type II usually occurs 5–10 years later than type I cancer. Type I often has a good prognosis whereas type II is associated with a poor prognosis due to a high frequency of distinct spread to pelvic lymph nodes. The morphological and clinical differences are paralleled by remarkable distinct genetic mutations in that type I and type II carry mutations of independent sets of genes [36,37]. Type I EC has defects in DNA-mismatch repair with microsatellite instability observed in 20–45% patients, and mutations in PTEN, K-ras, PIK3CA, and β-catenin encoded by CTNNB1 genes, and type II shows aneuploidy and p53, HER2/neu, p16 and E-cadherin mutations [36,37]. Although most EC patients are cured by surgery alone, about 15–20% with no signs of locally advanced or metastatic disease at primary treatment recurs, with limited responsiveness to systemic therapy. Our study has demonstrated that endometrial cancer had a significantly increased CA-125 in the serum and cervical and vaginal secretions. The increase of CA-125 content in serum, cervical and vaginal secretions was lesser significant in grade 3 cancer than that in grade 1 and 2 cancer. It appears that CA-125 synthesized by tumor cells is prevented from entry into the blood circulation in grade 3 cancer due to the presence of a membrane barrier.
Our study showed that endometrial CA-125 expression was significantly elevated in endometrial cancer compared to normal endometrium or endometrium with simple hyperplasia, with the tissue from all EC cases expressing CA-125. 47.62% and 66.67% of pathological grade 1 and 2 endometrial cancer expressed a score 3 staining of CA-125, but only 11.11% grade 3 endometrial cancer showed a score 3 staining of CA-125. Grade 3 endometrial cancer may express less CA-125 due to reduced production of this tumor antigen and/or increased degradation. Compared to low-grade ECs (grade 1 and 2), grade 3 ECs show a lower frequency of expression of estrogen receptor and progesterone receptor, with a higher frequency of expression of p16, p53 and insulin-like growth factor 2 mRNA-binding protein 3 (IMP3) [37]. CA-125 may be used in combination with these immunomarkers to distinguish between low grade (grade 1 and 2) and high grade (grade 3) ECs.
Notably, when all cases of endometrial cancer were pooled, there were significant correlations between serum CA-125 levels and cervical and vaginal secretions. However, there was no significant correlation between serum CA-125 contents and endometrial expression levels of CA-125 in our study, probably due to the presence of a barrier that blocks the entry of CA-125 from tumor cells into the circulation. This is in agreement with the results from previous studies [21].
Conclusions
In summary, there was a relatively high content of CA-125 in cervical and vaginal secretion but only a weak CA-125 expression in normal endometrium and simple endometrium hyperplasia. The levels of CA-125 in serum, and cervical and vaginal secretions were significantly increased in complex hyperplasia and endometrial cancer, but not in polyps and simple hyperplasia. There were significantly increased endometrial CA-125 expression in endometrial polyps, complex hyperplasia, and endometrial carcinoma. These findings indicate that endometrial CA-125 expression together with its levels in the serum and cervical and vaginal secretions can be used as a potential biomarker in the diagnosis of precancerous diseases and endometrial carcinoma in Chinese women. Further large-scale and long-term studies are required to evaluate the role of serum, cervical and vaginal secretions and tissue CA-125 levels in the diagnosis and prognosis prediction of endometrial cancer.
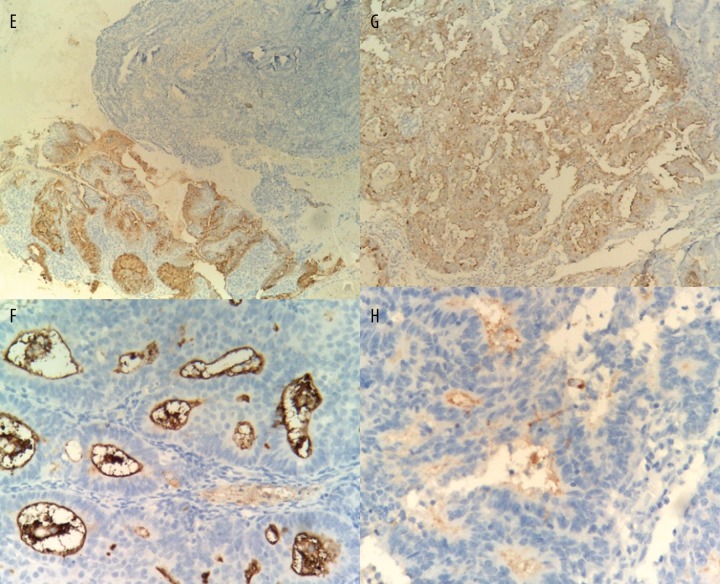
Figure 1E–H.
Endometrial staining of CA-125 in different groups of subjects (magnification × 400). (E) complex hyperplasia; (F), grade 1 endometrial carcinoma; (G), grade 2 endometrial carcinoma; and (H), grade 3 endometrial carcinoma.
Abbreviations
- CA-125
cancer antigen 125
- EC
endometrial cancer
- SD
standard deviation
Footnotes
Source of support: Departmental sources
References
- 1.O’Brien TJ, Tanimoto H, Konishi I, Gee M. More than 15 years of CA 125: what is known about the antigen, its structure and its function. Int J Biol Markers. 1998;13:188–95. doi: 10.1177/172460089801300403. [DOI] [PubMed] [Google Scholar]
- 2.Bast RC, Jr, Feeney M, Lazarus H, et al. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68:1331–37. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin BW, Dnistrian A, Lloyd KO. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. Int J Cancer. 2002;98:737–40. doi: 10.1002/ijc.10250. [DOI] [PubMed] [Google Scholar]
- 4.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–75. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal P, Kehoe S. Serum tumour markers in gynaecological cancers. Maturitas. 2010;67:46–53. doi: 10.1016/j.maturitas.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Duk JM, Aalders JG, Fleuren GJ, de Bruijn HW. CA 125: a useful marker in endometrial carcinoma. Am J Obstet Gynecol. 1986;155:1097–102. doi: 10.1016/0002-9378(86)90358-3. [DOI] [PubMed] [Google Scholar]
- 7.Bast RC, Jr, Spriggs DR. More than a biomarker: CA125 may contribute to ovarian cancer pathogenesis. Gynecol Oncol. 2011;121:429–30. doi: 10.1016/j.ygyno.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 8.Gundogdu F, Soylu F, Erkan L, et al. The role of serum CA-125 levels and CA-125 tissue expression positivity in the prediction of the recurrence of stage III and IV epithelial ovarian tumors (CA-125 levels and tissue CA-125 in ovarian tumors) Arch Gynecol Obstet. 2011;283:1397–402. doi: 10.1007/s00404-010-1589-8. [DOI] [PubMed] [Google Scholar]
- 9.Duk JM, Aalders JG, Fleuren GJ, et al. Tumor markers CA 125, squamous cell carcinoma antigen, and carcinoembryonic antigen in patients with adenocarcinoma of the uterine cervix. Obstet Gynecol. 1989;73:661–68. [PubMed] [Google Scholar]
- 10.Rose PG, Sommers RM, Reale FR, et al. Serial serum CA 125 measurements for evaluation of recurrence in patients with endometrial carcinoma. Obstet Gynecol. 1994;84:12–16. [PubMed] [Google Scholar]
- 11.Farias-Eisner G, Su F, Robbins T, et al. Validation of serum biomarkers for detection of early- and late-stage endometrial cancer. Am J Obstet Gynecol. 2010;202:73 e1–5. doi: 10.1016/j.ajog.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 12.Skates SJ, Horick NK, Moy JM, et al. Pooling of case specimens to create standard serum sets for screening cancer biomarkers. Cancer Epidemiol Biomarkers Prev. 2007;16:334–41. doi: 10.1158/1055-9965.EPI-06-0681. [DOI] [PubMed] [Google Scholar]
- 13.Chung HH, Kim JW, Park NH, et al. Use of preoperative serum CA-125 levels for prediction of lymph node metastasis and prognosis in endometrial cancer. Acta Obstet Gynecol Scand. 2006;85:1501–5. doi: 10.1080/00016340601022777. [DOI] [PubMed] [Google Scholar]
- 14.Takac I, Gorisek B. Serum CA 125 levels and lymph node metastasis in patients with endometrial cancer. Wien Klin Wochenschr. 2006;118(Suppl 2):62–65. doi: 10.1007/s00508-006-0554-9. [DOI] [PubMed] [Google Scholar]
- 15.Powell JL, Hill KA, Shiro BC, et al. Preoperative serum CA-125 levels in treating endometrial cancer. J Reprod Med. 2005;50:585–90. [PubMed] [Google Scholar]
- 16.Soper JT, Berchuck A, Olt GJ, et al. Preoperative evaluation of serum CA 125, TAG 72, and CA 15-3 in patients with endometrial carcinoma. Am J Obstet Gynecol. 1990;163:1204–9. doi: 10.1016/0002-9378(90)90692-z. [DOI] [PubMed] [Google Scholar]
- 17.Santala M, Talvensaari-Mattila A, Kauppila A. Peritoneal cytology and preoperative serum CA 125 level are important prognostic indicators of overall survival in advanced endometrial cancer. Anticancer Res. 2003;23:3097–103. [PubMed] [Google Scholar]
- 18.Denschlag D, Tan L, Patel S, et al. Stage III endometrial cancer: preoperative predictability, prognostic factors, and treatment outcome. Am J Obstet Gynecol. 2007;196:546 e1–7. doi: 10.1016/j.ajog.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Qiu F, Gao YH, Jiang CG, Tian YP, Zhang XJ. Serum proteomic profile analysis for endometrial carcinoma detection with MALDI-TOF MS. Arch Med Sci. 2010;6:245–52. doi: 10.5114/aoms.2010.13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo SS, Cheng DK, Ng TY, et al. Prognostic significance of tumour markers in endometrial cancer. Tumour Biol. 1997;18:241–49. doi: 10.1159/000218037. [DOI] [PubMed] [Google Scholar]
- 21.Ginath S, Menczer J, Fintsi Y, et al. Tissue and serum CA125 expression in endometrial cancer. Int J Gynecol Cancer. 2002;12:372–75. doi: 10.1046/j.1525-1438.2002.01007.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang XY, Pan ZM, Chen XD, et al. Accuracy of tumor grade by preoperative curettage and associated clinicopathologic factors in clinical stage I endometriod adenocarcinoma. Chin Med J (Engl) 2009;122:1843–46. [PubMed] [Google Scholar]
- 23.Metindir J, Dilek GB, Pak I. Staining characterization by immunohistochemistry of tumor cancer antigen in patients with endometrial cancer. Eur J Gynaecol Oncol. 2008;29:489–92. [PubMed] [Google Scholar]
- 24.Mylonas I, Makovitzky J, Richter DU, et al. Immunohistochemical expression of the tumour marker CA-125 in normal, hyperplastic and malignant endometrial tissue. Anticancer Res. 2003;23:1075–80. [PubMed] [Google Scholar]
- 25.de Bruijn HW, van Beeck Calkoen-Carpay T, Fleuren GJ, et al. The tumor marker CA 125 is a common constituent of normal cervical mucus. Am J Obstet Gynecol. 1986;154:1088–91. doi: 10.1016/0002-9378(86)90757-x. [DOI] [PubMed] [Google Scholar]
- 26.Martinez AR, Thomas CM, Segers MF, et al. CA-125 levels in cervical mucus during the menstrual cycle. Fertil Steril. 1994;61:843–49. doi: 10.1016/s0015-0282(16)56694-1. [DOI] [PubMed] [Google Scholar]
- 27.He SM, Xing FQ, Sui H, et al. CA 125 expression in cervical and vaginal secretions in women in normal reproductive period. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:173–75. [PubMed] [Google Scholar]
- 28.Bast RC, Jr, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309:883–87. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- 29.Dotters DJ. Preoperative CA 125 in endometrial cancer: is it useful? Am J Obstet Gynecol. 2000;182:1328–34. doi: 10.1067/mob.2000.106251. [DOI] [PubMed] [Google Scholar]
- 30.Kounelis S, Kapranos N, Kouri E, et al. Immunohistochemical profile of endometrial adenocarcinoma: a study of 61 cases and review of the literature. Mod Pathol. 2000;13:379–88. doi: 10.1038/modpathol.3880062. [DOI] [PubMed] [Google Scholar]
- 31.Lax SF. Molecular genetic changes in epithelial, stromal and mixed neoplasms of the endometrium. Pathology. 2007;39:46–54. doi: 10.1080/00313020601146822. [DOI] [PubMed] [Google Scholar]
- 32.Yao Y, Chen Y, Wang Y, et al. Molecular classification of human endometrial cancer based on gene expression profiles from specialized microarrays. Int J Gynaecol Obstet. 2010;110:125–29. doi: 10.1016/j.ijgo.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 33.Samarnthai N, Hall K, Yeh IT. Molecular profiling of endometrial malignancies. Obstet Gynecol Int. 2010;2010:162363. doi: 10.1155/2010/162363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bristow RE. Endometrial cancer. Curr Opin Oncol. 1999;11:388–93. doi: 10.1097/00001622-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 36.Clarke BA, Gilks CB. Endometrial carcinoma: controversies in histopathological assessment of grade and tumour cell type. J Clin Pathol. 2010;63:410–15. doi: 10.1136/jcp.2009.071225. [DOI] [PubMed] [Google Scholar]
- 37.Alkushi A, Kobel M, Kalloger SE, Gilks CB. High-grade endometrial carcinoma: serous and grade 3 endometrioid carcinomas have different immunophenotypes and outcomes. Int J Gynecol Pathol. 2010;29:343–50. doi: 10.1097/PGP.0b013e3181cd6552. [DOI] [PubMed] [Google Scholar]