Biotrophic fungi have developed a range of “life styles” in their relationship with plants from the mutualistic to the parasitic. Vesicular-arbuscular mycorrhizal fungi form mutualistic relationships with the roots of their plant hosts, in which the fungus obtains sugars from the plant and provides phosphates and other minerals in return. At the other extreme, powdery mildew and rust fungi form an obligately parasitic relationship in which the host plant becomes a source for sugars, amino acids, and other nutrients. These parasites develop a specialized organ, the haustorium (Fig. 1) within plant cells for transfer of nutrients from host cell to fungal thallus. The haustorium is assumed to have a key role in the ability of these parasites to compete with the developing plant for photoassimilates and other nutrients but basic questions remain regarding the function of the haustorium. These include: What are the major nutrients transported? What mechanisms are involved in the transport? How do individual components of the haustorium–host cell interface contribute to nutrient flow? And overall, how does haustorial function relate to the biotrophic relationship between host and parasite? The paper by Voegele et al. (1) in this issue of PNAS provides an important advance by characterizing a sugar transporter located at the haustorium–host interface.
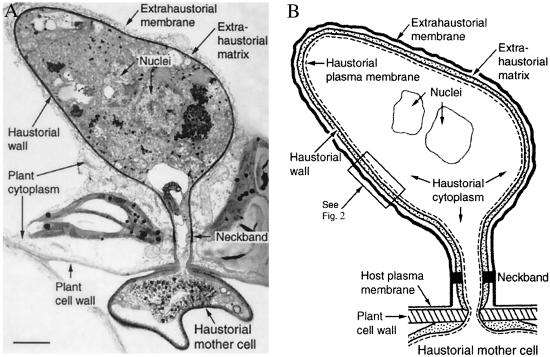
Figure 1.
Haustorial complex, a specialized feeding organ of biotrophic fungal parasites of plants. To move from host cell to fungus, nutrients must traverse the extrahaustorial membrane, the extrahaustorial matrix, the haustorial wall, and the haustorial plasma membrane. A neckband seals the extrahaustorial matrix from the plant cell wall region so that the matrix becomes a unique, isolated, apoplast-like compartment. The haustorium connects to intercellular fungal hyphae by way of a haustorial mother cell. Evidence from Voegele et al. (1) indicates that a proton symport system in the haustorial plasma membrane drives sugar transport from plant to parasite. (A) Transmission electron micrograph of a flax rust haustorium [Reproduced with permission from ref. 2 (Copyright 1972, NRC Research Press)]. (Bar, 1 μm.) (B) Drawing showing key features of the fungal haustorium.
A haustorium is formed when a specialized fungal hypha penetrates a plant cell wall and expands inside that cell (ref. 2; Fig. 1A). However, the haustorium is not located directly in plant cell cytoplasm; instead, it is surrounded by an extrahaustorial membrane, a thickened derivative of the plant cell plasma membrane. Lying between the extrahaustorial membrane and fungal haustorial wall is a gel-like layer enriched in carbohydrates called the extrahaustorial matrix. The haustorium itself contains a normal complement of cytoplasm, nuclei, mitochondria, and other organelles (Fig. 1A). The haustorial cytoplasm is bordered by a plasma membrane and by the haustorial wall (Fig. 1). To move from plant cytoplasm to haustorial cytoplasm, substances must pass sequentially through the extrahaustorial membrane and matrix, the haustorial wall, and the haustorial plasma membrane.
The development of the haustorium is the final step of an infection pathway in which the plant host plays a major role. In the case of rust fungi, infection typically begins with the germination of a spore on the leaf surface, followed by the development of an appressorium. The development of the appressorium depends on a thigmotrophic signal triggered by the specific topography of the host plant leaf surface (3). An infection peg formed by the appressorium enters the leaf through a pore (stoma), followed by the development of a substomatal vesicle, an infection hypha, a haustorial mother cell, penetration of a photosynthetic mesophyll cell by a peg and the establishment of a haustorium. Artificial membranes and etched surfaces have been used to mimic the topography of the leaf surface and induce the development of infection structures in vitro (4–6). However, the development is incomplete and haustoria are usually not formed unless carbohydrate is added (7).
Uptake studies have demonstrated that sugars and amino acids are transferred from the host plant into biotrophic parasites (8–11) and strongly support the idea that haustoria play a major role. However, because of the intracellular locations of haustoria and the complexities of the haustorium–host interface, it has been difficult to determine what transfer processes are involved and where they are located. In the last several years a different approach has been taken, in which cDNAs for developmentally expressed genes in infection structures and haustoria have been isolated (12, 13). Voegele et al. (1) demonstrated that one of the most abundantly expressed genes in the rust haustorium encodes a sugar transporter (HTX1). RNA analysis demonstrated that this gene was expressed in haustoria isolated from leaves, but not in other infection structures induced in vitro. Further, the authors demonstrated that this sugar transporter is localized in the haustorial membrane and not in membranes of intercellular hyphae, thus providing the first direct evidence that sugar uptake occurs in the haustorium and suggesting that the haustorium may be the sole site.
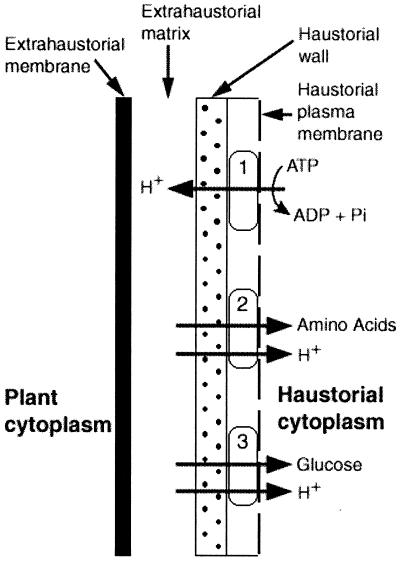
Sucrose is the primary sugar that is transported in plants and, therefore, it has been speculated that the haustoria may import sucrose directly. Recent studies with the powdery mildew fungus indicate that glucose, rather than sucrose, may be the sugar imported (14, 15). The data of Voegele et al. (1) demonstrate that the HXT1 sugar transporter has a specificity for d-glucose and d-fructose, confirming that glucose/fructose and not sucrose are the primary sugars imported by haustoria. In addition, the authors showed that glucose transport occurs through a proton symport mechanism. These results support a proton symport model for nutrient transport at the haustorial interface (ref. 16; Fig. 2). A membrane H+-ATPase generates a proton gradient across the haustorial plasma membrane, which provides the energy for transport of nutrients (glucose/fructose, amino acids) from the extrahaustorial matrix into the haustorium. The establishment of a proton gradient is possible because the extrahaustorial matrix is a sealed compartment, bounded by the extrahaustorial membrane on the plant side, the haustorial membrane on the fungal side, and the neck band, which seals it from the apoplast. In support of this model, a plasma membrane H+-ATPase (17) and an amino acid transporter (18) have been isolated.
Figure 2.
Proton symport model for nutrient transport across the haustorial plasma membrane, supplying glucose and amino acids to biotrophic fungi. 1, Protons are supplied by haustorial plasma membrane H+-ATPase (15); 2, Amino acid transporter (16); 3, Glucose/fructose transporter described by Voegele et al. (1). Redrawn from Hahn et al. (16).
The work of Voegele et al. (1) has provided an important piece of the puzzle regarding the nutrient transport pathway across the host–parasite interface in obligate parasitic fungi. However, many questions remain. Does sucrose diffuse out of the host plant cell into the extrahaustorial matrix, or is it first cleaved in the host cytosol into glucose and fructose? What enzyme breaks down the sucrose? Is it invertase, which is known to increase in infected leaf tissue (19, 20), or alternatively, sucrose synthase? Once glucose is transported into the haustorium, what sugar is then transported into intercellular hyphae? It has been speculated that mannitol may play this role (21). Further, photosynthetic mesophyll cells, usually producers of sucrose, which is actively exported, become net importers when colonized by rust fungi. How does the obligate parasite manipulate the host to change the flow of sugars? Are there gene regulators that are secreted by the fungal haustorium into the plant cell that alter these pathways? What other compounds traverse this interface? What methods are used by the fungus to elude or suppress the defense mechanisms of the host? The results of Voegele et al. (1) elegantly demonstrate that multifaceted experimental approaches can provide answers to the roles of haustoria in host–parasite interactions.
Acknowledgments
We thank Jacki Morrison for preparation of the figures, and Kurt Leonard and Richard Staples for their comments on the manuscript. This paper was supported by the Agricultural Research Service of the U.S. Department of Agriculture.
Footnotes
See companion article on page 8133.
References
- 1.Voegele R T, Struck C, Hahn M, Mendgen K. Proc Natl Acad Sci USA. 2001;98:8133–8138. doi: 10.1073/pnas.131186798. . (First Published June 5, 2001; 10.1073/pnas131186798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coffey M D, Palevitz B A, Allen P J. Can J Bot. 1972;50:231–240. [Google Scholar]
- 3.Staples R C, Hoch H C. Exp Mycol. 1987;11:163–169. doi: 10.1006/emyc.1995.1035. [DOI] [PubMed] [Google Scholar]
- 4.Maheshwari R, Hildebrandt A C, Allen P J. Can J Bot. 1967;45:447–450. [Google Scholar]
- 5.Hoch H C, Staples R C, Whitehead B, Comeau J, Wolf E D. Science. 1987;235:1659–1662. doi: 10.1126/science.235.4796.1659. [DOI] [PubMed] [Google Scholar]
- 6.Allen E A, Hazen B E, Hoch H C, Kwon Y, Leinhos G M E. Phytopathology. 1991;81:323–331. [Google Scholar]
- 7.Heath M C. Exp Mycol. 1990;14:84–88. [Google Scholar]
- 8.Manners J M, Gray J L. New Phytol. 1982;91:221–244. [Google Scholar]
- 9.Martin T J, Ellingboe A H. Physiol Plant Pathol. 1978;13:1–11. [Google Scholar]
- 10.Mendgen K. Arch Microbiol. 1979;123:129–135. [Google Scholar]
- 11.Mendgen K, Noss P. Planta. 1988;174:283–288. doi: 10.1007/BF00394782. [DOI] [PubMed] [Google Scholar]
- 12.Staples R C, Yoder O C, Hoch H C, Epstein L, Bhairi S. In: Biology and Molecular Biology of Plant-Pathogen Interactions. Bailey J A, editor. London: Springer; 1996. pp. 331–341. [Google Scholar]
- 13.Hahn M, Mendgen K. Mol Plant–Microbe Interact. 1997;10:427–437. doi: 10.1094/MPMI.1997.10.4.427. [DOI] [PubMed] [Google Scholar]
- 14.Sutton P N, Henry M J, Hall J L. Planta. 1999;208:426–430. [Google Scholar]
- 15.Clark J I M, Hall J L. New Phytol. 1998;140:261–269. doi: 10.1046/j.1469-8137.1998.00263.x. [DOI] [PubMed] [Google Scholar]
- 16.Hahn M, Deising H, Struck C, Mendgen K. In: Resistance of Crop Plants Against Fungi. Hartleb H, Heitefuss R, Hoppe H-H, editors. Stuttgart, Germany: Fischer; 1997. pp. 33–57. [Google Scholar]
- 17.Struck C, Siebels C, Rommel O, Wernitz M, Hahn M. Mol Plant–Microbe Interact. 1998;11:458–465. doi: 10.1094/MPMI.1998.11.6.458. [DOI] [PubMed] [Google Scholar]
- 18.Hahn M, Neef U, Struck C, Göttfert M, Mendgen K. Mol Plant–Microbe Interact. 1997;10:438–445. doi: 10.1094/MPMI.1997.10.4.438. [DOI] [PubMed] [Google Scholar]
- 19.Storr T, Hall J. New Phytol. 1993;121:535–543. [Google Scholar]
- 20.Scholes J D, Less P J, Horton P, Lewis D H. New Phytol. 1994;126:213–222. [Google Scholar]
- 21.Mendgen K, Struck C, Voegele R T, Hahn M. Physiol Mol Plant Pathol. 2000;56:141–145. [Google Scholar]