Summary
Background
Cytomegalovirus (CMV) is a risk factor for rejection and mortality soon after renal transplantation. Little is known about its consequences longer after transplantation. We prospectively investigated whether latent CMV infection is a risk factor for graft failure and mortality long after transplantation.
Material/Methods
Our study included 606 renal transplant recipients (RTR) with a functioning graft for >1 year. CMV serology was determined using ELISA. RTRs were divided into CMV-seronegative and latent CMV (seropositive + seroconverted).
Results
We measured CMV IgG at 6.0 [2.6–11.4] years post-transplant. During follow-up (7.0 [6.2–7.5] years), 54 (9%) RTRs experienced graft failure and 137 (23%) RTRs died. Risk for graft failure and mortality was significantly higher in RTRs with latent CMV compared to CMV-seronegative RTRs (HR=3.1, P=0.005 and HR=2.0, P=0.002, respectively). After adjustment for potential confounders, latent CMV infection remained an independent risk factor for graft failure (HR=4.6, P=0.001), but not for mortality (HR=1.4, P=0.2).
Conclusions
Latent CMV is an independent risk factor for graft failure long after renal transplantation and carries a higher risk for graft failure than for mortality. These findings confirm the notion that latent CMV can be harmful in transplanted kidneys.
Keywords: cytomegalovirus, chronic transplant dysfunction, recipient survival, renal transplantation
Background
Cytomegalovirus (CMV) has been established as the single most important pathogen after transplantation [1–3]. Several studies have shown that CMV reactivation from latency and primary infection shortly after transplantation are risk factors for both immunological rejection and mortality in the first year after transplantation [4–12]. The reactivation from latency that commonly occurs shortly after transplantation is the consequence of a temporary disruption of an otherwise existing balance between immunological surveillance and viral replication by treatment with cytotoxic drugs and antilymphocyte antibody therapy and by systemic infection and inflammation [13]. In both primary infection and reactivation, CMV as a medical problem slowly diminishes with time after transplantation in conjunction with return to latency. In most cases CMV latency is achieved within 1 year after transplantation; however, the virus may continuously smoulder in the vascular wall, in particular in inflamed tissues under conditions of chronic immunosuppression [14,15]. Latent CMV can be locally active in a transplanted organ with ongoing low-grade alloreactivity, without systemic signs of activity in the chronic phase after transplantation [16]. As a consequence, investigation of CMV reactivation and primary infection shortly after transplantation as a risk factor for graft loss or mortality may have negated the possibility that the situation in which CMV remains in latency in the early phase after transplantation can be accompanied by ongoing CMV-related inflammation locally in tissues longer after transplantation, especially in the transplanted kidney.
To investigate the late impact of latent CMV infection versus a persistent CMV-negative state on late outcome, we prospectively investigated the relation of CMV serology determined more than 1 year after transplantation with graft failure and mortality long after renal transplantation.
Material and Methods
Research design and subject
In this prospective cohort study, all renal transplant recipients (RTRs) who visited our out-patient clinic between August 2001 and July 2003 and had a functioning graft for at least 1 year were eligible to participate at their next visit to the out-patient clinic. Recipients were asked to participate at a later visit to the out-patient clinic if they were ill or had an infection. A total of 606 RTRs signed written informed consent, from a total of 847 eligibles (72% consent rate). The group that did not sign informed consent was comparable with the group that signed informed consent with respect to age, sex, body mass index (BMI), serum creatinine, creatinine clearance, and proteinuria. Of patients included, none had received a transplantation before 1960, 24 received their transplantation in the 1970s, 105 in the 1980s, 354 in the 1990s, and 123 between January 2000 and May 2002. Further details of this study have been published previously (17,18). The Institutional Review Board approved the study protocol (METc 01/039) which conformed to the Declaration of Helsinki [19].
Outcome events
All participating subjects visited the out-patient clinic at least once a year. Information on mortality and graft loss was recorded by our renal transplant center and through close contact with general practitioners and referring nephrologists. Graft failure was defined as return to dialysis or re-transplantation and was censored for death. Mortality and graft failure of all RTRs were recorded until August 2007. There was no loss to follow-up.
Renal transplant characteristics
Relevant transplant characteristics were taken from the Groningen Renal Transplant Database. This database contains information on all renal transplantations performed at our center since 1968, including the dialysis history of the individual RTRs. Standard immunosuppression consisted of the following: from 1968 until 1989, prednisolone and Azathioprine (100 mg/day); from January 1989 to February 1993, cyclosporin standard formulation (1288) (Sandimmune, Novartis Pharma b.v., Arnhem, The Netherlands; 10 mg/kg; trough levels of 175–200 mg/l in first 3 months, 150 mg/l between 3 and 12 months post-transplant, and 100 mg/l thereafter) combined with prednisolone (starting with 20 mg/day, rapidly tapered to 10 mg/day); and from March 1993 to May 1997, cyclosporin microemulsion (Neoral; Novartis Pharma b.v., Arnhem, The Netherlands; 10 mg/kg; trough levels idem) and prednisolone. From May 1997 to date, mycophenolate mofetil (Cellcept; Roche b.v., Woerden, The Netherlands; 2 g/day) was added. Current medication was extracted from the medical record.
BMI, waist circumference, body surface area (BSA), and blood pressure were measured as described previously [17]. Smoking status and cardiovascular history were recorded with a self-report questionnaire. Cardiovascular disease history was considered positive if there was a previous myocardial infarction (MI), transient ischemic attack (TIA) or cerebrovascular accident (CVA).
In our center we do not apply routine CMV prophylaxis. Prophylaxis for CMV is only applied in case of combined transplantation or use of anti-thymocyte globulin [20]. Instead, we perform frequent monitoring for CMV in blood, formerly – before and during the days that we performed the baseline measurements for the current study – by measuring CMV pp65 antigenemia, and presently by PCR. In our center, pp65 antigenemia was introduced for monitoring in 1986. CMV is monitored once a week in every transplant recipient during hospitalization and at every visit to the outpatient clinic until 3 months after transplantation, except when the donor and recipient are both seronegative for CMV. Beyond 3 months after transplantation, monitoring is continued for follow-up of previous demonstration of positive viremia or by medical indication. Guided by this monitoring, we start pre-emptive treatment, formerly by IV ganciclovir and presently by oral valganciclovir preferentially. In our hospital, IV ganciclovir became available for pre-emptive treatment in 1989. Data regarding whether RTRs received treatment with IV ganciclovir was retrieved from their individual charts. Oral valganciclovir was not yet available in our hospital at the time baseline measurements for this study were performed.
CMV disease was defined by detection of CMV in a clinical specimen, accompanied either by CMV syndrome with fever, muscle pain, leucopenia, and/or thrombocytopenia without other known causes, or by organ involvement such as hepatitis, gastrointestinal ulceration, pneumonitis, or retinitis. Leukopenia was defined as leukocyte count below 4×109 /L and thrombocytopenia when the cell count was less than 100×109 /L in peripheral blood. Hepatitis was defined as a rise in liver enzymes of at least twice the initial values without other known cause. Gastrointestinal CMV ulceration was confirmed by endoscopy and biopsy. Presence of CMV in tissue biopsies was detected by immunohistochemistry or growth of virus in cell cultures.
Laboratory measurements
Blood was drawn after an 8–12 h overnight fasting period. Anti-CMV IgG antibody levels were assessed by routine ELISA as described previously [21]. A detectable anti-CMV IgG titer indicated seropositivity. CMV in blood was monitored by measuring CMV pp65 antigenemia as described previously [18]. Serum creatinine levels were determined using a modified version of the Jaffé method (MEGA AU 510, Merck Diagnostica, Darmstadt, Germany). Serum total cholesterol, HDL cholesterol, triglycerides, high-sensitivity C-reactive protein (hsCRP), and urinary protein excretion were assessed as described previously [17]. Proteinuria was defined as urinary protein excretion ≥0.5 g/24 hr.
Statistical analysis
Analyses were performed with SPSS version 14.0 (SPSS Inc., Chicago, IL) and Sigma Plot version 10 (Systat Software Inc., Germany). Parametric variables are expressed as mean ±SD, whereas non-parametric variables are given as median (interquartile range). A 2-tailed P-value of less than P<0.05 indicated statistical significance.
Recipient characteristics are shown according to CMV serostatus >1 year after transplantation: CMV-seronegative (CMV IgG ≤1 U/mL at transplantation and beyond 1 year after transplantation), CMV-seroconverted (CMV IgG ≤1 U/mL at time of transplantation and CMV IgG >1 U/mL beyond 1 year after transplantation), and CMV-seropositive (CMV IgG >1 U/mL at time of transplantation and beyond 1 year after transplantation). Latent CMV infection was defined as CMV IgG >1 U/mL beyond 1 year after transplantation (= CMV seroconverted + CMV seropositive). Serologic analysis was done at inclusion and pre-transplant sera were not retested. To investigate which recipient and transplant-related variables were associated with CMV serostatus, we analyzed these factors by CMV serostatus beyond 1 year after transplantation. P for trend was calculated with chi-square, Kruskal-Wallis test and linear regression for dichotomous, ordinal and continuous variables, respectively. Skewed data were normalized by logarithmic transformation in all analyses.
In time-to-event analyses, we first investigated CMV serostatus (seronegative, seroconverted, and seropositive) as potential predictors of graft failure and mortality using Kaplan-Meier analyses. Statistical significance was tested by log-rank test. Finally, univariate and multivariate Cox-proportional hazard regression analyses were performed to judge whether the potential effect of latent CMV infection on graft failure and mortality was independent of potential confounders. In the multivariate analyses, the associations of latent CMV infection with graft failure and mortality were adjusted for recipient age and sex (Model 2) and for time between transplantation and inclusion date, creatinine clearance, and immunosuppressive era (Model 3). We subsequently adjusted for all other characteristics significantly associated with CMV serostatus >1 year after transplantation (Tables 1, 2, P<0.05, Model 4). As secondary analysis, the procedure was repeated with additional inclusion of variables with a P-value >0.05 and ≤0.1 (Model 5). Also as a secondary analysis, we investigated whether log-transformed quantitative anti-CMV antibody titers were associated with occurrence of graft failure.
Table 1.
Recipient-related characteristics of renal transplant recipients according to CMV serostatus >1 year after transplantation.
| CMV serostatus >1 year after transplantation | P | |||
|---|---|---|---|---|
| Negative | Seroconverted | Seropositive | ||
| n (%) | 174 (29) | 152 (25) | 280 (46) | |
| Recipient demographics | ||||
| Age (years) | 47.9±13.1 | 52.5±11.4 | 53.1±11.5 | <0.0001 |
| Male, n (%) | 103 (59) | 85 (56) | 144 (51) | 0.3 |
| Body composition measurements | ||||
| BMI (kg/m2) | 25.2±4.04 | 26.2±4.45 | 26.5±4.30 | 0.01 |
| Waist circumference (cm) | 94.8±13.5 | 97.8±14.5 | 98.3±13.2 | 0.03 |
| Blood pressure | ||||
| Systolic pressure (mmHg) | 151±21.4 | 149±23.1 | 157±23.0 | 0.001 |
| Diastolic pressure (mmHg) | 90.1±10.1 | 88.0±10.4 | 90.9±9.34 | 0.01 |
| Prior history of cardiovascular disease | ||||
| MI*, n (%) | 10 (6) | 20 (13) | 18 (6) | 0.02 |
| TIA/CVA**, n (%) | 6 (3) | 12 (8) | 15 (5) | 0.2 |
| Lipids | ||||
| Total cholesterol (mmol/L) | 5.6 [4.9–6.2] | 5.7 [4.9–6.3] | 5.5 [4.9–6.2] | 0.6 |
| LDL (mmol/L) | 3.6 [3.0–4.2] | 3.6 [3.0–4.2] | 3.5 [2.9–4.0] | 0.2 |
| HDL (mmol/L) | 1.0 [0.9–1.3] | 1.0 [0.8–1.3] | 1.1 [0.9–1.3] | 0.2 |
| Triglycerides (mmol/L) | 1.8 [1.3–2.4] | 1.9 [1.4–2.8] | 2.0 [1.4–2.6] | 0.02 |
| Use of statin, n (%) | 79 (45) | 68 (45) | 153 (55) | 0.06 |
| CRP (mg/L) | 2.0 [0.7–4.4] | 2.1 [0.8–4.9] | 2.0 [1.0–5.5] | 0.4 |
| CMV | ||||
| CMV IgG (U/mL) | 0 [0–0] | 110 [62–191] | 110 [62–198] | <0.0001 |
| CMV disease, n (%) | 0 (0) | 66 (43) | 66 (24) | <0.0001 |
| CMV curative treatment, n (%) | 0 (0) | 49 (32) | 61 (24) | <0.0001 |
Values are presented as mean ±standard deviation, median (interquartile range) or percentages. P for trend was calculated with chi-square, Kruskal-Wallis test and linear regression for dichotomous, ordinal and continuous variables, respectively. Skewed data were normalized by logarithmic transformation in all analyses.
MI – myocardial infarction;
TIA/CVA – transient ischaemic attack/cerebrovascular accident.
Table 2.
Transplant-related characteristics of renal transplant recipients according to CMV serostatus >1 year after transplantation.
| CMV serostatus >1 year after transplantation | P | |||
|---|---|---|---|---|
| Negative | Seroconverted | Seropositive | ||
| n (%) | 174 (29) | 152 (25) | 280 (46) | |
| Donor demographics | ||||
| Age (years) | 35.9±15.4 | 34.8±14.9 | 38.7±15.6 | 0.02 |
| Male, n (%) | 98 (56) | 82 (54) | 148 (53) | 0.8 |
| Renal allograft function | ||||
| Serum creatinine concentration (ìmol/L) | 136 [112–162] | 129 [111–170] | 134 [114–166] | 0.8 |
| Creatinine clearance (mL/min) | 66.5±21.2 | 59.6±21.3 | 60.5±23.5 | 0.007 |
| Proteinuria (g/24hr) | 0.2 [0.0–0.4] | 0.2 [0.0–0.5] | 0.2 [0.0–0.5] | 0.7 |
| Prior dialysis duration (mo) | 25 [12–47] | 26 [15–29] | 29 [16–53] | 0.09 |
| Transplantation type, n (%) | ||||
| Postmortem donor | 137 (79) | 134 (88) | 232 (83) | 0.06 |
| Living donor | 33 (19) | 15 (10) | 35 (12) | |
| Combined transplantation | 4 (2) | 3 (2) | 13 (5) | |
| Number of previous transplants, n (%) | ||||
| 0 | 163 (94) | 134 (88) | 245 (88) | 0.01 |
| 1 or more | 11 (6) | 18 (12) | 35 (12) | |
| Acute rejection, n (%) | 77 (44) | 77 (51) | 118 (42) | 0.2 |
| Immunosuppressive era, n (%) | ||||
| from 1968 to January 1989 | 30 (17) | 65 (43) | 17 (6) | <0.0001 |
| from January 1989 to February 1993 | 19 (11) | 17 (11) | 54 (20) | |
| from March 1993 to May 1997 | 42 (24) | 32 (21) | 83 (29) | |
| from May 1997 to date | 83 (48) | 38 (25) | 126 (45) | |
| Immunosuppresion | ||||
| Prednisolone dose, (mg/day) | 10.0 [7.5–10.0] | 10.0 [7.5–10.0] | 10.0 [7.5–10.0] | 0.04 |
| Calcineurine inhibitor, n (%) | 140 (81) | 94 (62) | 241 (86) | P=0.04 |
| Cyclosporin, n (%) | 112 (65) | 81 (53) | 197 (70) | 0.5 |
| Trough–level (μg/L) | 114 [82–140] | 101 [75–128] | 108 [80–143] | 0.2 |
| Tacrolimus, n (%) | 28 (16) | 13 (9) | 44 (16) | 0.5 |
| Trough–level (μg/L) | 8 [6–11] | 10 [7–11] | 9 [6–10] | 0.7 |
| Proliferation inhibitor, n (%) | ||||
| Azathioprine or Mycophenolate mofetil | 133 (76) | 107 (70) | 208 (74) | 0.5 |
Values are presented as mean ±standard deviation, median (interquartile range) or percentages. P for trend was calculated with χ-square, Kruskal Wallis test and linear regression for dichotomous, ordinal and continuous variables, respectively. Skewed data were normalized by logarithmic transformation in all analyses.
Results
A total of 606 RTRs (55% male, aged 51±12 years, 83% post-mortem donor transplants) were analyzed. Median time between transplantation and baseline measurements was 6.0 (2.6–11.4) years. Baseline median anti-CMV IgG was 72.0 (0.0–154.5) U/mL. Baseline characteristics according to CMV serostatus >1 year after transplantation are shown in Tables 1, 2; 174 (29%) RTRs were CMV-seronegative, 152 (25%) RTRs were CMV-seroconverted and 280 (46%) RTRs were CMV-seropositive. In the CMV-seronegative recipients group, 153 (88%) of the donors were CMV-negative, while 21 (12%) of the donors were CMV-seropositive. In the CMV-seroconverted recipients group, 6 (4%) of the donors were CMV-seronegative, while 146 (96%) were CMV-seropositive. In the CMV-seropositive recipients group, 124 (44%) of the donors were CMV-seronegative, while 154 (55%) were CMV-seropositive. We have no data regarding the CMV serostatus from 2 donors in this group. Recipient CMV serostatus was significantly associated with recipient age, BMI, waist circumference, systolic and diastolic blood pressure, myocardial infarction, triglyceride concentration, donor age, creatinine clearance, immunosuppressive era, dose of prednisolone, and use of calcineurin inhibitors. CMV disease was significantly associated with CMV serostatus (P<0.0001). In total, 132 RTRs experienced CMV disease – 66 (43%) of the 152 CMV-seroconverted RTRs and 66 (24%) of the CMV-seropositive RTRs. In our clinic, the cut-off level for starting pre-emptive treatment was when antigenemia tested positive for at least 20 cells/50 000 polymorphonuclear neutrophils, when consecutive increasing antigenemia values were detected or when symptoms suggestive of CMV infection were accompanied by any antigenemia positivity. Our relatively high cut-off with requirement for symptoms suggestive of CMV infection for start of pre-emptive treatment for lower levels of antigenemia may explain the relatively high rate of symptomatic infections.
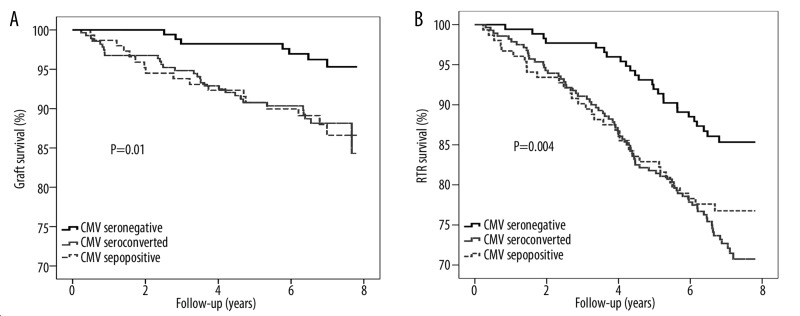
Median follow-up was 7.0 (6.2–7.5) years both for graft failure and mortality. During follow-up, 54 (9%) RTRs experienced graft failure and 137 (23%) RTRs died. In the CMV-seronegative group, 7 (4%) RTRs experienced graft failure and 25 (14%) died, while these numbers were 17 (11%) and 35 (23%) for the CMV-seroconverted RTRs and 30 (9%) and 77 (28%) for the CMV-seropositive RTRs (both log-rank test: P<0.02, Figure 1A, B).
Figure 1.
Kaplan-Meier analysis of (A) graft and (B) RTR survival according to CMV serostatus >1 year after transplantation. Tested with log-rank test.
Further analyses were performed for latent CMV infection (= CMV-seroconverted + CMV-seropositive) versus CMV-seronegative RTRs. RTRs with latent CMV infection were at significantly higher risk for graft failure (hazard ratio [HR] =3.1, 95% confidence interval [CI] 1.4–6.9, P=0.005) and death (HR=2.0, 95% CI 1.3–3.1, P=0.002) than CMV-seronegative RTRs (Model 1, Table 3). Adjustment for recipient age and recipient sex did not materially change these associations (Model 2, Table 3). After further adjustment for time between transplantation and inclusion date, creatinine clearance and immunosuppressive era (Model 3, Table 3), CMV latency remained significantly associated with graft failure (HR=3.2, 95% CI 1.4–7.2, P=0.006), while the association of CMV latency with death lost significance (HR=1.5, 95% CI 1.0–2.3, P=0.07). Additional adjustment for variables which were significantly associated with CMV serostatus (see Tables 1, 2, all variables with a P<0.05) did not materially change the outcomes (Model 4, Table 3). Subsequent adjustment for variables that were borderline significant associated with CMV serostatus (0.05<P<0.10, Tables 1, 2) did not materially change the outcomes (Model 5, Table 3). After multivariate analyses, the risk for graft failure (HR=4.6, 95% CI 1.8–11.9, P=0.001) in CMV IgG-positive RTRs was 3.3 times higher than the risk for death (HR=1.4, 95% CI 0.9–2.2, P=0.2, Model 5, Table 3). Final adjustment for CMV disease did not materially change the outcomes (data not shown).
Table 3.
Univariate and multivariate Cox-proportional hazards analyses of the effect of latent CMV infection on graft failure and mortality in RTR.
| Graft failure | Death | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Model 1 | 3.1 | 1.4–6.9 | 0.005 | 2.0 | 1.3–3.1 | 0.002 |
| Model 2 | 3.6 | 1.6–8.1 | 0.002 | 1.7 | 1.1–2.6 | 0.02 |
| Model 3 | 3.2 | 1.4–7.2 | 0.006 | 1.5 | 1.0–2.3 | 0.07 |
| Model 4 | 4.3 | 1.7–10.6 | 0.002 | 1.4 | 0.9–2.2 | 0.2 |
| Model 5 | 4.6 | 1.8–11.9 | 0.001 | 1.4 | 0.9–2.2 | 0.2 |
CI – confidence interval; Model 1 − crude model; Model 2 − model 1 + recipient age and sex; Model 3 − model 2 + time between transplantation and inclusion date, creatinine clearance and immunosuppressive era; Model 4 − model 3 + BMI, systolic blood pressure, myocardial infarction, concentration triglycerides, donor age, prednisolone dose, calcineurine inhibitor and CMV curative treatment; Model 5 − model 4 + use of statin, prior dialysis duration, transplantation type, and number of previous transplants.
As a secondary analysis, we investigated whether quantitative anti-CMV antibody titers were associated with graft outcome in RTRs with latent CMV infection. This appeared not to be the case (HR 1.3 [95% CI 0.6–2.3], P=0.4).
Discussion
To the best of our knowledge, our study is the first to prospectively investigate the impact of CMV serology determined >1 year after transplantation on graft and RTR survival long after renal transplantation. The main finding is that graft survival is significantly better in CMV-seronegative RTRs than in those with latent CMV infection. We furthermore found that RTRs with latent CMV infection are at 3.3 times higher risk for graft failure than for death.
CMV has been established as a major pathogen after renal transplantation, and as such is an important cause of morbidity and mortality after renal transplantation. CMV infection is highly prevalent in RTRs (up to approximately 80% in Western countries), whereas 25–33% of the infected RTRs develop a clinically overt disease after renal transplantation [22]. The CMV seroprevalence in this study is relatively low. There are several studies showing that the CMV seroprevalence in healthy Dutch women is approximately 35% [23,24]. It has also been shown that the CMV seroprevalence in the Netherlands is lower than in the United States and other western European countries, ranging from 40% to 83% [25]. Furthermore, there is a great variation in CMV seroprevalence according to socioeconomic status and ethnic composition [25]. These observations could be a good explanation for the low CMV seroprevalence in our study.
Numerous studies have shown that CMV infection and disease occurring in the first months after transplantation are risk factors for immunological rejection and mortality, both soon and long after transplantation [5–10]. However, use of CMV disease and infection soon after transplantation as predictors of late graft failure and mortality may lead to underestimation of risk held by CMV if it is the CMV-positive state itself rather than the severity of CMV disease or infection in the first phase after transplantation that is the risk factor. Inclusion in the control group of CMV-positive recipients that do not exhibit early CMV disease or infection will dilute the group of CMV-seronegative controls with subjects that are at increased risk. Results of our study are consistent with this notion because the CMV-seroconverted and the CMV-seropositive groups had similar increases in risk for late graft failure and mortality compared to recipients that remained CMV-seronegative.
In univariate analysis we found several risk factors for all-cause mortality (higher BMI, higher waist circumference, higher blood pressure, lower kidney function, more re-transplantations) to be associated with CMV serostatus. In view of the fact that each of these risk factors might be an explanation for the association of CMV serostatus with increased mortality, and the fact that CMV serostatus lost significance as a predictor of mortality in multivariate analyses adjusted for these factors, suggests that CMV serostatus is not a risk factor for mortality long after transplantation. However, although latent CMV infection lost significance as a risk factor for mortality after adjustment for other variables, it cannot be excluded that CMV actually acts on mortality, in part through these variables. The number of variables included in the multivariate Cox regression models may seem large compared to the existing rule of thumb that per 8–10 events 1 covariate can be added to a multivariate model [26]. This restriction, however, applies to the situation in which one wants to judge whether a covariate does not significantly add to a multivariate model, because multiple adjustment tends to “dilute” truly existing associations, thereby favoring falsely negative conclusions. When, however, adjustment for covariates does not materially change hazard ratios and confidence intervals, it is acceptable to adjust for more confounders than one would calculate on the basis of this rule of thumb, because the association is apparently so “robust” that it is not sensitive to the “dilution” effect that is introduced by adjustment for many covariates. The fact that the association of latent CMV with graft failure remained significant in multivariate analyses with multiple potential confounders and covariates included strengthens the conclusion that it is truly an independent risk factor. Obesity, which is an important cause of mortality and renal dysfunction [27–29], was not an important confounder in our study. CMV causing accelerated decline of renal function and/or accelerated atherosclerosis in RTRs may be potential mechanisms underlying an association of latent CMV infection with mortality. A potential role for CMV-related decline of renal function is supported by loss of significance of the association of CMV with mortality after adjustment for creatinine clearance. Active, but also latent CMV infection, may be associated not only with overexpression of major histocompatibility complex molecules and altered expression of growth factors and cytokines, but also with upregulation of pro-inflammatory adhesion molecules, which might lead to accelerated atherosclerosis in association with CMV [2,14,30]. The finding that CMV DNA is present in atherosclerotic plaques supports a role for CMV in atherogenesis [31–33], although some studies have failed to detect CMV in atherosclerotic tissue [34,35]. In a study performed shortly after transplantation, CMV has been suggested to play a role in the pathogenesis of post-transplant diabetes mellitus [36], which may also exert a pro-atherogenic effect.
The fact that we found that RTRs with latent CMV infection are at 3.3-fold higher risk for graft failure than for death is consistent with the recent finding that latent CMV may be locally active in a transplanted organ, without systemic signs of activity or consequences [16]. Latent CMV may be particularly active in organs and tissues with ongoing inflammation not directly related to CMV [15]. In transplantation, the allo-surrounding may provide the background inflammation which allows CMV expression to become harmful. After cardiac transplantation, it has been shown that CMV is associated with development of accelerated coronary artery sclerosis [37]. A similar process has been observed in transplanted kidneys in association with CMV infection [38,39]. In studies in rats, the interaction between CMV and the alloreactive response in the development of chronic rejection and transplant vascular sclerosis was investigated in small bowel and heart transplantation models [16]. It was shown that CMV infection accelerated the time to graft chronic rejection and increased the severity of transplant vascular sclerosis in both small bowel and heart allografts. We evaluated RTRs with respect to CMV serostatus. The fact that we found similar risks long after transplantation for seropositivity before transplantation and seroconversion after transplantation suggests that what is important is not whether subjects developed CMV disease after transplantation, but rather the CMV serostatus itself. Studies using repetitive CMV PCR analyses may provide additional information on this topic. In our study, adjustment for CMV treatment did not materially change the results of analyses. This also suggests that it is the CMV serostatus itself which is important, but CMV treatment was guided by symptoms of CMV disease and presence of detectable CMV in the circulation rather than prevention of deleterious effects on graft function later on. If biomarkers become available for identification and monitoring of chronic and deleterious CMV infection, this might pave the way for a trial with intensive and prolonged anti-CMV therapy. Such biomarkers might also be useful as intermediate end-points in trials with longer and/or more intensive prophylaxis. Intensive prophylaxis prevents acute rejection and cardiac allograft vascular disease after heart transplantation [40,41]. Interestingly, renal dysfunction is also an important problem after heart transplantation [42,43], and gancyclovir has been shown to inhibit ICAM-1 expression and proliferation in human coronary endothelial cells [44].
In our study CMV serostatus >1 year after transplantation was not associated with acute rejection. Most studies investigating the impact of CMV on acute rejection have found an association of CMV infection or disease with acute rejection soon after transplantation [5,8,9,45]. The absence of an association in our study may be explained by the fact that our study was designed to investigate the impact of CMV determined >1 year after transplantation on long-term graft and RTR survival. As a consequence of the fact that we only included RTRs with a kidney functioning for >1 year, RTRs who lost their kidney due to acute rejection in the first year(s) after transplantation were not invited to participate in this study. Therefore, in this study the number of RTRs who had an acute rejection is probably underestimated compared to studies in which RTRs were included from the moment of transplantation. Inclusion of every patient at a certain time point (e.g., 1 year after transplantation) would have been interesting, but it is artificial compared to what is actually happening at an outpatient clinic with renal transplant recipients. As we wanted to investigate late outcome rather than acute graft failure and early mortality, which often are defined as graft failure or mortality in the first year after transplantation, we excluded these recipients from this study. As it was our aim to investigate the potential impact of CMV serostatus on late outcome in a patient sample representative of the patients that are seen at an outpatient clinic, we included patients at more than 1 year after transplantation. If one would like to investigate it from the perspective of a certain time point (e.g., 1 year after transplantation) this might introduce “healthy survivor bias”, but from the perspective of a representative sample of the patients that are seen at an outpatient clinic it does not.
The present study has several limitations. First, because the study population almost entirely consisted of patients of Caucasian ethnicity, the applicability of our results to more racially diverse renal transplant populations may be limited. Furthermore, this study was a single-center study and the findings need to be confirmed in other centers and/or multicenter studies. Third, our study includes RTRs that were transplanted in multiple immunosuppressive eras. Although, immunosuppressive therapy was associated with CMV serostatus at baseline, adjustment for immunosuppressive era in the multivariate analyses did not materially change the association of CMV serostatus with outcomes. It may also be seen as a limitation that the fraction of CMV-seronegative RTRs of 54% at the time of transplantation in our population is higher than in other studies, in which, for instance, fractions of 49%, 45% and 52% have been reported [6,46]. It should, however, be noted that these studies included patients at the moment of transplantation, while we included patients at a median time of 6.0 years after transplantation. Because of this time point long after transplantation, our study differs from other studies in that it is not optimal for analyzing the association of CMV infection with graft loss or mortality, but rather for studying the potential association of latent CMV with graft loss or mortality. It has furthermore been reported in a large study on CMV seroconversion in CMV-seronegative organ transplant recipients that 2.6% of CMV-seronegative recipients transplanted with organs of CMV-seropositive donors have no seroconversion, despite documented CMV viremia [47]. The report noted that this may either be the consequence of absence of seroconversion or absence of serology data subsequent to CMV viremia. If some of these cases were the consequence of true absence of seroconversion, these subjects would be misclassified in our study and could potentially mitigate our findings, because they may then not be free of latent CMV. An important strength of this study is that follow-up was complete for all patients.
Conclusions
In conclusion, graft and recipient survival is significantly better in RTRs who are CMV-seronegative when compared to RTRs with latent CMV infection. Our results are consistent with the notion that what is important is not severity of infection soon after transplantation, but rather the CMV-positive state itself. Furthermore, RTRs with latent CMV infection are at 3.3-fold higher risk for graft failure than for death. This suggests that latent CMV is more active in a transplanted organ, potentially in association with chronic ongoing low-grade alloreactivity, or in kidneys in general. Future studies are needed to elucidate the mechanism underlying the link of CMV with graft failure and mortality long after renal transplantation.
Acknowledgements
We thank our physician assistants Marja van Kammeren, Erika Konneman-Zalk, and Saskia Vorderman for their work at the outpatient clinic and our lab technicians Simone Brandenburg and Jacko Duker. Lastly, we thank the Dutch Kidney Foundation for their support and funding (Nierstichting Nederland C00.1877).
Footnotes
Conflict of interest statement
None declared.
Source of support: This research was partially funded by the Dutch Kidney Foundation (Nierstichting Nederland C00.1877). APJ de Vries is supported by a Clinical Research Fellowship of the Netherlands Organization of Scientific Research (NWO-AGIKO 920-03-181) DM Zelle and SJL Bakker are supported by CTMM, the Centre for Translational Molecular Medicine (www.ctmm.nl), project PREDICCt (grant 01C-104)
References
- 1.Kotton CN. Viral infection in the renal transplant recipient. J Am Soc Nephrol. 2005;16:1758–74. doi: 10.1681/ASN.2004121113. [DOI] [PubMed] [Google Scholar]
- 2.Fishman JA. Infection in organ-transplant recipients. N Engl J Med. 1998;338:1741–51. doi: 10.1056/NEJM199806113382407. [DOI] [PubMed] [Google Scholar]
- 3.Sia I, Patel R. New Strategies for Prevention and Therapy of Cytomegalovirus Infection and Disease in Solid-Organ Transplant Recipients. Clin Microbiol Rev. 2000;13:83–121. doi: 10.1128/cmr.13.1.83-121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinke P, Fietze E, Ode-Hakim S, et al. Late-acute renal allograft rejection and symptomless cytomegalovirus infection. Lancet. 1994;344:1737–38. doi: 10.1016/s0140-6736(94)92887-8. [DOI] [PubMed] [Google Scholar]
- 5.Becker BN, Becker YT, Leverson GE, et al. Reassessing the impact of cytomegalovirus infection in kidney and kidney-pancreas transplantation. Am J Kidney Dis. 2002;39:1088–95. doi: 10.1053/ajkd.2002.32793. [DOI] [PubMed] [Google Scholar]
- 6.Nett PC, Heisey DM, Fernandez LA, et al. Association of cytomegalovirus disease and acute rejection with graft loss in kidney transplantation. Transplantation. 2004;78:1036–41. doi: 10.1097/01.tp.0000137105.92464.f3. [DOI] [PubMed] [Google Scholar]
- 7.Sagedal S, Hartmann A, Nordal KP, et al. Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney Int. 2004;66:329–37. doi: 10.1111/j.1523-1755.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- 8.Sagedal S, Nordal KP, Hartmann A, et al. The impact of cytomegalovirus infection and disease on rejection episodes in renal allograft recipients. Am J Transplant. 2002;2:850–56. doi: 10.1034/j.1600-6143.2002.20907.x. [DOI] [PubMed] [Google Scholar]
- 9.Pouteil-Noble C, Ecochard R, Landrivon G, et al. Cytomegalovirus infection – an etiological factor for rejection? A prospective study in 242 renal transplant patients. Transplantation. 1993;55:851–57. doi: 10.1097/00007890-199304000-00032. [DOI] [PubMed] [Google Scholar]
- 10.Sagedal S, Rollag H, Hartmann A. Cytomegalovirus infection in renal transplant recipients is associated with impaired survival irrespective of expected mortality risk. Clin Transplant. 2007;21:309–13. doi: 10.1111/j.1399-0012.2006.00639.x. [DOI] [PubMed] [Google Scholar]
- 11.Walker JD, Maier CL, Pober JS. Cytomegalovirus-infected human endothelial cells can stimulate allogeneic CD4+ memory T cells by releasing antigenic exosomes. J Immunol. 2009;182:1548–59. doi: 10.4049/jimmunol.182.3.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khedmat H, Taheri S. Characteristics and prognosis of post-transplant lymphoproliferative disorders within renal allograft: Report from the PTLD. Int Survey Ann Transplant. 2010;15:80–86. [PubMed] [Google Scholar]
- 13.Adams PL. Long-term patient survival: strategies to improve overall health. Am J Kidney Dis. 2006;47:S65–85. doi: 10.1053/j.ajkd.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 14.Stassen FR, Vega-Cordova X, Vliegen I, Bruggeman CA. Immune activation following cytomegalovirus infection: more important than direct viral effects in cardiovascular disease? J Clin Virol. 2006;35:349–53. doi: 10.1016/j.jcv.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Soderberg-Naucler C. HCMV microinfections in inflammatory diseases and cancer. J Clin Virol. 2008;41:218–23. doi: 10.1016/j.jcv.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Orloff SL, Streblow DN, Soderberg-Naucler C, et al. Elimination of donor-specific alloreactivity prevents cytomegalovirus-accelerated chronic rejection in rat small bowel and heart transplants. Transplantation. 2002;73:679–88. doi: 10.1097/00007890-200203150-00005. [DOI] [PubMed] [Google Scholar]
- 17.van Ree RM, De Vries AP, Oterdoom LH, et al. Abdominal obesity and smoking are important determinants of C-reactive protein in renal transplant recipients. Nephrol Dial Transplant. 2005;20:2524–31. doi: 10.1093/ndt/gfi052. [DOI] [PubMed] [Google Scholar]
- 18.Gross S, Homan van der Heide JJ, Van Son WJ, et al. Body mass index and creatinine clearance are associated with steady-state serum concentrations of the cell damage marker S100B in renal transplant recipients. Med Sci Monit. 2010;16(7):CR318–24. [PubMed] [Google Scholar]
- 19.Decleration of Helsinki revisited. IRB. 2000;22:10–11. No authors listed. [PubMed] [Google Scholar]
- 20.Mota C, Martins L, Costa T, et al. Nineteen years of experience utilizing anti-T-Lymphocyte globulin induction in pediatric kidney transplantation. Ann Transplant. 2010;15:84–91. [PubMed] [Google Scholar]
- 21.Van der Giessen M, van den Berg AP, van der Bij W, et al. Quantitative measurement of cytomegalovirus-specific IgG and IgM antibodies in relation to cytomegalovirus antigenaemia and disease activity in kidney recipients with an active cytomegalovirus infection. Clin Exp Immunol. 1990;80:56–61. doi: 10.1111/j.1365-2249.1990.tb06441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cainelli F, Vento S. Infections and solid organ transplant rejection: a cause-and-effect relationship? Lancet Infect Dis. 2002;2:539–49. doi: 10.1016/s1473-3099(02)00370-5. [DOI] [PubMed] [Google Scholar]
- 23.Gaytant MA, Galama JM, Semmekrot BA, et al. The incidence of congenital cytomegalovirus infections in The Netherlands. J Med Virol. 2005;76:71–75. doi: 10.1002/jmv.20325. [DOI] [PubMed] [Google Scholar]
- 24.Stelma FF, Smismans A, Goossens VJ, et al. Occupational risk of human Cytomegalovirus and Parvovirus B19 infection in female day care personnel in the Netherlands; a study based on seroprevalence. Eur J Clin Microbiol Infect Dis. 2009;28:393–97. doi: 10.1007/s10096-008-0635-y. [DOI] [PubMed] [Google Scholar]
- 25.Griffiths PD, Walter S. Cytomegalovirus. Curr Opin Infect Dis. 2005;18:241–45. doi: 10.1097/01.qco.0000168385.39390.1b. [DOI] [PubMed] [Google Scholar]
- 26.Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–79. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 27.Rymarz A, Durlik M, Rydzewski A. Intravenous administration of N-acetylcysteine reduces plasma total homocysteine levels in renal transplant recipients. Ann Transplant. 2009;14:5–9. [PubMed] [Google Scholar]
- 28.Naumnik B, Mysliwiec M. Renal consequences of obesity. Med Sci Monit. 2010;16(8):RA163–70. [PubMed] [Google Scholar]
- 29.Telles S, Balkrishna A. Yoga and diet change influence renal functions in the obese. Med Sci Monit. 2010;16(10):LE15. [PubMed] [Google Scholar]
- 30.Fishman JA, Emery V, Freeman R, et al. Cytomegalovirus in transplantation – challenging the status quo. Clin Transplant. 2007;21:149–58. doi: 10.1111/j.1399-0012.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 31.Hendrix MG, Salimans MM, van Boven CP, Bruggeman CA. High prevalence of latently present cytomegalovirus in arterial walls of patients suffering from grade III atherosclerosis. Am J Pathol. 1990;136:23–28. [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu B, Viira E, Tucker W, Fong IW. Chlamydia pneumoniae, cytomegalovirus, and herpes simplex virus in atherosclerosis of the carotid artery. Circulation. 1997;96:2144–48. doi: 10.1161/01.cir.96.7.2144. [DOI] [PubMed] [Google Scholar]
- 33.Radke PW, Merkelbach-Bruse S, Messmer BJ, et al. Infectious agents in coronary lesions obtained by endatherectomy: pattern of distribution, coinfection, and clinical findings. Coron Artery Dis. 2001;12:1–6. doi: 10.1097/00019501-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Daus H, Ozbek C, Saage D, et al. Lack of evidence for a pathogenic role of Chlamydia pneumoniae and cytomegalovirus infection in coronary atheroma formation. Cardiology. 1998;90:83–88. doi: 10.1159/000006824. [DOI] [PubMed] [Google Scholar]
- 35.Saetta A, Fanourakis G, Agapitos E, Davaris PS. Atherosclerosis of the carotid artery: absence of evidence for CMV involvement in atheroma formation. Cardiovasc Pathol. 2000;9:181–83. doi: 10.1016/s1054-8807(00)00040-5. [DOI] [PubMed] [Google Scholar]
- 36.Hjelmesaeth J, Sagedal S, Hartmann A, et al. Asymptomatic cytomegalovirus infection is associated with increased risk of new-onset diabetes mellitus and impaired insulin release after renal transplantation. Diabetologia. 2004;47:1550–56. doi: 10.1007/s00125-004-1499-z. [DOI] [PubMed] [Google Scholar]
- 37.Valantine HA. The role of viruses in cardiac allograft vasculopathy. Am J Transplant. 2004;4:169–77. doi: 10.1046/j.1600-6143.2003.00316.x. [DOI] [PubMed] [Google Scholar]
- 38.Helantera I, Koskinen P, Finne P, et al. Persistent cytomegalovirus infection in kidney allografts is associated with inferior graft function and survival. Transpl Int. 2006;19:893–900. doi: 10.1111/j.1432-2277.2006.00364.x. [DOI] [PubMed] [Google Scholar]
- 39.Helantera I, Loginov R, Koskinen P, et al. Persistent cytomegalovirus infection is associated with increased expression of TGF-beta1, PDGF-AA and ICAM-1 and arterial intimal thickening in kidney allografts. Nephrol Dial Transplant. 2005;20:790–96. doi: 10.1093/ndt/gfh714. [DOI] [PubMed] [Google Scholar]
- 40.Tu W, Potena L, Stepick-Biek P, et al. T-cell immunity to subclinical cytomegalovirus infection reduces cardiac allograft disease. Circulation. 2006;114:1608–15. doi: 10.1161/CIRCULATIONAHA.105.607549. [DOI] [PubMed] [Google Scholar]
- 41.Gil RJ, Gimeno FM. Abdominal aortic aneurysmectomy in renal transplant recipients. Arch Med Sci. 2011;5:111–14. [Google Scholar]
- 42.Przybylowski P, Malyszko J, Malyszko J. Kidney function assessed by eGFR, cystatin C and NGAL (neutrophil gelatinase-associated lipocalin) in relation to age in heart allograft recipients. Med Sci Monit. 2010;16(9):CR440–44. [PubMed] [Google Scholar]
- 43.Przybylowski P, Malyszko J, Malyszko J. Immunosuppressive regimen and prevalence of chronic kidney disease in orthotopic heart transplant recipients. Med Sci Monit. 2010;16(11):CR563–66. [PubMed] [Google Scholar]
- 44.Voisard R, Munder U, von Muller L, et al. Direct inhibitory effects of Ganciclovir on ICAM-1 expression and proliferation in human coronary vascular cells (SI/MPL-ratio: >1) Med Sci Monit. 2011;17(1):PI1–6. doi: 10.12659/MSM.881310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toupance O, Bouedjoro-Camus MC, Carquin J, et al. Cytomegalovirus-related disease and risk of acute rejection in renal transplant recipients: a cohort study with case-control analyses. Transpl Int. 2000;13:413–19. doi: 10.1007/s001470050723. [DOI] [PubMed] [Google Scholar]
- 46.Helantera I, Loginov R, Koskinen P, Lautenschlager I. Demonstration of HHV-6 antigens in biopsies of kidney transplant recipients with cytomegalovirus infection. Transpl Int. 2008;21:980–84. doi: 10.1111/j.1432-2277.2008.00716.x. [DOI] [PubMed] [Google Scholar]
- 47.Humar A, Mazzulli T, Moussa G, et al. Clinical utility of cytomegalovirus (CMV) serology testing in high-risk CMV D+/R− transplant recipients. Am J Transplant. 2005;5:1065–70. doi: 10.1111/j.1600-6143.2005.00797.x. [DOI] [PubMed] [Google Scholar]