Summary
Background
This study aimed to analyze the epidemiological and mycological profile of candidemia in intensive care unit (ICU) patients attending a tertiary care teaching hospital in the Himalayan region of northern India.
Material/Methods
A 15-bed medico-surgical ICU and a 5-bed pediatric ICU. Ninety-one consecutively admitted ICU patients were screened for the presence of candidemia by performing blood cultures at periodic intervals.
Results
The recovered Candida isolates were speciated and subjected to antifungal susceptibility testing using standard procedures. Forty-one of the recruited patients (45%) were found to be candidemic, with the majority of patients being in the extremes of age (13 neonates and 15 >65 years of age). Four risk factors were found to be significantly associated with the occurrence of candidemia in our patients – a period of hospitalization exceeding 7 days (p=0.0008), previous use of antibiotics (p=0.001), presence of chronic renal failure (p=0.003), and ongoing cancer chemotherapy (p= 0.041). Ninety-six Candida isolates were recovered from the 41 culture-positive patients, with Candida albicans being the commonest isolate recovered (n=75, 78.1%), followed by Candida tropicalis (n=15, 16%), and Candida glabrata (n=6, 6.5%). Fluconazole resistance was observed among 26% of all Candida isolates and 17.3% of C. albicans isolates.
Conclusions
Contrary to the majority of recent reports, species shift towards non-albicans candidemia has not been observed in our center, though the prevalence of azole resistance is alarmingly high even among the C. albicans isolates.
Keywords: candidiasis, intensive care unit, antifungal drug resistance
Background
Candidiasis is a common cause of bloodstream and invasive infection in critically ill and immunosuppressed patients throughout the world. In addition to its widespread occurrence, it is often acutely progressive, difficult to diagnose, unresponsive to treatment and associated with increased hospital stay and high mortality rates [1–5]. Although worldwide increase in the incidence of invasive Candida infections has been witnessed since the 1980s [6,7], the recent trends demonstrate a gradual change in its species distribution, with many countries experiencing a relative rise in the proportion of non-albicans Candida isolates [8–16]. In view of the intrinsic resistance to specific antifungal agents observed among several of these non-albicans Candida species, this changing trend bears important therapeutic implications. Moreover, there has been a documented increase in fluconazole resistance even among previously susceptible Candida spp., including Candida albicans (C.albicans) [17]C. lusitaniae, C. tropicalis and C. dubliniensis[18,19], which has been partially attributed to the widespread use of fluconazole as empirical antifungal therapy since the 1990s [20].
The increasing population of immuno-compromised patients, together with the rising incidence of non-albicans candidemia and the emergence of acquired antifungal resistance, necessitates the judicious administration of antifungal prophylaxis in at-risk patients and empirical antifungal therapy in patients suffering from candidemia. Sensitivity profiles of the locally prevalent Candida strains and knowledge regarding risk factors relevant for the patient profile attending a particular healthcare facility are key determinants in the selection of appropriate patients and antifungal agents for antifungal prophylaxis and empirical therapy. A recent survey on the importance of appropriate empirical therapy in invasive Candida infection has shown that adequate empirical therapy is received by only a quarter of patients and that inappropriate therapy is associated with increased mortality [21,22].
India has a high prevalence of invasive candidiasis owing to the presence of a number of contributory factors including favorable climatic conditions, a large population of immuno-compromised hosts including people with HIV/AIDS and diabetes mellitus, and widespread access to antibiotics and steroids without prescription [23]. Despite the availability of a few studies from national laboratories located in metropolitan cities [11], lack of adequate number of diagnostic mycology laboratories precludes the availability of representative data on the epidemiological and mycological characteristics of invasive candidiasis occurring in vast stretches of the country. Nevertheless, because of the immense eco-geographical heterogeneity in the country and in view of the geographical and temporal variation often observed in the species distribution of Candida associated with bloodstream infections [10], there is a need to investigate and monitor local epidemiological patterns of candidemia in India.
Since there is scant data on candidemia occurring in the vast Himalayan region of northern India, this prospective study was designed with the objectives of studying the local epidemiology of Candida infection in this region and determining the susceptibility of the Candida isolates to commonly used antifungal drugs.
Material and Methods
The study was conducted over a period of 18 months in a 15-bed general ICU and a 5-bed paediatric ICU, attached to the Himalayan Institute of Medical Sciences, a tertiary care teaching hospital in the Himalayan region of northern India. Febrile patients with bacteriologically sterile culture reports were recruited into the study. Known HIV-positive patients and patients with pre-existing fungal infection at the time of ICU admission were excluded from the study. The study protocol was approved by the institutional ethics committee and proper informed consent was obtained from each of the recruited patients.
Five milliliters and 2 ml of venous blood samples were collected from adult and paediatric patients, respectively, at the time of admission and on the 3rd, 7th, 10th and 14th days of ICU stay. The blood samples were inoculated in brain-heart infusion biphasic media and the culture bottles were tilted at periodic intervals until the appearance of fungal colonies. The bottles were incubated for 7 days before being declared negative. Growth on BHI Agar (brain-heart infusion agar) was sub-cultured on SDA (Sabouraud’s dextrose agar). All Candida isolates, characterized by smooth, creamy and pasty appearance of colonies on SDA, were subjected to species identification using standard tests (Germ tube test, Sugar fermentation test, Sugar assimilation test) and studying the morphological characters on corn meal agar and color production on CHROM agar media. Recovery of any Candida species from at least 1 blood culture sample was taken as evidence of candidemia. The recovered Candida isolates were then subjected to antifungal susceptibility testing using commercially procured antifungal discs (Hi- media), as per standard CLSI guidelines (document M-44A) [24] For interpretation of sensitivity or resistance, measurement of inhibition halos recommended by manufacturers was taken into consideration. The antifungal agents used in the study included Amphotericin B (10 U), Nystatin (100 U), Clotrimazole (10 μg), Fluconazole (25 μg), Ketoconazole (10 μg) and Itraconazole (10 μg). Standard ATCC strains (C. albicans ATCC 90028, C. parapsilosis 22019 and C. krusei 6258) were used as control. Isolates resistant to Fluconazole by disc diffusion method were tested by broth macrodilution method according to CLSI guidelines (M27 A2) [25] using Fluconazole powder procured from Sigma-Aldrich.
Statistical analysis
To examine the relative risk of developing candidemia when exposed to a particular risk factor, we calculated an odds ratio and constructed 95% confidence intervals for the same. Chi- square test and Fisher’s exact test (wherever appropriate) were performed to determine if the proportion of patients developing candidemia following exposure to a risk factor was significantly different from those not developing candidemia. Differences in antifungal sensitivity between C. albicans and non-albicans species were also examined for statistical significance using chi-square test and Fisher’s exact test. Controlling for the type of azole tested the relationship between the Candida species and azole-sensitivity was examined using Mantel-Haenszel analysis. SPSS version 17.0 was used for all statistical computations and p<0.05 was taken as significant.
Results
A total of 96 patients admitted in our ICUs were screened for the presence of candidemia − 32 neonates (mean age: 6 days), 8 children (mean age: 6 years), 29 adults (mean age: 36.5 years) and 27 patients above 65 years of age (mean age: 77.5years). The mean age of the entire study group was 30 years (SD of 27 years). Of the recruited patients, 41 patients (32 males) were culture-positive, which included 23 adults and 15 children. The majority of the candidemic patients were in the extremes of age, with 13 neonates and 15 over 65 years of age.
A total of 15 different co-morbid conditions and risk factors were identified among the recruited patients and analyzed for possible association with the development of candidemia (Table 1). Four of these risk factors were found to be significantly associated with the occurrence of candidemia in our patients – a period of hospitalization exceeding 7 days (p=0.0008), previous use of antibiotics (p=0.001), presence of chronic renal failure (p=0.003), and ongoing cancer chemotherapy (p=0.041). Among these risk factors, the odds of developing candidemia were highest for patients with chronic renal failure, with the risk being 8.3 times higher in these patients (95% confidence interval =6.9, 9.7). The odds ratios for the other significant risk factors were as follows: 5.5 for patients on antibiotics (95% confidence interval =4.5, 6.6), 5 for patients undergoing cancer chemotherapy (95% confidence interval =3.5, 6.4) and 4.6 for patients with a period of hospitalization exceeding 7 days (95% confidence interval =3.7, 5.6).
Table 1.
Distribution of co-morbid conditions and risk factors in the recruited patients.
| Co-morbid condition/ Risk Factor | No. of patients | Candidemia present | p value | Odds Ratio | 95% CI |
|---|---|---|---|---|---|
| Chronic Renal Failure | 11 | 9 | 0.003 | 8.3 | 6.9, 9.7 |
| Diabetes Mellitus | 32 | 5 | 0.0002 | 0.14 | −0.89, 1.17 |
| Anaemia (Hb <8%) | 22 | 5 | 0.04 | 0.32 | −0.72, 1.37 |
| Bronchial asthma | 6 | 2 | 0.68 | 0.7 | −0.83, 2.22 |
| Cancer Chemotherapy | 8 | 6 | 0.04 | 4.96 | 3.52, 6.41 |
| Road Traffic Accident | 29 | 6 | 0.005 | 0.24 | −0.75, 1.23 |
| Steroid Therapy | 19 | 4 | 0.43 | 0.30 | −0.8, 1.4 |
| Low Birth Weight | 15 | 3 | 0.003 | 0.08 | −1.6, 1.86 |
| Pre-maturity | 10 | 3 | 0.24 | 0.38 | −1.26, 2.02 |
| Hospitalization >7 days | 30 | 24 | 0.0008 | 4.64 | 3.7, 5.56 |
| Antibiotic Usage | 56 | 30 | 0.001 | 5.53 | 4.5, 6.6 |
| IV Cannula | 63 | 21 | 0.012 | 0.29 | −0.7, 1.3 |
| Urinary Catheter | 52 | 9 | <0.001 | 0.06 | −1.0, 1.11 |
| Endotracheal tube | 22 | 2 | 0.0003 | 0.09 | −1.19, 1.37 |
| CVP line | 13 | 3 | 0.149 | 0.375 | −0.85, 1.6 |
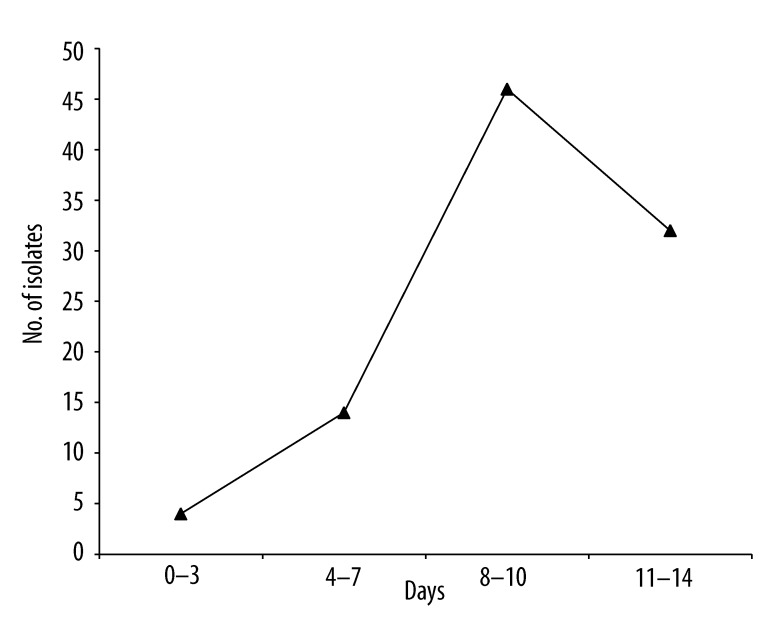
Ninety-six Candida isolates were recovered from the 41 culture-positive patients. The proportion of candidemic patients increased significantly beyond the 7th day of ICU stay (p= 0.0002). The maximum number of Candida isolates was recovered between the 8th to 10th days of ICU stay (Figure 1). Thirty-one (75.6%) of the candidemic patients yielded multiple culture-positive samples. Candida albicans was the commonest isolate recovered (n=75, 78.1%), followed by Candida tropicalis (n=15, 16%) and Candida glabrata (n=6, 6.5%). Multiple Candida species were not recovered from any patient. We next analyzed whether the distribution of risk factors was different between patients infected with C. albicans and those with non-albicans species. No statistically significant difference was observed between the 2 groups in terms of age, sex, length of hospitalization, administration of antibiotics or immunosuppressive agents, presence of indwelling devices and co-existence of the majority of co-morbid conditions and risk factors.
Figure 1.
Time kinetics of recovery of Candida isolates.
Table 2 depicts the results of in vitro antifungal susceptibility testing performed by disc diffusion technique on the recovered isolates. In all, 26% (25 out of 96) of all Candida isolates and 17.3% (13 out of 75) of C. albicans isolates were resistant to Fluconazole. Resistance to the other azoles varied, with 38.5% of all isolates being resistant to Ketoconazole, 18.7% recording resistance to Itraconazole and 10.4% showing resistance to Clotrimazole. While the C. glabrata isolates were invariably resistant to every azole tested, similar resistance to all azoles was observed among 1 isolate (1.3%) of C. albicans and 1 isolate (6.6%) of C. tropicalis. For each of the azole antifungal agents, the frequency of resistance among C. albicans isolates was significantly lower than that among non-albicans isolates (p=0.004 for Fluconazole; p≤0.001 for Ketoconazole; p≤0.001 for Itraconazole; p≤0.001 for Clotrimazole). Adjusting for the type of azole antifungal agent tested, resistance was significantly lower among the C. albicans isolates compared to the non-albicans isolates, χ2 (1, N=312)=71.36, p≤0.001. Resistance to Nystatin and Amphotericin B was, however, not observed in our study. Antifungal susceptibility testing of the resistant isolates by broth macrodilution method revealed 100% concordance with disc diffusion method. Eighteen out of 20 isolates subjected to broth macrodilution method had MIC ≥64 μg/ml to Fluconazole.
Table 2.
Antifungal sensitivity profile of the recovered isolates.
| Isolates | Number (%) | Resistance (%) | |||||
|---|---|---|---|---|---|---|---|
| Fu | Kt | It | Cc | Ns | AmpB | ||
| C. albicans | 75 (78.1) | 13 (20.6) | 21 (33.3) | 3 (4.8) | 1 (1.6) | 0 | 0 |
| C. tropicalis | 15 (15.7) | 6 (50) | 10 (83.3) | 9 (75) | 3 (25) | 0 | 0 |
| C. glabrata | 6 (6.2) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 0 | 0 |
Fu – Fluconazole; Kt – Ketoconazole; It – Itraconazole; Cc – Clotrimazole; Ns – Nystatin; AmpB – Amphotericin B.
Discussion
In this paper we have shown that C. albicans is the predominant cause of candidemia in our centre and the degree of resistance is significantly high even among the C. albicans isolates recovered in this study. We also identified the co-morbid conditions and risk factors associated with the development of candidemia in our patients. Interestingly, chronic renal failure was found to be the most important risk factor in this study.
Geographical variation is recognized to be an important feature in the species distribution of Candida. In sync with trends observed in the majority of studies from around the globe [10–16], a shift in the species distribution of Candida has been noted in several major Indian hospitals. Non-albicans Candida have been isolated from 30%–90% of cases of invasive candidiasis [12–16,26,27]. However, in our study C. albicans was isolated as the most prevalent isolate with no shift from albicans to non-albicans candidemia. We hypothesize that, as suggested by Bassetti et al. [28], this could be accounted for by the fact that prophylactic use of fluconazole is not a standard practice in our ICU and none of the patients recruited in this study were receiving antifungal prophylaxis. Similar to our findings, Narain et al also reported predominant isolation of C. albicans in their study from Mumbai, India [29]. Our hypothesis is further supported by similarity of findings in reports from countries with restricted usage of antifungal agents. In a retrospective evaluation of candidemia spanning a period of 6 years, in 5 university hospitals in the Netherlands it was found that the proportion of bloodstream infections caused by Candida albicans remained stable throughout the study period, with no signs of increasing rate of infections due to non-albicans Candida [30]. Likewise, in a Swiss study no shift was observed from C. albicans fungemia to those caused by non-albicans Candida [31,32].
C. tropicalis was the most frequently recovered non-albicans isolate in our study, while C. glabrata is the commonest non-albicans species worldwide [27,33–36]. This is in agreement with previous reports from India, Singapore and Taiwan where C. tropicalis has been reported to be the commonest non-albicans Candida isolated [23,37,38].
A large number of risk factors have been incriminated in the development of candidemia in studies from across the world [30,39–45]. The variation in these risk factors between studies is reflective of the recruited patient profile and the nature of treatment practices and therapeutic interventions observed in the reporting institutions. Knowledge of these risk factors is helpful in adopting centre-specific strategies for selective administration of antifungal prophylaxis. The differences in risk factors observed by us and those reported by other authors could be due to the fact that procedures like organ transplantation and bone marrow transplantation are not performed in our center. Patients with hematological malignancies and neutropenic patients and procedures like total body irradiation, central venous catheterization, arterial line insertion, and cardiothoracic surgery are also fewer compared to those reported in most other studies.
All C. glabrata isolates recovered in our study were azole resistant. This is similar to data published in recent years in which azole resistance has been found to be higher among C. glabrata. In a Scottish study, among the isolates of candidemia 55% of C. glabrata isolates showed reduced susceptibility to fluconazole, but azole resistance among other species of Candida was extremely low [40,38]. Similarly, Tan et al observed relatively higher fluconazole resistance among C. glabrata isolates [37]. The outstanding feature in the present study was the alarmingly high (17.3%) prevalence of fluconazole-resistance among the C. albicans isolates. This is in contrast to the findings in most of the studies that have reported 0–5.1% fluconazole resistance in C. albicans[37,46–48]. The reason for this difference remains unknown; this could be an interesting regional characteristic if this finding is validated in future studies. Work is currently underway in our laboratory wherein we are trying to corroborate these findings by blinded performance of broth dilution and disc diffusion in all our Candida isolates.
Unlike antibacterial susceptibility testing, antifungal susceptibility testing is not performed routinely and an empirical approach is usually followed in prescribing this class of drugs. The finding of 26% of fluconazole resistance among the Candida isolates in the present study underscores the importance of correct speciation and routine testing of antifungal susceptibility. The newer antifungals like voriconazole, posaconazole, and caspofungin, which have not been included in the present study, should also be tested for efficacy against the resistant isolates. Moreover, correlation needs to be explored between the results of antifungal sensitivity testing and host response to antifungal treatment.
Conclusions
This observational study, aimed at characterizing the profile of candidemia in the setting of a typical Indian ICU, could assist in alerting clinicians about the prevalence of this condition and in promoting adoption of important prophylactic and treatment guidelines for its improved management.
Footnotes
Source of support: Departmental sources
References
- 1.Abelson JA, Moore T, Bruckner D, et al. Frequency of fungemia in hospitalized inpatients over 11 years at a tertiary care institution. Pediatrics. 2005;116:61–67. doi: 10.1542/peds.2004-1605. [DOI] [PubMed] [Google Scholar]
- 2.Aquino VR, Lunardi W, Goldani LZ, et al. Prevalence, susceptibility profile for fluconazole and risk factors for candidemia in a tertiary care hospital in southern Brazil. Braz J Infect Dis. 2005;9:411–18. doi: 10.1590/s1413-86702005000500009. [DOI] [PubMed] [Google Scholar]
- 3.Lupetti A, Tavanti A, Davini P, et al. Horizontal transmission of Candida parapsilosis candidemia in a neonatal intensive care unit. J Clin Microbiol. 2002;40:2363–69. doi: 10.1128/JCM.40.7.2363-2369.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheng WH, Wang JT, Lin MS, et al. Risk Factors Affecting In-hospital Mortality in Patients with Nosocomial Infections. J Formos Med Assoc. 2007;106(2):110–18. doi: 10.1016/S0929-6646(09)60226-6. [DOI] [PubMed] [Google Scholar]
- 5.Morgan J, Meltzer MI, Plikaytis BD, et al. Excess mortality, hospital stay, and cost due to candidemia: a case-control study using data from population-based candidemia surveillance. Infect Control Hosp Epidemiol. 2005;26:540–47. doi: 10.1086/502581. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee SN, Emori TG, Culver DH, et al. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. National Nosocomial Infections Surveillance System. Am J Med. 1991;91(3B):S86–89. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- 7.Beck SC, Jarvis WR. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. National Nosocomial Infections Surveillance System. J Infect Dis. 1993;167(5):1247–51. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 8.Trick WE, Fridkin SK, Edwards JR, et al. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989–1999. Clin Infect Dis. 2002;35(5):627–30. doi: 10.1086/342300. [DOI] [PubMed] [Google Scholar]
- 9.Pfaller MA, Jones RN, Doern GV, et al. Bloodstream infections due to Candida species: SENTRY antimicrobial surveillance program in North America and Latin America, 1997–1998. Antimicrob Agents Chemother. 2000;44(3):747–51. doi: 10.1128/aac.44.3.747-751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfaller MA, Diekema DJ. Twelve years of fluconazole in clinical practice: global trends in species distribution and Fluconazole susceptibility of bloodstream isolates of Candida. Clin Microbiol Infect. 2004;10(Suppl 1):11–23. doi: 10.1111/j.1470-9465.2004.t01-1-00844.x. [DOI] [PubMed] [Google Scholar]
- 11.Eicher AD, Crofts N, Benjamin S, et al. A certain fate: spread of HIV among young injecting drug users in Manipur, north east India. AIDS Care. 2000;12:497–504. doi: 10.1080/09540120050123891. [DOI] [PubMed] [Google Scholar]
- 12.Krcmery V, Barnes AJ. Non albicans Candida spp. causing fungemia: pathogenicity and antifungal resistance. J Hosp Infect. 2002;50(4):243–60. doi: 10.1053/jhin.2001.1151. [DOI] [PubMed] [Google Scholar]
- 13.Chai YA, Wang Y, Khoo AL, et al. Predominance of C. tropicalis bloodstream infections in a Singapore teaching hospital. Med Mycol. 2007;45(5):435–39. doi: 10.1080/13693780701385868. [DOI] [PubMed] [Google Scholar]
- 14.Colombo AL. Epidemiology and treatment of hematogenous candidiasis: a Brazilian perspective. Braz J Infect Dis. 2000;4(3):113–8. [PubMed] [Google Scholar]
- 15.Meunier R, Aoun A, Bitar N. Candidemia in immunocompromised patients. Clin Infect Dis. 1992;14:120–25. doi: 10.1093/clinids/14.supplement_1.s120. [DOI] [PubMed] [Google Scholar]
- 16.Shivprakasha S, Radhakrishnan K, Karim PM. Candida spp. other than C. albicans: a major cause of fungaemia in a tertiary care centre. Ind J Med Microbiol. 2007;25:405–7. doi: 10.4103/0255-0857.37350. [DOI] [PubMed] [Google Scholar]
- 17.Hajjeh RA, Sofair AN, Harrison LH, et al. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J Clin Microbiol. 2004;42:1519–27. doi: 10.1128/JCM.42.4.1519-1527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estrella C, Rodriguez D, Almirante B, et al. In vitro susceptibilities of bloodstream isolates of Candida species to six antifungal agents: results from a population based active surveillance programme, Barcelona, Spain, 2002–2003. J Antimicrob Chemother. 2005;55:194–99. doi: 10.1093/jac/dkh548. [DOI] [PubMed] [Google Scholar]
- 19.Pfaller MA, Diekema DJ. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol. 2004;42:4419–31. doi: 10.1128/JCM.42.10.4419-4431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thean YT, Ling T, Nancy WT, et al. A Retrospective Analysis of Antifungal Susceptibilities of Candida Bloodstream Isolates from Singapore Hospitals. Ann Acad Med Singapore. 2008;37:835–40. [PubMed] [Google Scholar]
- 21.Parkins MD, Sabuda DM, Elsayed S, et al. Adequacy of empirical antifungal therapy and effect on outcome among patients with invasive Candida species infections. J Antimicrob Chemother. 2007;60(3):613–18. doi: 10.1093/jac/dkm212. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong JD. Invasive Candida species infection: the importance of adequate empirical antifungal therapy. J Antimicrob Chemother. 2007;60(3):459–60. doi: 10.1093/jac/dkm260. [DOI] [PubMed] [Google Scholar]
- 23.Chakrabarti A, Chatterjee SS, Shivprakash MR. Overview of opportunistic fungal infections in India. Jpn J Med Mycol. 2008;49(3):165–72. doi: 10.3314/jjmm.49.165. [DOI] [PubMed] [Google Scholar]
- 24.Clinical And Laboratory Standard Institute. Method for antifungal disc diffusion susceptibility testing for yeasts; approved guidelines. M44-A2. Wayne PA: CLSI; 2009. [Google Scholar]
- 25.National Committee for Clinical Labotatory Standards. Approved Standard M27–A2. Second Edition. NCCLS; Villanova, PA, USA: 2002. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. [Google Scholar]
- 26.Rani R, Mohapatra NP, Mehta G, Randhawa VS. Changing trends of Candida species in neonatal septicaemia in a tertiary North Indian hospital. Indian J Med Microbio. 2002;20(1):42–44. [PubMed] [Google Scholar]
- 27.Capoor MR, Nair D, Deb M, et al. Emergence of non-albicans Candida species and antifungal resistance in a tertiary care hospital. Jpn J Infect Dis. 2005;58(6):344–48. [PubMed] [Google Scholar]
- 28.Bassetti M, Righi E, Costa A, et al. Epidemiological trends in nosocomial candidemia in intensive care. BMC Infect Dis. 2006;6(10):21. doi: 10.1186/1471-2334-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narain S. Neonatal systemic Candidiasis in a tertiary care center. Ind J Med Microbiol. 2003;21:56–58. [PubMed] [Google Scholar]
- 30.Lunel FV, Koeleman JG, Spanjaard L, et al. Trends in fungaemia and antifungal susceptibility in the Netherlands. Neth J Med. 2006;64(7):236–42. [PubMed] [Google Scholar]
- 31.Garbino J, Kolarova L, Rohner P, et al. Secular trends of candidemia over 12 years in adult patients at a tertiary care hospital. Medicine. 2002;81(6):425–33. doi: 10.1097/00005792-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Marchetti O, Bille J, Fluckiger U, et al. Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991–2000. Clin Infect Dis. 2004;38(3):311–20. doi: 10.1086/380637. [DOI] [PubMed] [Google Scholar]
- 33.Dimopoulos G, Ntziora F, Rachiotis G, et al. C. albicans versus non-albicans intensive care unit-acquired bloodstream infections: differences in risk factors and outcome. Anesth Analg. 2008;106(2):523–29. doi: 10.1213/ane.0b013e3181607262. [DOI] [PubMed] [Google Scholar]
- 34.Pfaller MA, Diekema DJ. Twelve years of fluconazole in clinical practice: global trends in species distribution and Fluconazole susceptibility of bloodstream isolates of Candida. Clin Microbiol Infect. 2004;10(Suppl 1):11–23. doi: 10.1111/j.1470-9465.2004.t01-1-00844.x. [DOI] [PubMed] [Google Scholar]
- 35.Pfaller MA, Jones RN, Doern GV, et al. Bloodstream infections due to Candida species: SENTRY antimicrobial surveillance program in North America and Latin America, 1997–1998. Antimicrob Agents Chemother. 2000;44(3):747–51. doi: 10.1128/aac.44.3.747-751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boo TW, Reilly O, Leary O, et al. Candidaemia in an Irish tertiary referral hospital: epidemiology and prognostic factors. Mycoses. 2005;48(4):251–59. doi: 10.1111/j.1439-0507.2005.01134.x. [DOI] [PubMed] [Google Scholar]
- 37.Tan TY, Tan AL, Tee NW, et al. A retrospective analysis of antifungal susceptibilities of Candida blood stream isolates from Singapore hospitals. Ann Acad Med Singapore. 2008;37(10):835–40. [PubMed] [Google Scholar]
- 38.Kung HC, Wang JL, Chang SC, et al. Community-onset Candidemia at a university hospital, 1995–2005. J Microbiol Immunol Infect. 2007;40(4):355–63. [PubMed] [Google Scholar]
- 39.Stamos JK, Rowley AH. Candidemia in a pediatric population. Clin Infect Dis. 1995;20(3):571–75. doi: 10.1093/clinids/20.3.571. [DOI] [PubMed] [Google Scholar]
- 40.Odds FC, Hanson MF, Davidson AD, et al. One year prospective survey of Candida bloodstream infections in Scotland. J Med Microbiol. 2007;56:1066–75. doi: 10.1099/jmm.0.47239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal J, Bansal S, Malik GK, et al. Trends in neonatal septicemia: Emergence of non-albicans Candida. Indian Pediar. 2004;41:712–15. [PubMed] [Google Scholar]
- 42.Pasqualotto AC, Nedel WL, Machado TS, et al. A comparative study of risk factors and outcome among outpatient-acquired and nosocomial candidaemia. J Hosp Infect. 2005;60(2):129–34. doi: 10.1016/j.jhin.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Caggiano G, Iatta R, Laneve A, et al. Observational study on candidemia at a university hospital in southern Italy from 1998 to 2004. Mycosis. 2008;51(2):123–28. doi: 10.1111/j.1439-0507.2007.01452.x. [DOI] [PubMed] [Google Scholar]
- 44.Jędrzejowska AS, Niacka AW, Bartkowiak BD, et al. Yeast-like fungus infections in patients treated with low doses of immunosuppressive drugs. Med Sci Monit. 1997;3(3):369–72. [Google Scholar]
- 45.Soen GG, Sweed Y, Geva LL, et al. Nosocomial bloodstream infections in a pediatric intensive care unit: 3-year survey. Med Sci Monit. 2007;13(6):CR251–57. [PubMed] [Google Scholar]
- 46.Xu y, Chen L, Li C. Susceptibility of clinical isolates of Candida species to Fluconazole and detection of Candida albicans ERG11 mutations. J Antimicrob Chemother. 2008;61(4):798–804. doi: 10.1093/jac/dkn015. [DOI] [PubMed] [Google Scholar]
- 47.Lee JS, Shin JH, Lee K, et al. Species distribution and susceptibility to azole antifungals of candida bloodstream isolates from eight university hospitals in Korea. Yonsei Med J. 2007;48(5):779–86. doi: 10.3349/ymj.2007.48.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeplin MB, Kunz L, Ruchel R, et al. Epidemiology and antifungal susceptibilities of Candida spp. to six antifungal agents: results from a surveillance study on fungaemia in Germany from July 2004 to August 2005. J Antimicrob Chemother. 2007;60:424–28. doi: 10.1093/jac/dkm145. [DOI] [PubMed] [Google Scholar]