Summary
Background
The transplantation of neural stem cells (NSCs) has been accepted as a promising therapeutic strategy for central nervous system disorders. However, the beneficial effect of NSC transplantation upon functional recovery is limited due to the unfavorable microenvironment (niche) at the site of trauma or degenerative disease in the brain. Combination of transplantation of NSCs with neurotrophins may overcome the hurdles of impaired cell survival and neuronal differentiation.
Material/Methods
In the current study, the neurotrophin-3 (NT-3) gene was transduced into cultured mouse embryonic cortical NSCs via an AAV vector (NSC-NT-3). The effect of NT-3 over-expression on cell proliferation and differentiation in NSCs was observed by immunohistochemistry, cell culture and organotypic hippocampal slice cultures.
Results
The characteristics of self-renewal and multiple differentiation of NSCs were well-preserved. Cells in the NSC-NT-3 group proliferated faster and differentiated into more β-tubulin III-positive neurons compared to the control group in vitro. Furthermore, cells in the NSC-NT-3 group survived in a significantly higher percentage and undertook neuronal differentiation preferably in organotypic hippocampal slice cultures.
Conclusions
Our results suggest that the transduction of NT-3 into NSCs could effectively promote NSCs survival, proliferation, and neuronal differentiation in vitro without change of the stemness of NSCs. This work also offers evidence to better understand the safety and efficiency of combined treatment with NT-3 and NSCs for the central nervous system disorders.
Keywords: neurotrophin-3, gene transduction, cell proliferation and differentiation, neural stem cell, organotypic slice culture
Background
Stem cell therapy is a promising therapeutic strategy for central nervous system disorders, as stem cells can replace, repair, and enhance the function of damaged tissues or organs by differentiating into various cell types, secreting therapeutic substances, and stimulating the regeneration of endogenous stem cells [1–6]. The neural stem cell (NSC) has been considered as an ideal candidate for therapeutic cell transplantation because it is more committed to a neural lineage, and less tumorigenic and immunogenic in comparison with other stem cell types, such as embryonic stem cells and mesenchymal stem cells [2,5,7,8]. However, grafted NSCs do not sufficiently survive and perform their multiple functions in the unfavorable microenvironment that can occur at sites of trauma or degenerative disease [9–11]. Therefore, following NSC transplantation, the problem of retaining adequate numbers of cells with the proper phenotypes and integrative capacity remains to be solved.
Neurotrophic factors (NFs) such as nerve growth factor, brain-derived neurotrophic factor, and the neurotrophins, are well known to modulate a variety of neuronal processes during development [12–15]. In addition, NFs can reduce cell death, enhance neural regeneration, stimulate neuronal survival, and promote axonal growth [16–20]. The genetic modification of NSCs with NFs prior to transplantation enhances cell survival and consequently increases the therapeutic function of these cells following transplantation [19–23]. Existing data have demonstrated that the combination of NSCs and neurotrophin-3 (NT-3) dramatically promoted cell replacement and functional recovery after central nervous system (CNS) injuries such as stroke, spinal cord injury and multiple sclerosis [16,18,19,21–24], leading to NT-3 becoming a focus of great interest in therapeutic strategies for CNS disorders.
However, as for any new therapeutic approach, the safety and efficiency of treatment should be confirmed before clinical application. To fulfill the promise of genetically modified NSC-based treatment, it is vital to show that gene transduction will not alter the fundamental characteristic of the NSC, yet will promote cell survival and integrative capacity in vitro and ex vivo. As AAV vectors have emerged in recent years as powerful tools for therapeutic gene transfer and achieved successes in clinical trials, in the present study we genetically modify NSCs from mouse embryo cortex with the NT-3 gene via an AAV vector and observed the capacity of these modified NSCs for survival and differentiation.
Material and Methods
Construction of rAAV vector and production of recombinant AAV
The AAV Helper-Free System (Stratagene, USA) was used to produce recombinant AAV according to the manufacturer’s instructions. The full-length coding sequence of human NT-3, including the signal peptide, was amplified by PCR amplification in our laboratory. The NT-3 fragment was inserted into the pAAV-IRES-hrGFP using the BamHI and Xhol restriction sites under the control of CMV promoter. The recombinant plasmid pAAV-NT-3-IRES-hrGFP was restriction digested to further confirm the correct insertion of NT-3. AAV-293 cells were cultured in DMEM (High-Glucose, GIBCO, USA) with 10% fetal bovine serum (FBS, GIBCO, USA), until reaching 70–80% confluency, and passaged 2 days prior to transfection. Three plasmids, pAAV-RC, pHelper and the recombinant plasmid pAAV-NT-3-IRES-hrGFP or control plasmid pAAV-hrGFP, were transduced into AAV-293 cells at a 1:1:1 ratio by the calcium phosphate method. After green fluorescence of hrGFP was observed under fluorescent microscope at 72 h post-transfection, transfected AAV-293 cells were resuspended and subjected to 4 rounds of freeze-thaw with 20 s of sonication (Ultrasonic Cell Disrupter, China) between each round. The cellular debris was collected by centrifugation at 10 000 × g for 10 min at room temperature. rAAV-2 vector particles were concentrated by ammonium sulfate precipitation, resuspended in PBS, and stored in −20°C until further use. The recombinant AAVs were designated as AAV-NT-3 or AAV-GFP (control).
The physical titer of viral particles was measured by dot-blot assay (DIG High Prime DNA Labeling and Detection Starter kit II, Roche, USA) according to the kit instructions and 4×1011 vg/ml of AAV-NT-3 and 2×1011 vg/ml of AAV-GFP were yielded, respectively.
AAV-mediated NT-3 gene transduction of NSCs from mouse embryos cortex
All procedures involving animal work conformed to the ethical guidelines of the NIH Regulations for Experimentation on Laboratory Animals as set by the Xi’an Jiaotong University. NSCs were isolated from E14.5 mouse embryonic cortex and were cultured in serum-free DMEM/F12 (1:1) growth medium (GIBCO, USA) with N2 supplements (100×, GIBCO, USA) and 20 μg/L hEGF (GIBCO, USA), 10 μg/L bFGF (Sigma, USA) following the protocol of Gage et al. [25] and optimized in our lab [26]. These cells were passaged every 7 d. Upon passaging, the neurospheres were trypsinized into single cells and replated. To observe the differentiation of NSCs, cells were cultured on poly-lysine coated coverslips in 24-well plates in DMEM/F12 (1:1) containing 1% FBS.
Twelve hours prior to transduction, the neurospheres were trypsinized into single cells (passage 3) and subcultured into 6-well plates at a density of 2×105 cells/ml in 800 μl growth medium (serum-free DMEM/F12). Volumes of 10 μl, 20 μl and 50 μl AAV-NT-3 stocks were diluted with growth medium to a volume of 200 μl and added into individual wells of the 6-well plates with gentle swirling. Twenty-four hours later, an additional 1 ml fresh growth medium was added into each well; this time point was considered to be 1 d post-infection (DPI). AAV-GFP stock was diluted as above and used as a negative control. For the blank control, the same amount of fresh growth medium was added into each well. At 3 and 5 DPI, newly-formed neurospheres were observed using a fluorescent microscope (Olympus, Japan) to identify the expression of GFP. At this point, parallel neurospheres were trypsinized into single cells and analyzed by use of a Fluorescence-Activated Cell Sorter (FACS).
Determination of NT-3 expression
The expression NT-3 protein in the culture medium was detected by use of an ELISA kit (Raybio°R, USA). Culture medium of normal and transduced NSCs (NSC-NT-3) was changed 24 h prior to collection. A volume of 100 μl medium from 3 wells of each groups were collected at 3, 7, 14 and 28 DPI, respectively. According to the instruction, samples were added into appropriate wells and the whole kit was incubated for 2.5 h at room temperature. The results were read at 450 nm in an EL808 Ultra Microplate Reader (BIO-TEK instruments, USA).
Assessment of stemness, proliferation, and differentiation of NSCs following AAV transduction
We then assessed the NSCs for stemness, defined as the essential characteristics of stem cells that distinguish them from ordinary cells, such as the ability to form neurospheres and differentiate into multiple lineages. Upon the first passage following AAV transduction, NSCs were seeded into growth medium at a density of 2×105 cell/ml. The growth rate and number of neurospheres (diameter ≥100 μm) at day 6 after passage were compared between passage 4 and passage 10. Cells from these passage numbers were also cultured in differentiation medium (DMEM/F12 with 1% FBS) for 7 d, and immunocytochemical labeling was performed.
The effect of NT-3 upon NSC proliferation was assessed by charting the growth curve and measuring neurosphere diameter. NSCs from each group were seeded into 96-well plates at a density of 1000 cells per well and cultured in growth medium. Cell numbers from 3 separate wells of each group were counted manually every day for 8 d. At the same time, the diameters of 10 neurospheres in each well were measured under a microscope (Nikon TS100-F, Japan) equipped with an ocular micrometer. During this assessment, cells were kept in the culture medium without refreshing.
For the assessment of cell differentiation, neurospheres from each group were trypsinized into single cells and resuspended in differentiation medium, seeded onto polylysine-coated coverslips in 24-well plates at a density of 2×103 cells/well. Cell suspensions (100 μl per well) were placed onto each coverslip and cultured for 4 h at 37°C, followed by incubation in 500 μl differentiation medium for a period of 7 d. Half of the differentiation medium was exchanged on the third day for fresh medium. Coverslips were removed and fixed on days 4 and 7 post-plating with 4% paraformaldehyde (PFA) for 30 min at room temperature.
Culturing of organotypic hippocampal slice culture and transplantation of NSCs
The whole brains of newborn mice (on postnatal day 3) were removed aseptically and hippocampal slices were cultured following the protocol of the Stoppini method and optimized in the authors’ laboratory [27,39]. Briefly, hippocampus was dissected in 4° Hank’s balanced salt solution (HBSS; Invitrogen, UK) with HEPEs and then chopped into 250 to 400 μm slices on a MclIwain tissue chopper. The culture medium was composed of 10% minimum essential media (MEM; Invitrogen, UK), 10% newborn calf serum (NCS; Invitrogen, UK), 5% sodium bicarbonate (Sigma, UK) and 1% L-Glutamine (Sigma, UK). Slices were routinely observed under a phase-contrast microscope at 2-day intervals and half the medium was replaced every 3–4 days.
AAV-NT-3-transduced NSCs (NSC-NT-3) and non-transduced control NSCs were labeled using CellTrackerTM lipophilic fluorochrome chloromethylbenzamido dialkylcarbocyanine (CM-DiI, Invitrogen, UK) for 5 min at 37° and then further 15 min at 4° in dark. After 4 days culture, a volume of 10 μl solution containing CM-DiI labeled NSCs was added to the surface of the slices that had been cultured for 6 days. The slices were maintained in culture for another 4 days in the same conditions and then fixed by 4° 2% PFA.
Immunocytochemical labeling and quantification
Immunocytochemistry was employed to characterize NSCs, neurons, and astrocytes utilizing mouse anti-nestin (monoclonal antibody, Abcam, 1:500), rabbit anti-β tubulin III (monoclonal antibody, Chemicon, 1:200), rabbit anti-GFAP (polyclonal antibody, DAKO, 1:1000) following a standard protocol optimized in the authors’ laboratory [28]. Cells were incubated with a blocking solution containing 5% normal goat serum (NGS) and 0.25% Triton X-100 in PBS. Primary antibodies were diluted in PBS containing 2% NGS at 4°C overnight. FITC-conjugated (1:2000) goat anti-mouse and goat anti-rabbit IgG (Beijing Zhongshan Biotechnology Co. Ltd.) secondary antibodies were used. Cell nuclei were counterstained with DAPI-containing mounting media (Vector Labs, UK) and visualized under a fluorescent microscope (Olympus BX51, Japan) equipped with a DP70 digital camera and the DPManager (DPController, Olympus, Japan) software. For the negative control, primary antibody was replaced by PBS.
Quantification and statistical analysis
For quantification, immunoreactive cells from 6 random fields (2 coverslips per group, 3 fields per coverslip) were counted under the microscope. Cell counting was performed using a 20× objective lens by an observer blind to the treatment groups. All data were analyzed with the Prism 4.0 software. Student’s T test, one-way or two-way ANOVA, and Bonferroni multiple comparison tests were used, and a p value <0.05 was considered significant.
Results
AAV transduction and the expression of NT-3 in NSC-NT-3
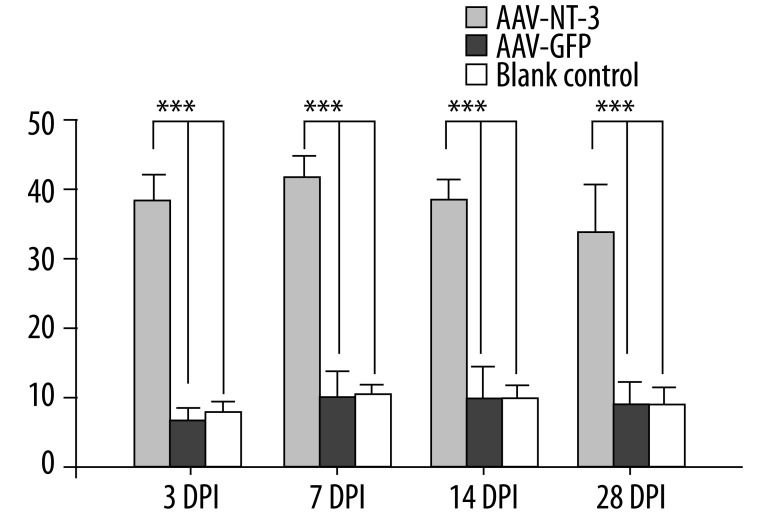
NSCs isolated from E14.5 mouse embryo cortex were cultured in growth medium. A large number of neurospheres emerged after 5–7 d in vitro (DIV; Figure 1A) and immunocytochemistry confirmed that most of these cells were nestin-positive NSCs (Figure 1B). Neurospheres (passage 3) were trypsinized into single cells 12 h prior to transduction. During the first 24 h following transduction, cell debris was observed in both AAV-NT-3 and AAV-GFP-transduced groups. At 72 h following transduction, results of FACS demonstrated that 15.3% and 16.1% of NSCs expressed GFP in AAV-NT-3- and AAV-GFP-transduced groups, respectively, indicating successful transduction (Figure 1C,D). The concentration of NT-3 protein, determined by ELISA, in the medium of NSC-NT-3 was 37.6 pg/ml at 3 DPI. It elevated slightly at 7 DPI (40.7 pg/ml) and then remain at a relatively high level until 28 DPI (33.8 pg/ml); it was significantly higher than that in control groups. (Figure 2, n=3, p<0.001).
Figure 1.
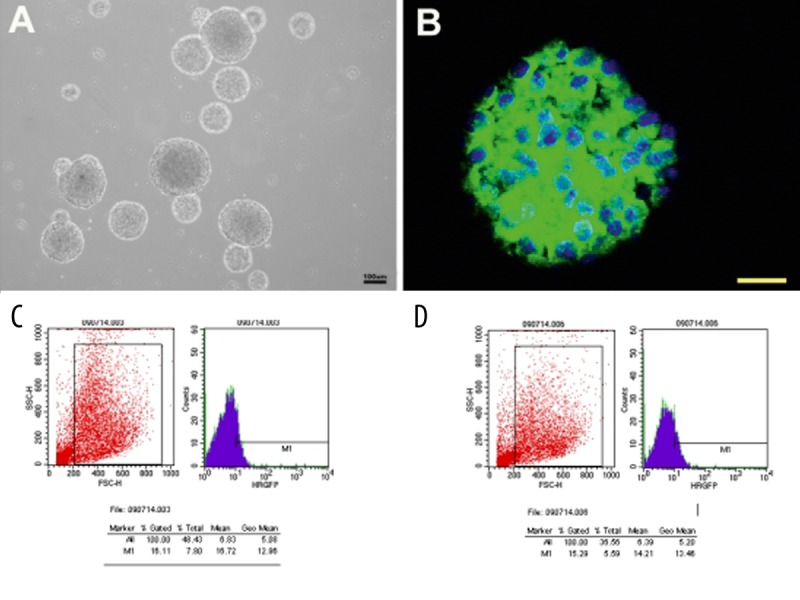
Successful transduction of NSCs by AAV Cells isolated from E14.5 mouse embryo cortex proliferated into neurospheres at 7 DIV (A) and most of these cells were nestin positive NSCs (B). Neurospheres were trypsinized into single cells 12 h prior to AAV transduction. At 3 DPI, up to (B) 16.1% of NSCs were transduced by AAV-GFP and (C) 15.3% of NSCs were transduced by AAV-NT-3. E: embryonic day. DIV: day in vitro. DPI: day post-infection. B Scale bar, 30 um.
Figure 2.
Expression of NT-3 Secreted NT-3 in the medium of AAV-NT-3, AAV-GFP and blank control groups was detected by ELISA at 3, 7, 14 and 28 DPI, respectively. AAV-NT-3-transduced NSCs produced a higher level of NT-3 compared with control groups. No significant difference was found between AAV-GFP and blank control. DPI: day post infection. *** p<0.001.
Stemness of NSCs after NT-3 gene transduction
The ability of NSCs to form neurospheres and undergo differentiation was determined following AAV transduction. In both the AAV-NT-3- and AAV-GFP-transduced groups, abundant spherical cell clusters emerged after 5–7 d in growth medium. After seeding in differentiation medium, cells in both groups differentiated into various cell types as defined by different morphological appearance (Figure 3A–D). Immunocytochemistry further confirmed that the differentiated cells were GFAP-positive astrocytes and β-tubulin III-positive neurons (Figure 3E,F).
Figure 3.
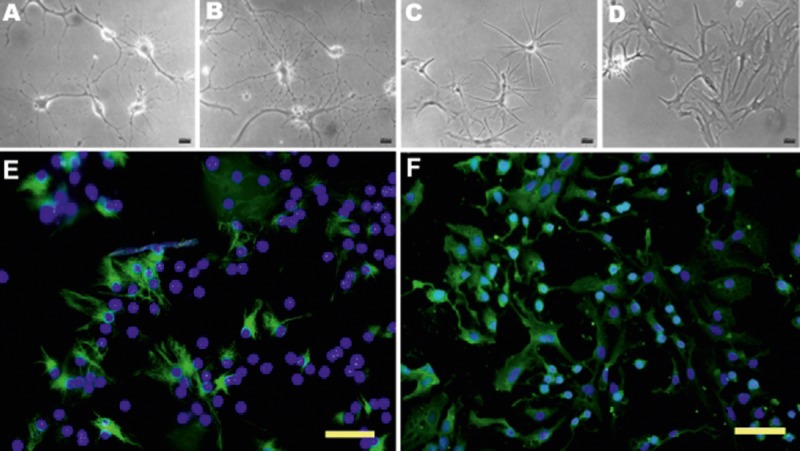
Multiple differentiation of NSCs following AAV transduction (A–D) NSCs differentiated into various cell types with various morphological appearances following 7 days in differentiation medium. Immunocytochemistry further confirmed that these cells were: GFAP-positive astrocytes (E) and β-tubullin III-positive neurons (F). E,F Scal bar, 100 um
The survival, proliferation and differentiation of NSC after NT-3 gene transduction in vitro
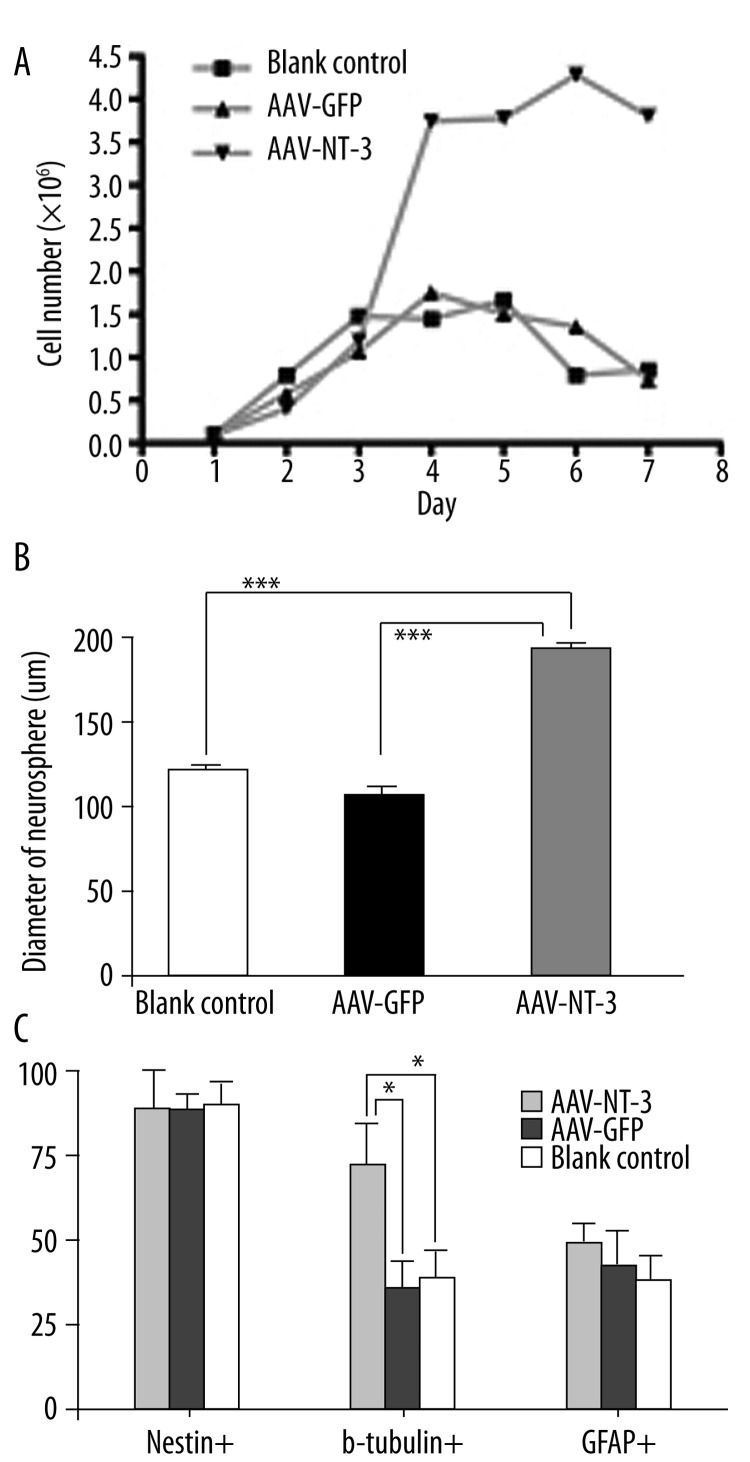
During the assessment of cell survival and proliferation, NSCs were kept in culture for 8 days without medium replacement. Dark-colored neurospheres which contained more dead cells surrounded by abundant cell debris were observed in both blank controls and the AAV-GFP-transduced groups at 5 DIV. However, in the AAV-NT-3-transduced group, neurospheres were lighter colored and surrounded by little debris, even at 10 DIV. The trypan blue staining during the cell counting further confirmed that NSC-NT-3 contained more viable cells compared with the controls. The growth curve of the NSCs (passage 4), as well as the neurosphere diameter, was measured in the different groups throughout the 8-d growth period to evaluate cell proliferation. NSC-NT-3 proliferated at a higher rate compared with the NSC-GFP (AAV-GFP-transduced) and blank control cultures at 4 DIV (Figure 4A, n=3, p<0.05). At 6 DIV, more and larger-sized spherical cell clusters were visible in NSC-NT-3 than controls, likely due to the greater increase in cell numbers (Figure 4B, n=30, p<0.001).
Figure 4.
NT-3 genetic modification promotes cell viability, proliferation and neuronal differentiation in vitro NSCs-NT-3 exhibited a high rate of proliferation (A) and produced significantly larger neurospheres (B, 7 DIV) than the control group. *** p<0.001. The majority of NSCs-NT-3 remained nestin-positive after 7 days in differentiation medium (C). Some of them were nestin/β-tubullin III double-positive and nestin/GFAP double-positive. Nearly two-thirds of NSCs-NT-3 (67.7%) were immunoreactive with β-tubullin III, which is significantly higher than controls (31.2% and 28.4%, respectively; p<0.05). Nevertheless, no significant difference was observed between NSCs-NT-3 and controls in the proportion of GFAP-positive cells (47.6% vs. 43.5% and 40.3%; p>0.05).
Cells were fixed after 7 d in differentiation medium, and processed for immunofluorescence to assess the effect of NT-3 on the differentiation of NSCs. A proportion of NSCs-NT-3 and controls differentiated into β-tubulin III-, GFAP-positive neurons and glia. Nearly 90% of cells remained undifferentiated at this time point, as showed by nestin+ and nestin+/β-tubulin III+, nestin+/GFAP+ immunoreactivity. It was noted that 67.7% of NSCs-NT-3 were immunoreactive for β-tubulin III; this percentage was significantly higher than that of NSCs-GFP and blank controls (28.4% and 31.2%, respectively, n=6, p<0.05; Figure 4C). No significant difference was observed among these groups in the proportion of GFAP-positive astrocytes, which were 47.6%, 43.5% and 40.3%, respectively.
The survival, proliferation and differentiation of NSC after NT-3 gene transduction in organotypic hippocampal slice cultures
All slices remained healthy after addition of CM-DiI labeled neurospheres (Figure 5A). CM-DiI labeled NSCs appeared throughout the slices. It was noticed that more NSCs-NT-3 survived after being transplanted into organotypic hippocampal slices than controls (Figure 5B). After 4 days of co-culture, abundant GFAP-positive glia and β-tubulin III positive neurons were observed throughout the slices. Some of them were labeled with both CM-DiI and β-tubulin or CM-DiI and GFAPs (Figure 5C, D), indicating the differentiation of transplanted NSCs in slice cultures. The proportion of neuron and glia differentiation was similar to that observed in vitro.
Figure 5.
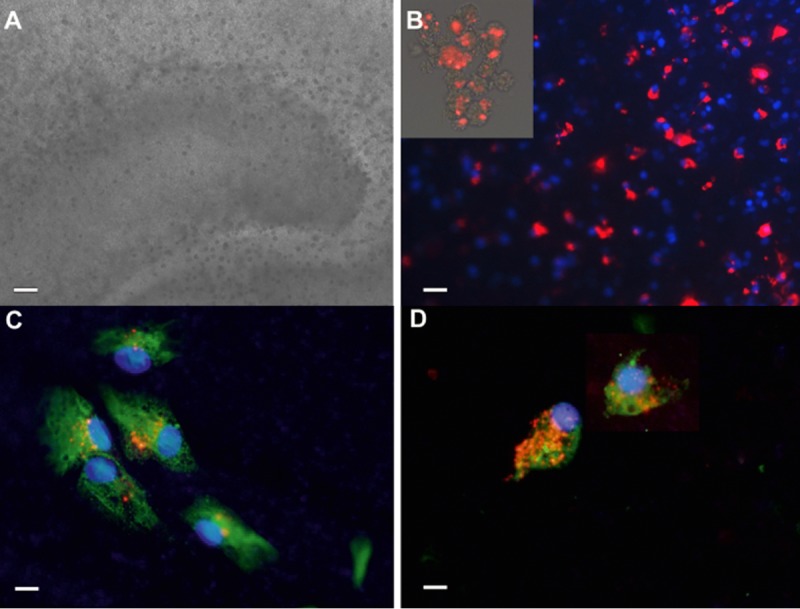
The survival and differentiation of NSCs-NT-3 in orgnaotypic hippocampal slice cultures Hippocampal slice cultures remained healthy after addition of CM-DiI labeled neurospheres (A). CM-DiI labeled neurospheres (insert in B) appeared to all over the slices and more NSCs-NT-3 survived after transplanted into organotypic hippocampal slices than controls (B). Some of them differentiated into β-tubulin positive neurons (C) and GFAP positive astrocytes (D). A Scal bar, 100 um; B Scal bar, 50 um; C and D Scal bar, 20 um.
Discussion
In the present study, the AAV Helper-Free System produced a high titer of AAV particles. The stemness of NSCs derived from mouse embryo cortex was well preserved after being successfully transduced with AAV. In addition, NSCs transduced with AAV-NT-3 had increased survival and proliferation, as well as preferential neuronal differentiation in comparison with control groups. Two conclusions may be drawn from our results: 1) the therapeutic NT-3 gene can be successfully delivered into NSCs via AAV and that this modification does not alter the characteristics of NSCs, and 2) the autocrine and/or paracrine NT-3 significantly increases NSC viability and neuronal differentiation.
The NSC microenvironment plays a very important role in cell proliferation and differentiation. However, after injury, the local microenvironment may become unfavorable to cell survival and function due to inflammation and cellular stress [2,29–31]. Therefore, it is crucial to regulate NSC proliferation and differentiation so that a sufficient quantity of the proper cell type may be obtained prior to therapeutic transplantation into the patient. Neurotrophic factors, including NT-3, have been proven to promote NSC proliferation, induce neuronal differentiation, and enhance axonal elongation. Also, the genetically modified Schwann cells and the human immortalized cell line C17.2 to express NT-3 have been transplanted into spinal cord injury animal models to enhance functional recovery [16,18,19,21–24], Nevertheless, the combination of neurotrophic factor gene and neural stem cell-based therapy has not yet achieved clinical application due to various problems, including concerns about the safety and efficiency of gene transfection, and the potential adverse effects of exogenous gene expression.
The AAV vector is an attractive tool for the gene therapy of neural disorders due to its low frequency of genome integration, lack of pathogenesis and immunogenesis, safety in clinical trials, and its preferential neuronal tropism [32–34]. The transduction rate of AAV is variable among different cell types [35,36], and there is no published data showing the transduction efficiency of AAV on NSCs. In the present study, 4×1011 vg/ml of AAV-NT-3 particles were produced, and 15.3% of NSCs (passage 4) were transduced by this vector. Our results are consistent with existing data regarding the titer of recombinant AAV and transduction efficiency on other cell types, such as blood cells, motor neuron-like cells and HEK-293, [36–38] and fulfilled the requirements of our experiment.
The present study aimed to promote NSC viability and induce neuronal differentiation by genetically transducing NT-3 into NSCs, with the hope that such modified NSCs will be applicable to the treatment of CNS disorders. It is, therefore, vital that the transduced NSCs maintain their characteristics of self-renewal and multiple differentiation potential. We have not found any other discussion of the maintenance of NSC stemness after genetic modification. To address this issue, we performed the current experiments, and found that the stemness of NSCs is well-preserved following AAV transduction. These cells proliferate and form neurospheres for at least 10 passages, and also differentiate into different neural cell types after exposure to differentiation medium, as determined by morphological appearance and immunoreactivity for different cell-type markers.
Additionally, in the present study, mouse NSCs were observed to proliferate at a higher rate and nearly two-thirds of NSCs differentiated into β-tubulin III-positive cells following AAV-NT-3 transduction, in comparison with about one-third of NSCs in the non-transduced group and AAV-GFP-transduced group; this was further confirmed in organotypic hippocampal slice cultures. In unrefreshed medium, NSC-NT-3 survived well and proliferated, likely benefiting from the NT-3 which has been secretively expressed by the infected cells, whereas dark-colored neurospheres which contained more dead cells were observed in AAV-GFP-transduced and blank control groups, might be due to the consumption of nutrition in the medium. This result is especially important, as it indicates that application of genetically modified NSCs with NT-3 will not only contribute to cell replacement via NSC, but may also improve the local environment via NT-3 secretion.
Conclusions
The present study suggests that the AAV-mediated NT-3 gene transduction did not alter the basic characteristics of NSCs, but promoted NSC survival, proliferation, and neuronal differentiation in vitro, offering the prospect of a combined gene and stem cell therapy for CNS disorders. These findings must, of course, be confirmed in an in vivo model, which should include the characterization of the specific phenotypes of neurons which were differentiated from NT-3-modified NSCs. Research on regulation of exogenous NT-3 gene expression by hypoxic response element and the confirmation of its effect in ischemic stroke animal model are being undertaken in the authors’ laboratory currently.
Acknowledgement
The authors would like to thank Qi-Chen from the School of International Study, Xi’an Jiaotong University for her brilliant English editing of our manuscript.
Abbreviations
- DIV
days in vitro
- DMEM
Dulbecco’s modified eagle medium
- DPI
days post-infection
- FBS
fetal bovine serum
- hrGFP
human recombinant green fluorescent protein
- NFs
neurotrophic factors
- NSC
neural stem cell
- NT-3
neurotrophin-3
- PCR
polymerase chain reaction
- rAAV
recombinant adeno-associated virus
Footnotes
Source of support: This work was supported by grants from the National Natural Science Foundation of China ((81070998 and 31070943) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (2008)
References
- 1.Wang Y, Chen S, Yang D, Le W-d. Stem cell transplantation: a promising therapy for Parkinson’s disease. J Neuroimmune Pharmacol. 2007;2:243–50. doi: 10.1007/s11481-007-9074-2. [DOI] [PubMed] [Google Scholar]
- 2.Imitola J. Prospects for neural stem cell-based therapies for neurological diseases. Neurotherapeutics. 2007;4:701–14. doi: 10.1016/j.nurt.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joannides AJ, Chandran S. Human embryonic stem cells: an experimental and therapeutic resource for neurological disease. J Neurol Sci. 2008;265:84–88. doi: 10.1016/j.jns.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Bithell A, Williams BP. Neural stem cells and cell replacement therapy: making the right cells. Clinical science (London, England: 1979) 2005;108:13–22. doi: 10.1042/CS20040276. [DOI] [PubMed] [Google Scholar]
- 5.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 6.Park DH, Borlongan CV, Eve DJ, Sanberg PR. The emerging field of cell and tissue engineering. Med Sci Monit. 2008;14(11):RA206–20. [PubMed] [Google Scholar]
- 7.Imitola J, Comabella M, Chandraker AK, et al. Neural stem/progenitor cells express costimulatory molecules that are differentially regulated by inflammatory and apoptotic stimuli. Am J Pathol. 2004;164:1615–25. doi: 10.1016/S0002-9440(10)63720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Los A, Fraga G, Hernandez S, et al. Prolonged survival and expression of neural markers by bone marrow-derived stem cells transplanted into brain lesions. Med Sci Monit. 2009;15(2):BR47–54. [PubMed] [Google Scholar]
- 9.Lepore AC, Fischer I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp Neurol. 2005;194:230–42. doi: 10.1016/j.expneurol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Lepore AC, Han SS, Tyler-Polsz CJ, et al. Differential fate of multipotent and lineage-restricted neural precursors following transplantation into the adult CNS. Neuron Glia Biol. 2004;1:113–26. doi: 10.1017/s1740925x04000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shindo T, Matsumoto Y, Wang Q, et al. Differences in the neuronal stem cells survival, neuronal differentiation and neurological improvement after transplantation of neural stem cells between mild and severe experimental traumatic brain injury. JMI. 2006;53:42–51. doi: 10.2152/jmi.53.42. [DOI] [PubMed] [Google Scholar]
- 12.Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clinical Science (London, England: 1979) 2006;110:167–73. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- 13.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–81. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 14.Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Developmental Neurobiology. 2010;70:271–88. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dicou E. Neurotrophins and neuronal migration in the developing rodent brain. Brain Res Rev. 2009;60:408–17. doi: 10.1016/j.brainresrev.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Gu S, Huang H, Bi J, Yao Y, Wen T. Combined treatment of neurotrophin-3 gene and neural stem cells is ameliorative to behavior recovery of Parkinson’s disease rat model. Brain Res. 2009;1257:1–9. doi: 10.1016/j.brainres.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Lee S-T, Chu K, Jung K-H, et al. Slowed progression in models of Huntington disease by adipose stem cell transplantation. Ann Neurol. 2009;66:671–81. doi: 10.1002/ana.21788. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Gu S, Zhao C, Wen T. Combined treatment of neurotrophin-3 gene and neural stem cells is propitious to functional recovery after spinal cord injury. Cell transplantation. 2007;16:475–81. doi: 10.3727/000000007783464902. [DOI] [PubMed] [Google Scholar]
- 19.Grill R, Murai K, Blesch A, et al. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci. 1997;17:5560–72. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson DJ, Longhi L, Lee EB, et al. Genetically modified NT2N human neuronal cells mediate long-term gene expression as CNS grafts in vivo and improve functional cognitive outcome following experimental traumatic brain injury. J Neuropathol Exp Neurol. 2003;62:368–80. doi: 10.1093/jnen/62.4.368. [DOI] [PubMed] [Google Scholar]
- 21.Park KI, Himes BT, Stieg PE, et al. Neural stem cells may be uniquely suited for combined gene therapy and cell replacement: Evidence from engraftment of Neurotrophin-3-expressing stem cells in hypoxic-ischemic brain injury. Exp Neurol. 2006;199:179–90. doi: 10.1016/j.expneurol.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Himes BT, Liu Y, Solowska JM, et al. Transplants of cells genetically modified to express neurotrophin-3 rescue axotomized Clarke’s nucleus neurons after spinal cord hemisection in adult rats. J Neurosci Res. 2001;65:549–64. doi: 10.1002/jnr.1185. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Himes BT, Solowska J, et al. Intraspinal delivery of neurotrophin-3 using neural stem cells genetically modified by recombinant retrovirus. Exp Neurol. 1999;158:9–26. doi: 10.1006/exnr.1999.7079. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Zeng Y, Zhang W, et al. Co-transplantation of neural stem cells and NT-3-overexpressing Schwann cells in transected spinal cord. J Neurotrauma. 2007;24:1863–77. doi: 10.1089/neu.2007.0334. [DOI] [PubMed] [Google Scholar]
- 25.Gage FH, Coates PW, Palmer TD, et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci USA. 1995;92:11879–83. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Tian Y, Yao L, et al. Hypoxia stimulates proliferation of rat neural stem cells with influence on the expression of cyclin D1 and c-Jun N-terminal protein kinase signaling pathway in vitro. Neuroscience. 2010;165:705–14. doi: 10.1016/j.neuroscience.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–82. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 28.Lu HX, Levis H, Melhem N, Parker T. Toxin-produced Purkinje cell death: a model for neural stem cell transplantation studies. Brain Res. 2008;1207:207–13. doi: 10.1016/j.brainres.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 29.Conover JC, Notti RQ. The neural stem cell niche. Cell Tissue Res. 2008;331:211–24. doi: 10.1007/s00441-007-0503-6. [DOI] [PubMed] [Google Scholar]
- 30.Jiang W, Xiao L, Wang J-C, et al. Effects of nitric oxide on dentate gyrus cell proliferation after seizures induced by pentylenetrazol in the adult rat brain. Neurosci Lett. 2004;367:344–48. doi: 10.1016/j.neulet.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 31.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science (New York, N Y) 2003;302:1760–65. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 32.Taymans J-M, Vandenberghe LH, Haute CVD, et al. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum Gene Ther. 2007;18:195–206. doi: 10.1089/hum.2006.178. [DOI] [PubMed] [Google Scholar]
- 33.Vasileva A, Jessberger R. Precise hit: adeno-associated virus in gene targeting. Nature reviews Microbiology. 2005;3:837–47. doi: 10.1038/nrmicro1266. [DOI] [PubMed] [Google Scholar]
- 34.Howard DB, Powers K, Wang Y, Harvey BK. Tropism and toxicity of adeno-associated viral vector serotypes 1, 2, 5, 6, 7, 8, and 9 in rat neurons and glia in vitro. Virology. 2008;372:24–34. doi: 10.1016/j.virol.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veldwijk MR, Schiedlmeier B, Kleinschmidt JA, et al. Superior gene transfer into solid tumour cells than into human mobilised peripheral blood progenitor cells using helpervirus-free adeno-associated viral vector stocks. Eur J Cancer, (Oxford, England: 1990) 1999;35:1136–42. doi: 10.1016/s0959-8049(99)00075-1. [DOI] [PubMed] [Google Scholar]
- 36.Nathwani AC, Hanawa H, Vandergriff J, et al. Efficient gene transfer into human cord blood CD34+ cells and the CD34+CD38− subset using highly purified recombinant adeno-associated viral vector preparations that are free of helper virus and wild-type AAV. Gene Ther. 2000;7:183–95. doi: 10.1038/sj.gt.3301068. [DOI] [PubMed] [Google Scholar]
- 37.Keir SD, Xiao X, Li J, Kennedy PG. Adeno-associated virus-mediated delivery of glial cell line-derived neurotrophic factor protects motor neuron-like cells from apoptosis. J Neurovirol. 2001;7:437–46. doi: 10.1080/135502801753170291. [DOI] [PubMed] [Google Scholar]
- 38.Hildinger M, Baldi L, Stettler M, Wurm FM. High-titer, serum-free production of adeno-associated virus vectors by polyethyleneimine-mediated plasmid transfection in mammalian suspension cells. Biotechnol Lett. 2007;29:1713–21. doi: 10.1007/s10529-007-9441-3. [DOI] [PubMed] [Google Scholar]
- 39.Lu HX, Levis H, Liu Y, Parker T. Organotypic slices culture model for cerebellar ataxia: potential use to study Purkinje cell induction from neural stem cells. Brain Res Bull. 2010;84:169–73. doi: 10.1016/j.brainresbull.2010.12.001. [DOI] [PubMed] [Google Scholar]