Summary
Background
Aging is a highly complex process that affects various tissues and systems in the body. Senescent changes are relatively more prevalent and severe in the postmitotic cells. Mitochondria play an important role in the aging process. Recently, cell cultures have been widely used as an in vitro model to study aging. The present study was designed to investigate mitochondrial dysfunction associated with aging in a long-term cell culture system.
Material/Methods
Rat hippocampal neurons were maintained in culture in serum-free medium for 30 days in vitro (DIV). The morphology and development of hippocampal neurons was observed by phase contrast microscope. The levels of cellular senescence were evaluated by cytochemical staining of senescence-associated β-galactosidase (SA-β-Gal) at DIV 5, 10, 15, 20, 25 and 30. In addition, we investigated the changes in mitochondrial membrane potential (Δψm) and intracellular reactive oxygen species (ROS) generation of hippocampal neurons by flow cytometry at different ages.
Results
The proportion of the senescent cells steadily increased with age in neuron cultures. Δψm decreased gradually with age in long-term culture, while ROS generation increased.
Conclusions
This study indicates an age-related decrease in mitochondrial function in long-term hippocampal neuronal culture and suggests that DIV 25 neurons could possibly serve as a platform for the future study of anti-aging from the perspective of mitochondrial function.
Keywords: senescence, mitochondrial membrane potential, reactive oxygen species, long-term primary neuronal culture
Background
Aging is a highly complex process that affects various tissues and systems in the body [1,2], and which is regulated by many divergent pathways and on many levels. Cell cultures are widely used as an in vitro model to study aging. The cell culture does model intriguing aspects of aging, but it may be a little preliminary to call changes over time in culture “aging” per se. Recently, some researchers have used long-term primary cultures of differentiated cells in aging studies [3–7].
It is widely thought that senescent changes are relatively more prevalent and severe in postmitotic cells. Senescent cells show a series of morphological and physiological alterations, including a flat and enlarged morphology [8], mitochondrial alterations [9], chromatin condensation [10], and changes in gene expression pattern [11]. Replicative senescence is defined as a state where normal somatic cells lose their replicative capacity in vitro after prolonged division in culture, an occurrence which results in irreversible growth arrest [9,12,13]. The brain contains large numbers of postmitotic cells particularly vulnerable to “normal” age-related changes, which affect its function. Moreover, aging is a major risk factor in most neurodegenerative diseases [14]. Therefore, establishment of a model for neuronal cell aging in vitro may produce valuable information to explore aging at the cellular and molecular levels.
Several lines of evidence suggest that mitochondria play an important role in the aging process [15,16] and are affected by aging [17]. One of the hallmarks of age-related mitochondrial function is associated with decreased mitochondrial membrane potential (Δψm) and increased reactive oxygen species (ROS) levels in aging tissues [18–21] or cells from animals of different ages [22]. SA-beta-Gal activity is a widely used marker for replicative cellular senescence in vivo and in vitro. Recently, several studies have indicated that SA-beta-Gal activity can be used as a biomarker for senescence of neurons in vivo and in vitro[23–25].
Previous studies have investigated the relationships between changes in Δψm and [Ca2+]i during and after a toxic glutamate challenge in cultured rat hippocampal neurons [26]. In addition, Parihar et al. [22] compared ROS production while simultaneously monitoring Δψm before and after glutamate treatment of live neurons from embryonic, middle-aged, and old rats. Age-dependent changes of neuronal survival, protein oxidation, and creatine kinase BB expression in long-term hippocampal cell culture have been examined [3]. In this study, we conducted a systematic parallel observation of the changes in Δψm and ROS levels and examined the senescence of neurons by β-galactosidase staining. Moreover, we have attempted to identify on which day in culture the ROS, delta psi or beta-gal suggest irreversible or accelerated deterioration in long-term culture based on the findings.
Material and Methods
Chemicals and reagents
Culture reagents were purchased from Gibco. All other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
Cell culture
Primary cultures of hippocampal neurons were prepared according to the published protocols from the laboratories of Nelson [27] with modification. Briefly, hippocampi were dissected from newborn (P0, 0–24 h) Sprague-Dawley rats in ice-cold dissection solution containing sucrose/glucose/HEPES (DISGH solution: 136 mM NaCl, 5.4 mM KCl, 0.2 mM Na2HPO4, 2 mM KH2PO4, 16.7 mM glucose, 20.8 mM saccharose, 0.0012% phenol red, and 10mM HEPES, pH7.4). Isolated hippocampi were mechanically triturated, and then digested in solution containing 0.25% trypsin and 0.02% EDTA at 37° for 15 min. Single cell suspension was obtained by repeated passages of the dissociated tissues through flame-polished pipette in plating medium (DMEM supplemented with 10% heat inactivated FBS and 1% penicillin-streptomycin). Cells were finally plated on poly-L-lysine (0.1 mg/ml) coated plates at optimal cell density. High density cultures (1×106 cells, 1000 cells/mm2) and lower density cultures (3×105 cells, 310 cells/mm2), plated onto 6-well culture plates, were used for measuring mitochondrial function and cytochemical studies, respectively. The serum-containing plating medium was replaced by growth medium (serum-free DMEM/F12 medium supplemented with 2% B27 and 1% penicillin-streptomycin) within 24 h after plating. Half of the growth medium was changed every 3 days thereafter. Cultures were kept at 37° in a humidified 5% CO2-containing atmosphere. More than 95% of cells were neurons on 10 day in vitro (DIV), verified by positive staining of mouse monoclonal anti-NSE (neuron-specific enolase) (data not shown). Neuronal cultures were maintained for up to DIV 30. All animal procedures were carried out with the approval of the local Animal Care and Use Committee.
Senescence-associated β-galactosidase (SA-β-Gal) staining
The senescent status was detected by the method of Dimri et al. [28]. In brief, the monolayers of cells were washed 2 times with phosphate-buffered saline (PBS), fixed with 3% formaldehyde for 3–5 min, washed 2 times in PBS, and then stained for 18 h at 37° in a CO2-free atmosphere with fresh β-galactosidase staining solution [1 mg/mL 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-Gal), 40 mM citric acid/sodium phosphate, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, 2 mM MgCl2, pH 6.0]. After staining, cells were washed twice with PBS and photographed. The percentage of SA-β-Gal-positive cells was determined by counting the number of blue cells within a sample of 1000 cells.
Measurement of Δψm
Δψm of hippocampal neurons was measured by uptake of lipophilic cation Rhodamine 123 (Rh123) into mitochondria. About 5×105 cells were collected at DIV 5, 10, 15, 20, 25 and 30 and incubated with 10 μg/ml Rh123 at 37° for 30 min. Then the cells were washed twice with PBS and resuspended in 500 μl PBS. The samples were analyzed for fluorescence using a flow cytometer.
Measurement of intracellular ROS generation
Intracellular ROS production was measured by using a non-fluorescent compound 2′, 7′-dichlorofluorescin diacetate (DCFH-DA), which can be converted to DCFH by esterases when taken up. DCFH reacts with ROS to generate a new highly fluorescent compound, dichlorofluorescein, which can be analyzed with flow cytometry. About 5×105 cells at different ages were incubated with 20 μM DCFH-DA at 37° for 30 min, washed twice with PBS, and then measured with flow cytometry.
Statistical analysis
Each experiment was carried out in triplicates with at least 3 separated cultures. Data are presented as mean ± standard deviation (SD) calculated from at least 3 separate experiments. The data were analyzed by one-way ANOVA followed by Student-Newman-Keuls test using SPSS 11.0 software. P<0.05 was considered statistically significant.
Results
Long-term hippocampal cell culture
Dissociated cells from the hippocampus of newborn rat brain were plated at a cell density of 310 cells/mm2. Within the first 2 days after plating, cell density decreased to 260 cells/mm2. Once stabilized, the number of surviving neurons remained constant up to 25 days and then started to decline. Photomicrographs of rat hippocampal neurons at different ages in culture are shown in Figure 1. Hippocampal neurons at DIV 5 extended prominent neurite (Figure 1A). The first 15 days was a period of maturation of young neurons in culture, during which they formed an extensive network of processes and increased the size of their cell bodies (Figure 1A–C). Mature cells exhibited an increase in the size of the cell bodies, thick dendritic processes, and an extremely dense and extensive network (Figure 1C, D). Neurons with vacuolated soma and beaded or fragmented neurites were observed after DIV 20, and then the number of neurons decreased after DIV 25 (Figure 1E, F).
Figure 1.
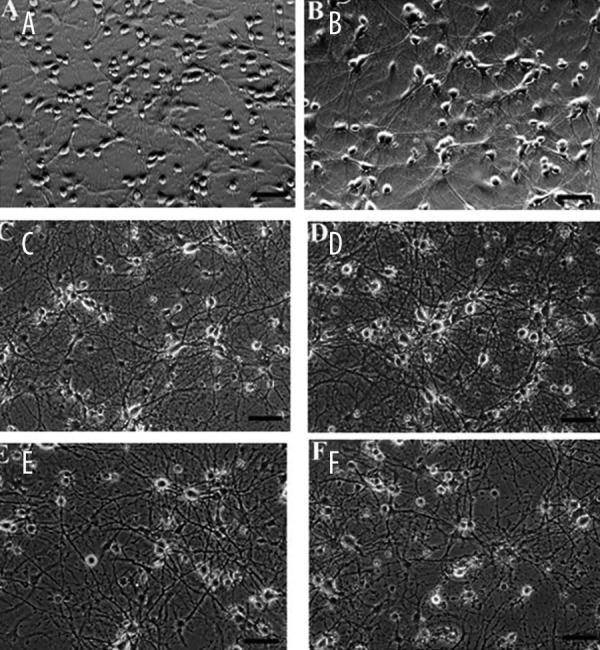
Phase contrast photomicrographs of live rat primary hippocampal neurons in culture plated at 310 cells/mm2. A-F show neurons of ages 5, 10, 15, 20, 25 and 30 DIV, respectively. Scale bar =100 μm.
SA-β-Gal staining with age in long-term culture
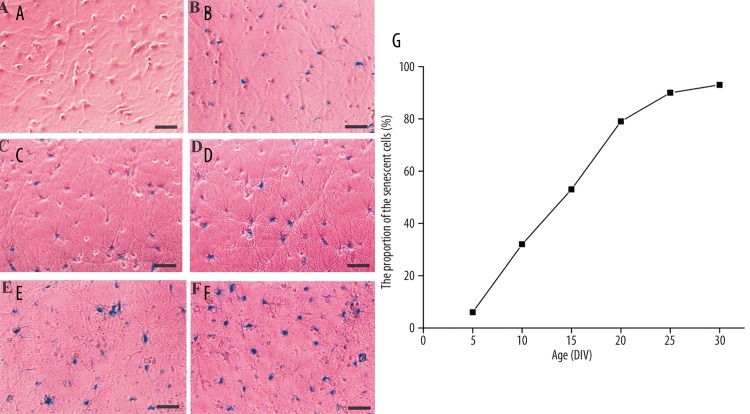
Senescent cells display visibly increased β-galactosidase activity at pH 6.0 when measured in situ[28]. In order to establish a link between senescence and culture time, hippocampal neurons were cultured in serum-free medium until 30 days. The activity of SA-β-Gal was detected every 5 days. The proportion of the senescent cells steadily increased in neuron cultures (Figure 2A–F). More than 90% of cells stained SA-β-Gal positive at DIV 25 and DIV 30 (Figure 2G).
Figure 2.
Detection of SA-β-Gal activity in primary culture of hippocampal neurons on day 5 (A), day 10 (B), day 15 (C), day 20 (D), day 25 (E) and day 30 (F). (G) The proportion of neurons stained positive for SA-β-Gal at different ages. These estimates and those illustrated were deemed representative of repeated cultures from different preparations of neurons. Scale bar =100 μm.
Δψm with age in long-term culture
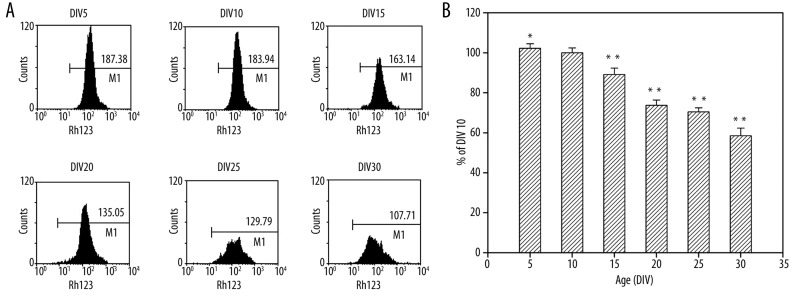
To analyze the alteration in Δψm in long-term culture, we examined the Δψm by using the mitochondria-specific dye Rh123 at DIV 5, 10, 15, 20, 25 and 30. As shown in Figure 3, there was an age-related decrease of Δψm in neurons. The fluorescence intensity of Rh123 of DIV 25 neurons and DIV 30 neurons decreased to 71% and 59%, respectively, of the levels of DIV 10 neurons.
Figure 3.
Detection of Δψm with Rh123. (A) Mean Rh123 fluorescence intensity detected by flow cytometry. (B) The results were expressed as the relative fluorescence intensity (%) with respect to neurons at DIV 10. *P<0.05 vs. DIV 10, **P<0.01 vs. DIV 10.
Intracellular ROS generation with age in long-term culture
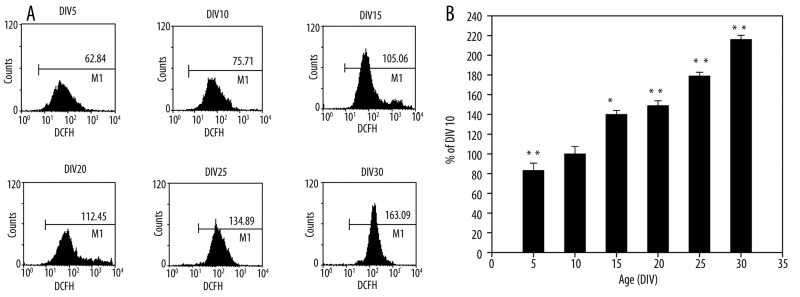
We examined ROS production at different time points in long-term culture. As shown in Figure 4, there was a time-associated increase of ROS generation in neurons. The fluorescence intensity of DCFH of DIV 25 neurons and DIV 30 neurons increased to 178% and 215%, respectively, of the levels of DIV 10 neurons. These results indicate that ROS generation obviously increased in aging neurons.
Figure 4.
ROS production in long-term culture hippocampal neurons. (A) Mean DCFH fluorescence intensity. (B) The results were expressed as the relative fluorescence intensity (%) with respect to cells at DIV 10. ** P<0.01 vs. DIV 10.
Discussion
Our results demonstrate that primary hippocampal neurons in prolonged culture develop characteristics of senescence. Furthermore, these results indicate that long-term culture primary hippocampal neurons may serve as a mitochondria dysfunction model associated with aging, and that DIV 25 neurons could possibly represent aging neurons in culture.
There has been a certain similarity between whole animal neuron aging and neuronal aging in culture. Long-term culture of cerebellar granule neurons showed changes in [Ca2+]i that were strikingly similar to the age-dependent changes recorded in cerebellar brain slice preparations [29,30]. Long-term culture of hippocampal neurons reproduced the pattern of changes in Ca2+ channel density observed in brain slices obtained from aging rats [31]. The morphological changes of mitochondria in long-term primary neuronal culture were consistent with the results in vivo[32]. In addition, Parihar et al. [22] monitored Δψm simultaneously with measuring ROS on an individual mitochondrial basis in single live hippocampal neuronal cells isolated from embryonic (E18), middle-aged (12 months), and old (24 months) rats, and maintained in a uniform culture environment. The results showed an age-related increase in ROS production and an age-related depolarization at rest in single somal and axonal/dendritic mitochondria. However, Potter et al. [33] used FEP (fluorinated ethylene-propylene)-sealed culture dishes for culturing cells that maintains their health and sterility for over a year. They reduced or eliminated problems with infection and hyperosmolality, while maintaining pH and O2 homeostasis. Using conventional techniques, increases in the osmotic strength of media due to evaporation are a large and underappreciated contributor to the gradual decline in the health of primary neuron cultures. Osmolarity changes in the medium are an uncontrolled but causal factor.
We applied SA-β-Gal staining to identify senescent hippocampal neurons in long-term culture, and our results show that SA-β-Gal activity gradually increased. These results, in agreement with those obtained in other studies [23,24], support the use of SA-beta-Gal activity as a biomarker for senescence of neurons in vivo and in vitro.
Δψm has often been used as a marker for mitochondrial integrity [34]. In addition, depolarized Δψm has been regarded as a function of age [35,36]. Depolarized Δψm in aged cells appears to be one of the features of age-dependent alterations of mitochondrial function. One proposed mechanism to explain depolarized Δψm in aged cells is the alteration in the activity of the respiratory chain complexes. The results showed that Δψm depolarized in senescent neurons, which was in agreement with previous reports on exocrine cells [37], hepatocytes [38] and neurons [22 39]. Moreover, depolarized Δψm produces more ROS on cardiac myocytes [40], neurons [22], and Hela cells [41].
Mitochondria, by virtue of their intense respiratory chain activity, are the major source of ROS [34], and also constitute a main target of cumulative oxidative stress. A large body of experimental evidence suggests that ROS production is increased in aging tissues [42,43]. Hence, mitochondria-generated ROS have been implicated as a common feature that connects aging of organisms and age-related diseases [44,45]. Our data showed that ROS levels increased with age in vitro, which is consistent with the observations in vivo[20,21]. The maintenance of low ROS levels is critical to normal cell functions. A decrease in Δψm has been shown to be associated with an increase in ROS in a variety of experimental models [22,46,47]. Our results further support the idea that depolarized Δψm produces more ROS.
Conclusions
In summary, our results demonstrate a decrease in mitochondrial function with days in culture. Furthermore, we identify that DIV 25 neurons could be an ideal time point for modeling age-related mitochondrial dysfunction in culture under our experimental conditions, which may contribute to the exploration of relationship between mitochondria dysfunction and aging, and may be useful for future anti-aging studies.
Footnotes
Source of support: This work was supported by grants from the National Natural Science Foundation of China (No. 30472248)
References
- 1.Kneser M, Kohlmann T, Pokorny J, Tost F. Age related decline of microvascular regulation measured in healthy individuals by retinal dynamic vessel analysis. Med Sci Monit. 2009;15(8):CR436–41. [PubMed] [Google Scholar]
- 2.Petrofsky JS, McLellan K, Bains GS, et al. The influence of ageing on the ability of the skin to dissipate heat. Med Sci Monit. 2009;15(6):CR261–68. [PubMed] [Google Scholar]
- 3.Aksenova MV, Aksenov MY, Markesbery WR, Butterfield DA. Aging in a dish: age-dependent changes of neuronal survival, protein oxidation, and creatine kinase BB expression in long-term hippocampal cell culture. J Neurosci Res. 1999;58:308–17. [PubMed] [Google Scholar]
- 4.Lesuisse C, Martin LJ. Long-term culture of mouse cortical neurons as a model for neuronal development, aging, and death. J Neurobiol. 2002;51:9–23. doi: 10.1002/neu.10037. [DOI] [PubMed] [Google Scholar]
- 5.Brewer LD, Thibault O, Staton J, et al. Increased vulnerability of hippocampal neurons with age in culture: temporal association with increases in NMDA receptor current, NR2A subunit expression and recruitment of L-type calcium channels. Brain Res. 2007;1151:20–31. doi: 10.1016/j.brainres.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Kim MJ, Oh SJ, Park SH, et al. Neuronal loss in primary long-term cortical culture involves neurodegeneration-like cell death via calpain and p35 processing, but not developmental apoptosis or aging. Exp Mol Med. 2007;39:14–26. doi: 10.1038/emm.2007.3. [DOI] [PubMed] [Google Scholar]
- 7.Tajes M, Gutierrez-Cuesta J, Ortuno-Sahagun D, et al. Anti-aging properties of melatonin in an in vitro murine senescence model: involvement of the sirtuin 1 pathway. J Pineal Res. 2009;47:228–37. doi: 10.1111/j.1600-079X.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- 8.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 9.Hwang ES, Yoon G, Kang HT. A comparative analysis of the cell biology of senescence and aging. Cell Mol Life Sci. 2009;66:2503–24. doi: 10.1007/s00018-009-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funayama R, Ishikawa F. Cellular senescence and chromatin structure. Chromosoma. 2007;116:431–40. doi: 10.1007/s00412-007-0115-7. [DOI] [PubMed] [Google Scholar]
- 11.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–22. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Hayflick L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp Cell Res. 1965;37:614–36. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 13.Wright WE, Shay JW. Historical claims and current interpretations of replicative aging. Nat Biotechnol. 2002;20:682–88. doi: 10.1038/nbt0702-682. [DOI] [PubMed] [Google Scholar]
- 14.Farooqui T, Farooqui AA. Aging: An important factor for the pathogenesis of neurodegenerative diseases. Mech Ageing Dev. 2009;130:203–15. doi: 10.1016/j.mad.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Carretero M, Escames G, Lopez LC, et al. Long-term melatonin administration protects brain mitochondria from aging. J Pineal Res. 2009;47:192–200. doi: 10.1111/j.1600-079X.2009.00700.x. [DOI] [PubMed] [Google Scholar]
- 16.Modi HR, Katyare SS, Patel MA. Ageing-induced alterations in lipid/phospholipid profiles of rat brain and liver mitochondria: implications for mitochondrial energy-linked functions. J Membr Biol. 2008;221:51–60. doi: 10.1007/s00232-007-9086-0. [DOI] [PubMed] [Google Scholar]
- 17.Hutter E, Renner K, Pfister G, et al. Senescence-associated changes in respiration and oxidative phosphorylation in primary human fibroblasts. Biochem J. 2004;380:919–28. doi: 10.1042/BJ20040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagen TM, Yowe DL, Bartholomew JC, et al. Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. Proc Natl Acad Sci USA. 1997;94:3064–69. doi: 10.1073/pnas.94.7.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kashiwagi K, Shinkai T, Kajii E, Kashiwagi A. The effects of reactive oxygen species on amphibian aging. Comp Biochem Physiol C Toxicol Pharmacol. 2005;140:197–205. doi: 10.1016/j.cca.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Petrosillo G, Matera M, Casanova G, et al. Mitochondrial dysfunction in rat brain with aging Involvement of complex I, reactive oxygen species and cardiolipin. Neurochem Int. 2008;53:126–31. doi: 10.1016/j.neuint.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Petrosillo G, Matera M, Moro N, et al. Mitochondrial complex I dysfunction in rat heart with aging: critical role of reactive oxygen species and cardiolipin. Free Radic Biol Med. 2009;46:88–94. doi: 10.1016/j.freeradbiomed.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 22.Parihar MS, Brewer GJ. Simultaneous age-related depolarization of mitochondrial membrane potential and increased mitochondrial reactive oxygen species production correlate with age-related glutamate excitotoxicity in rat hippocampal neurons. J Neurosci Res. 2007;85:1018–32. doi: 10.1002/jnr.21218. [DOI] [PubMed] [Google Scholar]
- 23.Chernova T, Nicotera P, Smith AG. Heme deficiency is associated with senescence and causes suppression of N-methyl-D-aspartate receptor subunits expression in primary cortical neurons. Mol Pharmacol. 2006;69:697–705. doi: 10.1124/mol.105.016675. [DOI] [PubMed] [Google Scholar]
- 24.Geng YQ, Guan JT, Xu XH, Fu YC. Senescence-associated beta-galactosidase activity expression in aging hippocampal neurons. Biochem Biophys Res Commun. 2010;396:866–69. doi: 10.1016/j.bbrc.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Dong WG, Huang F, Fan WG, et al. Differential effects of melatonin on amyloid-beta peptide 25–35-induced mitochondrial dysfunction in hippocampal neurons at different stages of culture. J Pineal Res. 2010;48:117–25. doi: 10.1111/j.1600-079X.2009.00734.x. [DOI] [PubMed] [Google Scholar]
- 26.Vergun O, Keelan J, Khodorov BI, Duchen MR. Glutamate-induced mitochondrial depolarisation and perturbation of calcium homeostasis in cultured rat hippocampal neurones. J Physiol. 1999;519(Pt 2):451–66. doi: 10.1111/j.1469-7793.1999.0451m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson PG. Nerve and muscle cells in culture. Physiol Rev. 1975;55:1–61. doi: 10.1152/physrev.1975.55.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–67. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirischuk S, Verkhratsky A. Calcium homeostasis in aged neurones. Life Sci. 1996;59:451–59. doi: 10.1016/0024-3205(96)00324-4. [DOI] [PubMed] [Google Scholar]
- 30.Toescu EC, Verkhratsky A. Neuronal ageing in long-term cultures: alterations of Ca2+ homeostasis. Neuroreport. 2000;11:3725–29. doi: 10.1097/00001756-200011270-00027. [DOI] [PubMed] [Google Scholar]
- 31.Porter NM, Thibault O, Thibault V, et al. Calcium channel density and hippocampal cell death with age in long-term culture. J Neurosci. 1997;17:5629–39. doi: 10.1523/JNEUROSCI.17-14-05629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savitha S, Panneerselvam C. Mitochondrial membrane damage during aging process in rat heart: potential efficacy of L-carnitine and DL alpha lipoic acid. Mech Ageing Dev. 2006;127:349–55. doi: 10.1016/j.mad.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Potter SM, DeMarse TB. A new approach to neural cell culture for long-term studies. J Neurosci Methods. 2001;110:17–24. doi: 10.1016/s0165-0270(01)00412-5. [DOI] [PubMed] [Google Scholar]
- 34.Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol Rev. 2000;80:315–60. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- 35.Unterluggauer H, Hutter E, Voglauer R, et al. Identification of cultivation-independent markers of human endothelial cell senescence in vitro. Biogerontology. 2007;8:383–97. doi: 10.1007/s10522-007-9082-x. [DOI] [PubMed] [Google Scholar]
- 36.Sugrue MM, Tatton WG. Mitochondrial membrane potential in aging cells. Biol Signals Recept. 2001;10:176–88. doi: 10.1159/000046886. [DOI] [PubMed] [Google Scholar]
- 37.Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Age-related alterations in Ca2+ signals and mitochondrial membrane potential in exocrine cells are prevented by melatonin. J Pineal Res. 2008;45:191–98. doi: 10.1111/j.1600-079X.2008.00576.x. [DOI] [PubMed] [Google Scholar]
- 38.Sastre J, Pallardo FV, Garcia de la Asuncion J, Vina J. Mitochondria, oxidative stress and aging. Free Radic Res. 2000;32:189–98. doi: 10.1080/10715760000300201. [DOI] [PubMed] [Google Scholar]
- 39.Xiong H, Camello PJ, Verkhratsky A, Toescu EC. Mitochondrial polarisation status and [Ca2+](i) signalling in rat cerebellar granule neurones aged in vitro. Neurobiol Aging. 2004;25:349–59. doi: 10.1016/S0197-4580(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 40.Zorov DB, Filburn CR, Klotz LO, et al. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–14. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belousov VV, Fradkov AF, Lukyanov KA, et al. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006;3:281–86. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 42.Cocuzza M, Athayde KS, Agarwal A, et al. Age-related increase of reactive oxygen species in neat semen in healthy fertile men. Urology. 2008;71:490–94. doi: 10.1016/j.urology.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 43.Dkhar P, Sharma R. Effect of dimethylsulphoxide and curcumin on protein carbonyls and reactive oxygen species of cerebral hemispheres of mice as a function of age. Int J Dev Neurosci. 2010;28:351–57. doi: 10.1016/j.ijdevneu.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Van Remmen H, Jones DP. Current thoughts on the role of mitochondria and free radicals in the biology of aging. J Gerontol A Biol Sci Med Sci. 2009;64:171–74. doi: 10.1093/gerona/gln058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shipounova IN, Svinareva DA, Petrova TV, et al. Reactive oxygen species produced in mitochondria are involved in age-dependent changes of hematopoietic and mesenchymal progenitor cells in mice. A study with the novel mitochondria-targeted antioxidant SkQ1. Mech Ageing Dev. 2010;131:415–21. doi: 10.1016/j.mad.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Green PS, Simpkins JW. Estradiol protects against ATP depletion, mitochondrial membrane potential decline and the generation of reactive oxygen species induced by 3-nitroproprionic acid in SK-N-SH human neuroblastoma cells. J Neurochem. 2001;77:804–11. doi: 10.1046/j.1471-4159.2001.00271.x. [DOI] [PubMed] [Google Scholar]
- 47.Odagiri K, Katoh H, Kawashima H, et al. Local control of mitochondrial membrane potential, permeability transition pore and reactive oxygen species by calcium and calmodulin in rat ventricular myocytes. J Mol Cell Cardiol. 2009;46:989–97. doi: 10.1016/j.yjmcc.2008.12.022. [DOI] [PubMed] [Google Scholar]