Summary
Background
To investigate plasma IL-17 level and the expression of Th17 cell transcription factor RORγt in the pathogenesis of Behçet’s Disease (BD).
Material/Methods
Blood samples were collected from 73 patients with BD (45 patients were in active stage), 20 systemic lupus erythematosus (SLE) and 12 multiple sclerosis patients (MS). Twelve patients with BD were investigated both in their active and remission stages. Samples were processed to detect IL-17A level in plasma by enzyme-linked immunosorbent assay (ELISA). Related gene expression was assessed by real-time reverse transcription polymerase chain reaction. Function of Th17 cells in active BD patients with erythema nodosum (EN)-like eruption was studied in relation to human umbilical vein endothelial cells (HUVECs).
Results
We demonstrated the presence of Th17 cells and RORγt among the peripheral blood mononuclear cells (PBMC). The percentage of circulating Th17 cells and the ability to produce interleukin-17A (IL-17A) were increased in samples derived from patients with active BD, MS and SLE patients. We observed that IL-17A from patients with active BD could induce adhesion molecule messenger RNA expression in HUVECs.
Conclusions
RORγt determined Th17 cell might be involved with increased IL-17A in BD. Our results indicate that IL-17 contributes to the active proinflammatory pattern that is characteristic of inflammatory diseases and patients with active BD.
Keywords: Behçet’s Disease, interleukin-17A, RORγt mRNA, Th17 cell
Background
Behçet’s disease (BD) is a vasculitis characterized by oral and genital ulcers and uveitis. Additional target organ, including vascular, neurological, and gastrointestinal manifestations, were added to the disease spectrum [1]. The pathogenesis of BD is still unclear, but immune dysfunction, viral and bacterial agents, such as Staphylococcus spp. and herpes simplex virus, have been postulated [2]. Behçet’s disease is an inflammatory disease characterized by local tissue injury caused by immunocompetent cells, in particular CD4+ T lymphocytes, that are involved in the pathogenesis of this syndrome via the production of distinctive sets of cytokines like IFN-γ, IL-6, TNF-α, TGF-β [3–5]. The results from a cytokine production and gene profile analysis in different autoimmune/inflammatory diseases identified a population of in vivo differentiated retinoid-related orphan receptor γ-expressing T cells, producing high levels of IL-17 that can represent up to 30% of infiltrating T lymphocytes [6]. Various in vitro differentiation systems have confirmed that IL-17 producing T cells were a distinct linage cells from Th1 or Th2 cells [7,8].
Th17 cell has been shown to be a new lineage of proinflammatory T helper cells and plays a role in some autoimmune diseases [9,10]. Interleukin-17A (IL-17) has been described as Th17 cell-derived cytokine and is highly expressed in autoimmune disorders and inflammatory diseases [11]. The investigation about Th17 could be a solution to explain the Th1/Th2 imbalance. Increased local levels of released IL-17 have been reported for a number of chronic inflammatory diseases such as allergic asthma [12,13] rheumatoid arthritis [14,15] and inflammatory bowel disease [16].
The mechanisms by which IL-17 induces the expression of proinflammatory mediators may be cell type-dependent, and appear to involve gene transcription [17,18] and possibly modulation of mRNA processing [19,20]. The specific transcription factor of Th17 cell lineage is RORγt [21] which has also been shown to correlate with autoimmune diseases. A functional role for Th17 cells in inflammatory/autoimmune diseases was proposed based on the demonstration that the level of IL-17A was elevated, which induced elevated expression of messenger RNA (mRNA) for adhesion molecules in human umbilical vein endothelial cells (HUVECs) and elicited T cell adherence to HUVEC [22]. IL-17A-induced signaling of adhesion molecules might play a key role in the inflammatory reaction of inflammatory/autoimmune diseases by eliciting T cell adhesion. Circulating IL-17A and IL-17A-induced signaling of adhesion molecules might play a key role in the inflammatory reaction by eliciting T cell adhesion.
Material and Methods
Patients
The study was approved by the Ethical Committee of our University. A total of 73 patients with BD (21 females, 52 males) fulfilling the International Study Group Criteria for BD [23] were enrolled into this study (Table 1), along with 40 normal volunteers (10 females, 30 males). Patients with active BD (n = 45 patients; aged: 42 years; range 20–47 years) and the mean disease duration was 76 months (range 10–141 months). Twenty eight BD patients were in remission (aged: 43 years; range 28–49 years) and they have lost the majority of their symptoms. Disease activity was evaluated according to published criteria [24]. Patients with active disease were treated with steroids and colchicines. Twelve patients with BD were investigated for plasma IL-17, circulating Th17 cell frequencies and RORγt mRNA before and after treatment, when all their symptoms were lost (All 12 patients were in complete remission). Laboratory findings included erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP).
Table 1.
Clinical characteristics of patients with active Behçet’s Disease.
| Clinical characteristics | Patients (n=45) | (%) |
|---|---|---|
| Oral ulcer | 40 | 88.9 |
| Genital ulcer | 37 | 82.2 |
| Erythema nodosum | 26 | 57.8 |
| Ocular symptoms | 16 | 35.5 |
| Thrombophelibitis | 12 | 26.6 |
| Arthritis | 27 | 60 |
| Neurologic involvement | 4 | 8.9 |
| Pulmonary manifestation | 8 | 17.8 |
| The skin pathergy test (+) | 32 | 71.1 |
Twenty SLE and 12 MS patients (all in active stage) acted as control diseases. SLE patients were aged: 48 years; (range 32–55 years). The diagnosis of SLE patients were established according to the 1982 revised American College of Rheumatology criteria Disease activity was evaluated by the SLE disease activity index score (SLEDAI) [25]. MS patients were aged: 52 years; (range 36–49 years). The diagnosis of MS was established according to the McDonald et al. criteria [26]. All SLE patients were in inflammatory attack of the disease. Normal volunteers matched for age and sex included as control subjects did not show any signs of acute infection or chronic disease (e.g., other autoimmune or atopic disorders).
Blood samples
Venous blood samples (15 ml) were collected from all participants aseptically into tubes with anti-coagulant. Peripheral blood mononuclear cells (PBMC) were prepared by Ficoll-Hypaque density gradient centrifugation (Amersham Biosciences) from heparinized blood for real-time polymerase chain reaction (PCR). Plasma was obtained after centrifugation and stored at −20°C for the measurement of IL-17A.
IL-17A Measurement
Concentration of IL-17A in plasma was measured by enzyme-linked immunosorbent assay following the manufacturer’s instructions using reagent kits of human IL-17A (purchased from Bender MedSystems Gmbh, BMS2017, Austria).
PBMC culture
PBMCs were cultured in RPMI 1640 (Gibco, Grand Island, NY) supplemented with 300 mg L-glutamine, 100 units/ml penicillin, 100 units/ml streptomycin (Sigma-Aldrich), and 10% fetal calf serum (Gibco). PBMCs from patients and healthy controls were incubated for 5 hours with 50 ng/ml PMA and 750 ng/ml ionomycin. Supernatants were then collected for later use.
Analysis of cytokine and transcription factor mRNA expression
Total RNA was extracted using TRIZOL® reagent (Invitrogen) according to manufacturer’s instructions. Complementary DNA (cDNA) samples were synthesized using random hexamer primers and RNase H-reverse transcriptase ((Fermentas)). The reaction system includes 2.5 μl of cDNA template, 12.5 μl of SYBR Green mix (Fermentas), and 8 μl of distilled H2O, 1 μl (10 μM) of each forward and backward primer set. Real-time PCR Detection System (Fermentas) was used for amplification and employed cycling program as follows: denaturation at 94°C for 2 min; then 40 cycles of denaturation, 15 s at 94°C; annealing 45 s at 59°C and extension 45 s at 72°C; and extension at 72°C for 10 min. The purity of PCR products was assessed by dissociation curve plots. Amplification plots were used to assign values for the “cycle threshold” (Ct) by SLAN software. The differences of gene expression in each sample were evaluated by 2−ΔΔCt, ΔΔCt = (Cttarget gene − Ctβ-actin) − (Ctnegative control − Ctβ-actin). The primer pairs used are shown in Table 2.
Table 2.
Primer pairs used for analysis.
| Gene | Forwar (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| RORγt | TGAGAAGGACAGGGAGCCAA | CCACAGATTTTGCAAGGGATCA |
| IL-17A | AAAGTGGCCCGGATGTGAGA | GACATTGTGCCCTGCCCTTCT |
| E-cadherin | TGCCCAGAAAATGAAAAAGG | AATGGCAGGAATTTGCAATC |
| ICAM-1 | ATCTGTGTCCCCCTCAAAAG | GGTCTCTATGCCCAACAACT |
| VCAM-1 | TACAACCGTCTTGGTCAGCC | CCACAGGATTTTCGGAGCA |
IL-17A – interleukin-17A; RORγt – retinoic acid-related orphan receptor γt; ICAM-1 – intercellular adhesion molecule-1; VCAM-1 – vascular cell adhesion molecule 1.
Flow cytometric analysis
PE-conjugated anti-IL-17 mAbs were purchased from eBiosciences (San Diego, CA). For Th17 detection, whole blood samples (200 μl) were activated with phorbol-12-myristate 13-acetate (PMA, 50 ng/ml) and ionomycin (1 μg/ml) for 5 h. After surface staining for CD3+CD4+ T cells and lysis of red blood cells, the remaining cells were permeabilized and stained with FITC-conjugated anti-human IFN-γ and PE-conjugated anti-human IL-17. The cells were fixed in 1% of paraformaldehyde and flow cytometric analyses were performed using FACSCalibur and CELLQuest software (Becton Dickinson, San Jose, CA).
Immunohistochemical staining
Seven patients with erythema nodosum (EN)-like eruption were studied [27]. Paraffin-embedded, formalin-fixed skin tissue lesions were cut into 5-μm sections and placed on polylysine-coated slides. Goat anti-human IL-17 (R&D Systems, Minneapolis, MN) and biotinylated donkey anti-mouse immunoglobulin (Ig) or biotinylated rabbit anti-goat-Ig (Becton Dickinson, Biopole Lab Tunisia) were used for IL-17 staining. The substrate was 3-amino-9-ethyl-carbazole (AEC) followed by counterstaining with hematoxylin for single staining. Quantitative evaluation of lymphocytes was done by analyzing 10 different high-powered fields (hpf, ×400) by two independent observers.
Statistical analysis
Data were tested for normal distribution using Kolmogorov–Smirnov test. Correlation was estimated by Pearson’s (r) correlation coefficient. Data were analyzed using the Statistical Package for the Social Sciences (SPSS) software (Advanced Statistics, version 17.0), Chicago, IL. A probable value of P<0.05 was considered to be statistically significant.
Results
Plasma IL-17A leve in BD and
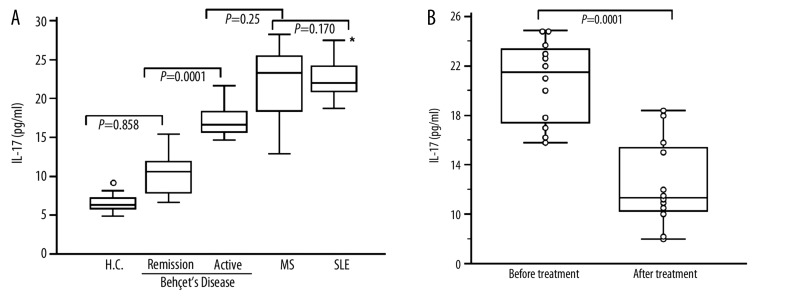
Plasma IL-17A level in BD patients, SLE patients, MS patients and normal controls were shown in (Figure 1A). Patients with active BD expressed similar level of IL-17A (20.94±3.96 pg/ml) compared to MS patients (22.45±4.21 pg/ml; P=0.25). However active BD patients expressed low IL-17A level when compared to SLE patients (24.26±3.02 pg/ml; P=0.0015). No significant difference was observed between MS and SLE patients (P=0.170). Significant differences were observed between active BD and remission BD (11.22±3.49 pg/ml; P=0.0001). No differences in IL-17 levels were found between healthy controls (11.045±4.33; pg/ml) and remission BD patients (P=0.858).
Figure 1.
Plasma IL-17A level in Behçet’s Disease (BS), systemic lupus erythematosus (SLE) patients, multiple sclerosis patients (MS) and healthy controls (HC). (A): Box indicating IL-17A level in active BD patients (n=45), remission BD stage (n=28), SLE patients (n=20), MS patients (n=12) and in HC (n=40). The control diseases (SLE and MS patients) were in active disease (B): Box plot indicating elevated levels of IL-17A in 12 BD patients studied respectively in active and in remission stages after 8 to 10 months of treatment. The medians are indicated by a line inside each box, the 25th and 75th percentiles by the box limits, the lower and upper error bars represent the 10th and 90th percentiles, respectively. (*): Comparison between active BD patients and SLE patients, (P=0.0015).
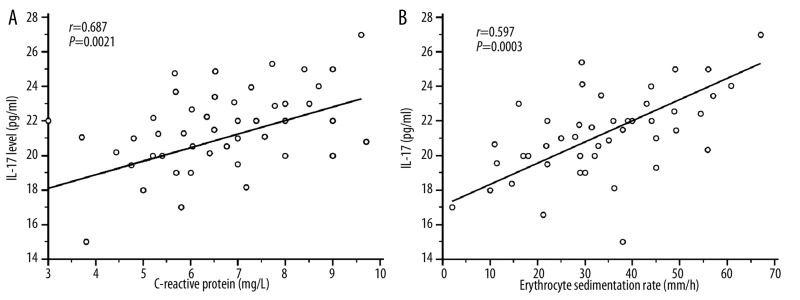
Twelve patients with BD were studied for IL-17 levels during active and remission stages (Figure 1B). These patients have oral ulcer, genital ulcer, ocular symptoms and arthritis. During their remission stage, they exhibited a decreased IL-17 level which contrasted with their IL-17A levels during the active stage: (active stage: 20.73±3.16 pg/ml; remission stage: 12.45±3.52 pg/ml; P=0.0001). None of these patients had vascular lesions, CNS involvement or pulmonary manifestation. This result indicates the role of IL-17 levels in the inflammatory process in BD as reported in other inflammatory diseases [28]. Significant correlation were observed between CRP (r=0.687; P=0.0021), ESR (r=0.597; P=0.003) and plasma IL-17 levels (Figure 2A, B).
Figure 2.
Correlation between plasma IL-17 levels in 45 active BD patients and biological parameters CRP and ESR using Pearson’s correlation coefficient. Erythrocyte sedimentation rate (ESR: mm/h) and C-reactive protein (CRP: mg/L) expressed as median (range) values in active BD patients were significantly increased in active BD patients [ESR: 35.76 (2–90); CRP: 107.5 (3–260)] compared to healthy controls [ESR: 6 (2–17); CRP: 3.2 (3–9); P<0.0001]. (A): Positive correlation between plasma IL-17A level and CRP (r=0.687; P=0.0021) in active Behçet’s disease (BD). (B): Significant positive correlation was observed between plasma IL-17A level and ESR (r=0.597; P=0.003).
Increased circulating Th17 cell frequencies are corrlelated with disease activity
To determine whether Th17 cells are present in BD (45 patients with active BD and 28 patients in remission BD), PBMC were isolated and stimulated with PMA and ionomycin in the presence of brefeldin A (Figure 3A, B). We compared the Th17 cell proportions in BD, in healthy controls and in control diseases (MS and SLE patients). There was a significantly higher frequency of circulating Th17 cells in active BD patients (2.87±1.2%) compared to healthy controls (0.64±0.37%; P=0.0001) and remission BD patients (0.75±0.37%; P=0.0001). No Significant differences were observed between active BD patients, MS patients (2.96±1.21%; P=0.52) and SLE patients (2.75±1.31; P=0.47). No difference was also observed between MS and SLE patients (P=0.663).
Figure 3.
Increased circulating Th17 frequency is correlated with Behçet’s diseases (BD) activity. (A): Representative figures of Th17 cells from a normal control and one active BD patient. CD4+IL-17+ Th17 were gated from CD3+ T cells. (B): Percentage of Th17/CD4+ cells from 45 active BD patients, 28 remission BD patients, 40 healthy controls (HC), 12 multiple sclerosis (MS) and 20 systemic lupus erythematosus (SLE) patients. The frequencies of Th17 cells in active BD patients and all two patient groups are significantly higher than for normal controls and remission BD patients. The medians are indicated by a line inside each box, the 25th and 75th percentiles by the box limits, the lower and upper error bars represent the 10th and 90th percentiles. (C1): Histologic feature of middle dermis of erythema nodosum (EN)-like eruption taken from active BD patient. Mononuclear cells are mainly infiltrated around and in the walls of vessels in the middle dermis (HE, 200×) [30]. Panels on the right show immunochemical histology of the EN-like eruption with a predominance of CD3+ lymphocytes around vessels. (C2): Lesional skin was further stained with IL-17. Bars =100 μm.
Th17 cell proportions in 12 BD patients were also compared in their active and remission stages for circulating Th17 cell frequencies. There was a significantly higher frequency of circulating Th17 cells in active BD patients (2.43±0.97%) compared to the same patients in remission stage (0.86±0.52%; P=0.0001).
Using immunohistochemistry, we analyzed erythema nodosum (EN)-like eruption from 7 patients with active BD (Figure 3C) compared to controls (5 biopsy samples removed for other reasons from patients with non specific inflammation). EN-like eruption samples from active BD exhibited typical pathologic changes, with a large number of CD3+ T cells infiltrating, especially around vessels (Figure 3C1). To better understand these CD3+ T cells from EN-like eruption samples, staining with antibodies to IL-17A was performed. The results confirmed the presence of an important population of IL-17+ cells infiltrating the BD skin lesions (26.8±2.7%) compared to control biopsies (0.6±0.13%; P=0.001) (Figure 3C2).
These data indicated that Th17 cells are present among PBMCs and at sites of inflammation, supporting the high levels of plasma IL-17 levels in active BD, as observed in our control inflammatory diseases SLE and MS patients.
Relative expression of IL-17 and RoR-γt from PBMC
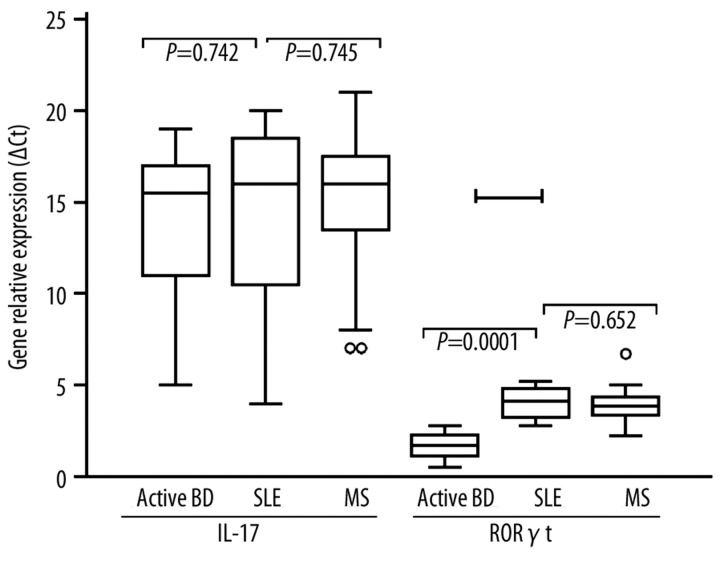
There were no significant differences in the mean IL-17 mRNA between active BD patients (15.9±3.4), MS (16.7±8.5) and SLE (16.4±6.3) patients (Figure 4). RoR-γt (RORC) mRNA was expressed in active BD patients (1.78±0.72; P=0.0001) at lower levels than MS (4.5±0.63) and SLE (4.3±0.52) patients. No significant difference was observed between MS and SLE patients (P=0.0652).
Figure 4.
Relative expression of IL-17 and RoR-γt from PBMC of Behçet’s Disease (BD). Relative expression of IL-17 and RoR-γt as indicated on graphs, was calculated as described in methods. Expression of IL-17 and RORγt mRNA expression in BD patients, MS and SLE patients. Box with whiskers represent the interquartile range and extremes of ΔCt values, horizontal line within box represents median.
Gene relative expression (ΔCt) for IL-17 and RORC mRNA were significantly increased in active BD patients when compared to healthy controls (IL-17 mRNA: 3.82±1.63; RORC mRNA: 0.82±0.17; P=0.001) and to remission BD patients (IL-17 mRNA: 4.32±0.57; RORC mRNA: 0.93±0.23; P=0.001).
Th-17 cytokine-mediated expression of adhesion molecule mRNA
To determine whether IL-17A derived from patients with active BD can induced increased expression of adhesion molecule mRNA, we prepared sera from patients with active BD and supernatants from culture of stimulated PBMC from patients with active BD, and compared their effects on the gene expression of adhesion molecules in HUVECs.
PBMCs from patients with active BD (n=40), with MS patients (n=12) and with SLE patients (n=20) and control subjects (n=12) were stimulated for 5 hours with phorbol myristate acetate and ionomycin, and supernatants were collected to detect IL-17A by enzyme-linked immunosorbent assay (Figure 5A). IL-17A secretion from stimulated PBMC from patients with active BD was increased (1092±270 pg/ml) compared with that in samples from healthy controls (580±178 pg/ml; P=0.0001). No significant difference was observed between active BD and MS patients (1130±270 pg/ml; P=0.685). However low significant difference was observed when active BD were compared to SLE patients (1500±310 pg/ml; P=0.0048).
Figure 5.
Up-regulation of adhesion molecule mRNA expression in human umbilical vein endothelial cells (HUVECs) by interleukin-17A (IL-17A) derived from patients with active Behçet’s disease (BD). (A): PBMCs from patients with active BD (n=20), MS (n=12), SLE (n=20) and control subjects (n=12) were stimulated for 5 hours with phorbol myristate acetate and ionomycin, and supernatants were collected to detect IL-17A by enzyme-linked immunosorbent assay. (B): Quantitative reverse transcription-polymerase chain reaction was performed to determine the expression of adhesion molecule mRNA in HUVECs induced by supernatants from active BD patients from the culture of peripheral blood mononuclear cells (PBMCs) derived from patients with active BD (n=10).
Quantitative reverse transcription-polymerase chain reaction was performed to determine the expression of adhesion molecule mRNA in HUVECs induced by the sera from patients with active BD or supernatants from the culture of peripheral blood mononuclear cells (PBMCs) derived from patients with active BD (n=10). The supernatants from culture of PBMC derived from patients with active BD or sera from patients with active BD could promote expression of mRNA for E-cadherin, intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in HUVECs (Figure 5B). Neutralization of IL-17A in culture medium was able to inhibit the expression of adhesion molecule mRNA. Our data indicate that HUVEC may be responsive to stimulation of IL-17A produced by PBMC derived from active BD patients.
Discussion
Th17 cells constitute the third effector arm of Th cells, complementing the Th1 and Th2 lineages. The secretion of IL-17 is a primary defining characteristic of Th17 cells. The orphan nuclear receptor RORγt/RORC2 (mice/humans) is the master transcription factor that can drive Th17 cell differentiation in human. We have found that plasma IL-17 level was significantly increased in active BD, MS and SLE patients. Our data showed that patients with active BD exhibit an increased proportion of Th17 cells compared with remission BD and healthy individuals. IL-17 level was associated with disease activity. A positive correlation was found between CRP, ESR and plasma IL-17 level in active BD patients, which suggested that Th17 cells may sustain inflammation in these patients. Because we detected enhanced IL-17A production in single T cells from patients with active BD, we next examined in vivo concentrations of IL-17A in BD, which was produced at higher levels in patients with active disease. Taken together, these data demonstrate that both the proportion of Th17 cells and the ability to produce IL-17A are enhanced in the setting of active BD despite low expression of RoR-γt mRNA (compared to MS and SLE patients), suggesting that the population of Th17 cells might be expanded as a result of disease activity. The increased plasma IL-17 level and circulating Th17 cell frequencies in active BD patients was supported by data obtained at the molecular levels (IL-17 mRNA and RORC mRNA) indicating an inflammatory conditions as observed in our control diseases MS and SLE patients.
The inflammatory process observed in active BD was corroborated by the presence of multitude inflammatory mediators in the peripheral circulation and in the inflammatory sites [29–33]. Taken together, these data suggest that expansion of the Th17 cell population is related to distinct cytokine milieu in active BD, confirming the recent finding that the IL-23-IL-17 axis is important for the inflammatory reaction in BD [31]. Increased mRNA expression and protein production of IFN-γ was detected in patients with active BD [4]. IFN-γ might promote the expansion of Th17 cells through the generation of an inflammatory milieu in humans [34].
Previous findings supported the notion that Th1 cytokines could play an important role in inflammation and tissue injury and are correlated with active BD [29,35]. Th17 cells also have a specific role in immune function through coordinated effector cytokine action [36]. IL-17A is a key cytokine produced by Th17 cells that can induce the expression of adhesion molecules in several cell lines [37] and our findings indicate that Th17 cell numbers are correlated with BD disease activity. We hypothesized that Th17 cells might contribute to inflammation due to increased secretion of IL-17A.
Our data showed that increasing gene expression of ICAM-1, VCAM-1, and E-cadherin in HUVECs was induced by supernatants of stimulated PBMCs derived from patients with active BD. Treatment with antibodies to IL-17A suppressed the production of adhesion molecules. Taken together, these data emphasized that IL-17A was present in the sera of patients with active BD, and that the level was elevated in culture supernatants of stimulated PBMCs from these patients. These results suggest that microenvironmental Th17 cells and IL-17A might be involved in inflammation in active BD. Although a substantial population of IL-17+ cells could be detected in skin lesion from patients with active BD, and IFNγ cells infiltrated to a lesser extent [38–40]. We should not conclude that Th1 cells were not implicated in such tissue inflammation. Both the Th17 and Th1 cytokines might be involved in the pathogenesis of active BD albeit via different mechanisms. Recent studies showed that Th17 and Th1 cells might be involved at different stages during the development of the inflammation [41]. Th17 cells might be generated more rapidly than Th1 cells during inflammation. Through the potent induction of chemokines and adhesion molecules, Th17 cells might attract Th1 cells to sites of inflammation at later stages of the inflammation process, which could further propagate inflammation and tissue damage [33,39,42,43]. However, sequential involvement and different functions of Th17 and Th1 cells during the development of BD have not been demonstrated. Given that Th17 cells appear at sites of inflammation at the early stage, it is meaningful to inhibit the inflammation by blocking the function of Th17 cells, which might delay or block the subsequent recruiting of Th1 cells.
The decreased levels of Th17 in remission BD compared to active BD could be explained by a probable conversion of Th17 into regulatory T (Treg) cells. Several recent studies have indicated the existence of a close interplay between Treg and Th17 cells in regulating some autoimmune conditions [44]. Differentiation of Treg into Th17 cells involved down-regulation of Foxp3 expression and suppressor functions. Foxp3 has been suggested to inhibit Th17 differentiation by antagonizing RORγt function [45].
Conclusions
We cannot draw conclusions regarding the observed expansion of the Th17 cell population as a function of disease flare. Regardless of whether this is a cause or a consequence, the expansion of Th17 cells is related to distinct cytokine environments in active BD that influence the extent inflammation within tissues. IL-17A from patients with active BD can elevate the expression of adhesion molecule mRNA and induces adherence of T cells to HUVEC, which underscores the role of Th17 cells and IL-17A-induced adhesion molecule signaling in vascular inflammation in active BD. Understanding the intricate regulatory mechanisms that govern T cell subsets at the molecular level in BD as well as in inflammatory/autoimmune diseases, will undoubtedly yield therapies for dysregulated immune responses.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Source of support: This work was supported by a grant from the “Minister of Science and Technology of Tunisia”. UR/99/08-40
References
- 1.Yazici H, Yurdakul S, Hamuryudan, Fresko V. Behçet’s syndrome. In: Hochberg MC, Silman JA, Smolen JS, et al., editors. Rheumatology. London: Mosby; 2003. pp. 1665–69. [Google Scholar]
- 2.Onder M, Gurer MA. The multiple faces of Behçet’s disease and its etiological factors. J Eur Acad Dermatol Venereol. 2001;15:126–36. doi: 10.1046/j.1468-3083.2001.00251.x. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki N, Nara K, Suzuki T. Skewed Th1 responses caused by excessive expression of Txk, a member of the Tec family of tyrosine kinases, in patients with Behcet’s disease. Clin Med Res. 2006;4:147–51. doi: 10.3121/cmr.4.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamzaoui K, Hamzaoui A, Guemira F, et al. Cytokine profile in Behçet’s disease patients. Relationship with disease activity. Scand J Rheumatol. 2002;31:205–10. doi: 10.1080/030097402320318387. [DOI] [PubMed] [Google Scholar]
- 5.Saruhan-Direskeneli G, Yentur SP, Akman-Demir G, et al. Cytokines and chemokines in neuro-Behçet’s disease compared to multiple sclerosis and other neurological diseases. J Neuroimmunol. 2003;145:127–34. doi: 10.1016/j.jneuroim.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 6.Pène J, Chevalier S, Preisser L, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180:7423–30. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 7.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 8.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–57. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–73. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 11.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–77. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 12.Molet S, Hamid Q, Davoine F, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–38. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 13.Wong CK, Ho CY, Ko FW, et al. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol. 2001;125:177–83. doi: 10.1046/j.1365-2249.2001.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–52. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chabaud M, Durand JM, Buchs N, et al. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–70. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 16.Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andoh A, Takaya H, Makino J, et al. Cooperation of interleukin-17 and interferon-gamma on chemokine secretion in human fetal intestinal epithelial cells. Clin Exp Immunol. 2001;125:56–63. doi: 10.1046/j.1365-2249.2001.01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kao CY, Chen Y, Thai P, et al. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol. 2004;173:3482–91. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 19.Faour WH, Mancini A, He QW, Di Battista JA. T-cell-derived interleukin-17 regulates the level and stability of cyclooxygenase-2(COX-2) mRNA through restricted activation of the p38 mitogen-activated protein kinase cascade: role of distal sequences in the 3′-untranslated region of COX-2 mRNA. J Biol Chem. 2003;278:26897–907. doi: 10.1074/jbc.M212790200. [DOI] [PubMed] [Google Scholar]
- 20.Shimada M, Andoh A, Hata K, et al. IL-6 secretion by human pancreatic periacinar myofibroblasts in response to inflammatory mediators. J Immunol. 2002;168:861–68. doi: 10.4049/jimmunol.168.2.861. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Chu Y, Yang X, et al. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1472–83. doi: 10.1002/art.24499. [DOI] [PubMed] [Google Scholar]
- 23.International Study Group for Behçet’s disease. Criteria for diagnosis of Behçet’s disease. Lancet. 1990;335:1078–80. [PubMed] [Google Scholar]
- 24.Lawton G, Bhakta BB, Chamberlain A, Tenant A. The Behçet’s disease activity index. Rheumatol. 2004;43:73–78. doi: 10.1093/rheumatology/keg453. [DOI] [PubMed] [Google Scholar]
- 25.Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 26.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–27. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 27.Hamzaoui K, Houman H, Hamzaoui A. Serum BAFF levels and skin mRNA expression in patients with Behçet’s disease. Clin Exp Rheumatol. 2008;26(4 Suppl 50):S64–71. [PubMed] [Google Scholar]
- 28.Smith E, Prasad KM, Butcher M, et al. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121(15):1746–55. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raziuddin S, Al-Dalaan A, Bahabri S, et al. Divergent cytokine production profile in Behcet’s disease: altered Th1/Th2 cell cytokine pattern. J Rheumatol. 1998;25:329–33. [PubMed] [Google Scholar]
- 30.Hamzaoui K, Borhani Haghighi A, Ben Ghorbel I, Houman H. RORC and Foxp3 in cerebrospinalk fluid of patients with neuro-Behçet’s disease. J Neuroimmunol. 2011 doi: 10.1016/j.jneuroim.2011.01.012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Chi W, Zhu X, Yang P, et al. Upregulated IL-23 and IL-17 in Behçet patients with active uveitis. Invest Ophthalmol Vis Sci. 2008;49:3058–64. doi: 10.1167/iovs.07-1390. [DOI] [PubMed] [Google Scholar]
- 32.Kulaber A, Tugal-Tutkun I, Yentür SP, et al. Pro-inflammatory cellular immune response in Behçet’s disease. Rheumatol Int. 2007;27(12):1113–18. doi: 10.1007/s00296-007-0367-9. [DOI] [PubMed] [Google Scholar]
- 33.Dilek K, Ozçimen AA, Saricaoǧlu H, et al. Cytokine gene polymorphisms in Behçet’s disease and their association with clinical and laboratory findings. Clin Exp Rheumatol. 2009;27:S73–78. [PubMed] [Google Scholar]
- 34.Jandus C, Bioley G, Rivals JP, et al. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58:2307–17. doi: 10.1002/art.23655. [DOI] [PubMed] [Google Scholar]
- 35.Alayli G, Aydin F, Coban AY, et al. T helper 1 type cytokines polymorphisms: association with susceptibility to Behçet’s disease. Clin Rheumatol. 2007;26:1299–305. doi: 10.1007/s10067-006-0503-z. [DOI] [PubMed] [Google Scholar]
- 36.Steinman L. A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–45. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 37.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 38.Melikoglu M, Uysal S, Krueger JG, et al. Characterization of the divergent wound-healing responses occurring in the pathergy reaction and normal healthy volunteers. J Immunol. 2006;177:6415–21. doi: 10.4049/jimmunol.177.9.6415. [DOI] [PubMed] [Google Scholar]
- 39.Aridogan BC, Yildirim M, Baysal V, et al. Serum Levels of IL-4, IL-10, IL-12, IL-13 and IFN-gamma in Behçet’s disease. J Dermatol. 2003;30:602–7. doi: 10.1111/j.1346-8138.2003.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 40.Keller M, Spanou Z, Schaerli P, et al. T cell-regulated neutrophilic inflammation in autoinflammatory diseases. J Immunol. 2005;175:7678–86. doi: 10.4049/jimmunol.175.11.7678. [DOI] [PubMed] [Google Scholar]
- 41.Korn T, Reddy J, Gao W, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dalghous AM, Freysdottir J, Fortune F. Expression of cytokines, chemokines, and chemokine receptors in oral ulcers of patients with Behcet’s disease (BD) and recurrent aphthous stomatitis is Th1-associated, although Th2-association is also observed in patients with BD. Scand J Rheumatol. 2006;35:472–75. doi: 10.1080/03009740600905380. [DOI] [PubMed] [Google Scholar]
- 43.Imamura Y, Kurokawa MS, Yoshikawa H, et al. Involvement of Th1 cells and heat shock protein 60 in the pathogenesis of intestinal Behcet’s disease. Clin Exp Immunol. 2005;139:371–78. doi: 10.1111/j.1365-2249.2005.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deknuydt F, Bioley G, Valmori D, Ayyoub M. IL-1beta and IL-2 convert human Treg into T(H)17 cells. Clin Immunol. 2009;131:298–307. doi: 10.1016/j.clim.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Zhou L, Lopes JE, Mark MW, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]