Summary
Background
There is evidence that myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is characterized by activation of immune, inflammatory, oxidative and nitrosative stress (IO&NS) pathways. The present study was carried out in order to examine whether ME/CFS is accompanied by increased levels of plasma peroxides and serum oxidized LDL (oxLDL) antibodies, two biomarkers of oxidative stress.
Material/Methods
Blood was collected from 56 patients with ME/CFS and 37 normal volunteers. Severity of ME/CFS was measured using the Fibromyalgia and Chronic Fatigue Syndrome (FF) Rating Scale.
Results
Plasma peroxide concentrations were significantly higher in patients with ME/CFS than in normal controls. There was a trend towards significantly higher serum oxLDL antibodies in ME/CFS than in controls. Both biomarkers contributed significantly in discriminating between patients with ME/CFS and normal controls. Plasma peroxide and serum oxLDL antibody levels were both significantly related to one of the FF symptoms.
Conclusions
The results show that ME/CFS is characterized by increased oxidative stress.
Keywords: myalgic encephalomyelitis, chronic fatigue syndrome, CFS, inflammation, oxidative stress, antioxidantsas a marker of oxidative stress
Backgroiund
There is evidence that myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is accompanied by disorders in inflammatory, oxidative and nitrosative stress (IO&NS) pathways. We have discussed elsewhere that an increased production of intracellular inflammatory mediators, like nuclear factor κB (NFκB), and consequently of cyclo-oxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) are key factors in ME/CFS [1–4]. The inflammatory response in ME/CFS may explain the various findings on increased levels of pro-inflammatory cytokines ([5)]; immune activation, with Th-1-like or Th-2-like responses and an increased expression of activation markers, e.g. CD38 ([6)]; ex vivo immunossuppression as exemplified by lowered natural killer cell activity and decreased expression of activation markers, e.g. CD69 ([7,8)]; dysregulation of the 2″–5″ oligoadenylate synthetase/RNase L pathway ([9)]; mitochondrial dysfunctions ([10,11)] and even apoptosis pathways ([12,13)].
Inflammatory reactions, like the increased production of NFκB, iNOS and cytokines may instigate the O&NS pathways [3,4]. Increased isoprostane, thiobarbituric acid reactive substances (TBARS), protein carbonyl levels, and urinary excretion of 8-OH-deoxyguanosine suggest that ME/CFS is accompanied by increased O&NS and that fatty acids, lipids and DNA are damaged by oxidation [14–18]. An increased production of nitric oxide and peroxynitrite may cause damage to proteins by nitration and nitrosylation, as reactive oxygen and nitrogen species (ROS/RNS) attack fatty acids, proteins, and mitochondria and mitochondrial DNA (mtDNA). The latter may cause mutagenic mtDNA lesions and accumulations of these lesions [19]. ME/CFS has been shown to be accompanied by mitochondrial damage [10,11,20,21] and mitochondrial dysfunctions and structural changes [22,23], which is important in that these mitochondrial and mtDNA lesions may cause lowered activity of the mitochondrial respiratory chain, which produces ATP and accounts for 98% of cellular energy [19].
In addition to damaging cells and tissues, increased O&NS can cause an autoimmune response [4,24–27]. During oxidation and nitration, the chemical structures of self epitopes may be changed to generate new epitopes, or neoepitopes, which are highly immunogenic ([4,24–27)]. Thus, oxidation of fatty acid autoepitopes or membrane lipids may generate neoepitopes that are no longer hidden from the immune system. Similarly, during nitration of proteins, neoepitopes may be formed which are strongly immunogenic, such as nitrotyrosine (NO-tyrosine) [24]. Following the initial damage, the immune system may mount an IgG or IgM-mediated autoimmune response against these epitopes. There is evidence that ME/CFS is accompanied by an IgM-mediated autoimmune response against membrane fatty acids, like oleic, palmitic and myristic acid; by-products of lipid peroxidation, such as malondialdehyde (MDA) and azelaic acid; and functional lipid structures, such as phosphatidyl inositol (Pi) [25,26]. ME/CFS is also accompanied by a mounted IgM-mediated autoimmune responses against NO-derivates, like nitro-tyrosine, nitro-phenylalanine, nitro-arginine, nitro-tryptophan, nitro-cysteinyl and NO-albumin [25,27].
The aim of the present study was to examine two biomarkers of O&NS:: plasma peroxides and serum oxidized low density lipoprotein (oxLDL) IgG antibodies. Peroxides are one type of ROS that can be found in peripheral blood that indicates the presence of oxidative stress. Increased oxLDL IgG autoantibodies are a footprint for lipid peroxidation and the consequent immune responses that take place in vivo.
Material and Methods
Subjects
Ninety-three subjects participated in the present study, 56 ME/CFS patients and 37 normal controls.
All subjects with ME/CFS were outpatients admitted to the Maes Clinics, Antwerp, Belgium. Subjects with a life-time diagnosis of psychiatric disorders, according to the DSM-IVR [28], including depression, bipolar disorder, anxiety disorders, psychotic and organic mental disorders were excluded from this study.
Additional exclusionary criteria for study participation included any subjects who: a) had been treated with anti-psychotic drugs, anticonvulsants or mood stabilizers; b) had medical illnesses, such as inflammatory bowel disorders, diabetes type 1 or type 2, hypertension, and arteriosclerosis; c) abnormal blood tests, e.g. thyroid stimulating hormone (TSH), total protein and positive IgM antibody titers for EBV or CMV; d) had acute infections within two months of the study; e) were treated with statins and beta-blockers; and f) had been taking dietary supplements with antioxidants. Patients and controls gave written informed consent after the study protocol was fully explained. The study was approved by the local ethical committee.
The diagnosis “ME/CFS” was made using the Centres for Disease Control and Prevention (CDC) criteria [29]. The severity of ME/CFS was measured by means of the Fibromyalgia and Chronic Fatigue Syndrome Rating Scale (FF scale) [30]. This scale measures pain, muscular tension, fatigue, concentration difficulties, failing memory, irritability, sadness, sleep disturbances, autonomic disturbances, irritable bowel, headache, and subjective experience of infection. The total sum on this scale was employed as a measure of the severity of illness. The total sum on the FF scale is computed as a measure for severity of illness. All diagnostic assessments in the patients were carried out by physicians. The normal controls were recruited from laboratory personnel or family members of the personnel.
Methods
Fasting blood was sampled between 8.30 a.m. and 11.30 a.m. for the assay of peroxides and oxLDL antibodies. Plasma peroxide levels were determined by means of the colorimetric assay Oxystat (Biomedica Medizinproducte GmBH & Co KG, A-1210 Wien) for the quantitative determination of peroxides in EDTA plasma (Cat No BI-5007). This method is based on the reaction of the biological peroxides with the enzyme peroxidase and a subsequent color reaction using tetra-methyl-benzidine (TMB) as substrate. After addition of a stop solution, the developed color is measured photometrically at 450 nm. A calibrator is measured in parallel and used to calculate the concentration of circulating biological peroxides in the sample, in a one point calibration protocol. The detection limit of our assay is 7 μmol/l and the interassay coefficient of variation 5.1%. The oxLDL antibodies were measured by means of the enzyme immunoassay (EIA) for the quantitative determination of human IgG autoantibodies to oxLDL, Biomedica Medizinproducte GmbH & Co (A-1210 Wien, Austria; Cat. no: BI-20032; 12×8 tests; conventional 96-well ELISA format). This assay is based on a microtiter plate solid phase which is coated with oxLDL after which diluted samples and calibrators are added to the microtiter plate wells, incubated for 1.5 hours at 37°C, washed, incubated 30 minutes at room temperature with the conjugate i.e. a monoclonal anti-human IgG-HRPO, washed again after incubation an reacted for 15 minutes with TMB substrate. The absorbance measured at 450 nm is proportional to the amount of oxLDL antibodies in the sample or calibrator. The detection limit of this assay is 48 mU/ml, while the standard range is between 37–1200 mU/ml and the interassay coefficient of variation is 4.0%.
Statistics
Analysis of variance (ANOVA) or covariance (ANCOVA) were employed to analyze the differences between group means. Multiple comparisons between group means were assessed by means of Least Significant Difference (LSD) analysis. In order to assess the biological differences between the diagnostic groups we employed a stepwise linear discriminant analysis (LDA) with an F-to-enter of p=0.05. Relationships between variables were computed by Pearson’s product-moment correlation coefficient or linear regression analysis. Associations between classification systems were checked by means of analysis of contingence tables (χ2-test) and Fisher’s exact probability test. The diagnostic performance of the oxidative stress variables was computed by means of receiver operating characteristics (ROC) analysis with computation of the area under the ROC curve, sensitivity, specificity and predictive value of a positive test result (PV+) and with kappa statistics. In order to normalize the data distribution of the oxidative data a Box-Cox transformation was used where necessary. The significance was set at p=0.05 (two tailed).
Results
Table 1 shows the mean age, the gender ratio, and the mean and median peroxide and oxLDL antibody values in both patients and controls. There were no significant differences in age between normal controls and ME/CFS patients. In the total study group, we found no significant correlations between age and plasma peroxides (r=−0.07, p=0.50) and age and serum oxLDL antibodies (r=−0.18, p=0.09). There were more females than males in the study sample of ME/CFS patients compared to the normal controls. In the total study group, women (mean=527.5±363.2 μmol/L) had significantly higher (F=13.6, df=1/86, p=0.0007) plasma peroxide levels than men (mean=177.9±81.7 μmol/L). There were no significant differences (F=0.14, df=1/86, p=0.7) in serum oxLDL antibodies between women (mean=373.8±371.0 mU/mL) and men (mean=335.8±293.1 mU/mL). In order to adjust for possible effects of age and gender on the results, ANCOVAs were carried out with age as an independent variable and sex as a second factor. In addition, correlation analyses on plasma peroxides were performed on the residualized peroxide values after partialling out the effects of sex by means of regression analysis (residualized peroxide values). The oxLDL values were assessed in Box-Cox transformation. Table 1 shows the mean (±SD) of the untransformed peroxide and oxLDL antibody levels as well as the median values with corresponding q25 and q75 values.
Table 1.
Measurements of age and gender ratio, and plasma peroxide and serum oxidized LDL (oxLDL) antibody levels in patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) versus controls.
| Variables | Controls | ME/CFS | F or χ2 | Df | P |
|---|---|---|---|---|---|
| Age | 42.5 (11.4)* | 38.2 (14.0)* | 2.5 | 1/91 | 0.10 |
| Female/Male Ratio | 25/12 | 50/6 | 5.4 | 1 | 0.02 |
| Peroxides (mol/L) | 349.9 (246.3)* 300 (176–379)** |
550.9 (398.9)* 465 (286–663)** |
– | – | – |
| oxLDL (mU/mL) | 270.3 (297.7)* 167 (116–237)** |
424.9 (378.9)* 302 (122–540)** |
– | – | – |
Results shown as mean (±SD) values;
Results shown as median values (q25 – q75 values).
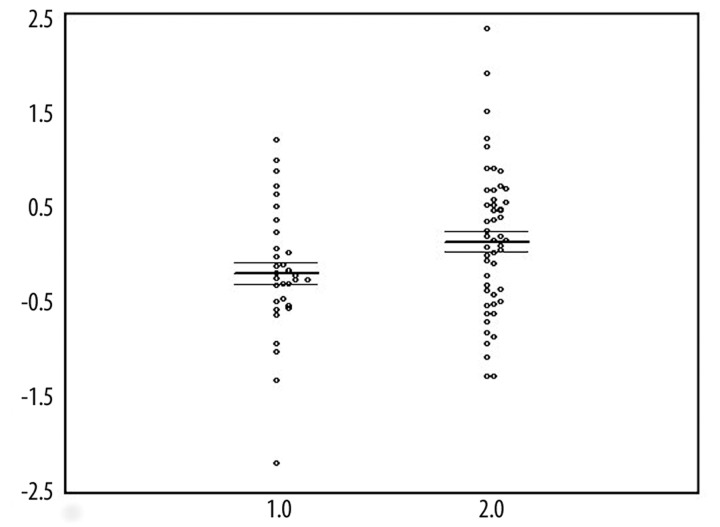
Figure 1 shows that the residualized plasma peroxide levels are significantly higher in ME/CFS patients than in normal controls. A factorial ANCOVA with diagnosis and gender as factors and age as covariate showed significantly higher peroxide values in ME/CFS patients than in controls (F=5.48, df=1/83, p=0.02). There were significant gender differences (F=17.9, df=1/83, p=0.0001), and no significant diagnosis X sex interaction (F=0.91, df=1/83, p=0.70) and no significant effects of age (F=0.32, p=0.6). Least Significant Difference (LSD) analysis at p<0.05 showed that the plasma peroxide levels were significantly higher in ME/CFS males and females as compared to their healthy counterparts. The residualized plasma peroxide values showed no significant diagnostic performance for ME/CFS, where the area under the ROC curve was only 62.6%.
Figure 1.
Scatter plot of the residualized peroxide values (after correction for gender; in mol/L and in Box-Cox transformation) in normal controls (1.0) and ME/CFS patients (2.0).
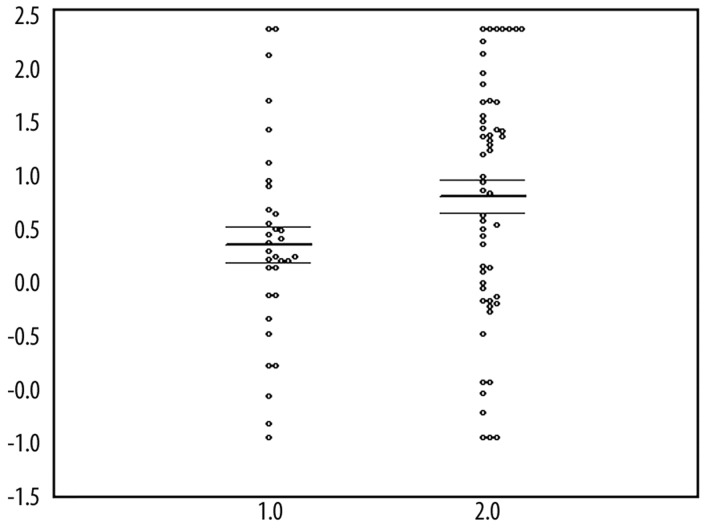
Figure 2 shows that there is a trend towards higher oxLDL antibodies in ME/CFS patients than in normal controls. A factorial ANCOVA with diagnosis and gender as factors and age as covariate showed no significant differences in serum oxLDL antibodies between ME/CFS and controls (F=1.8, df=1/83, p=0.2), no significant gender (F=0.1, df=1/83, p=0.70) and age (F=2.5, df=1/83, p=0.09) effects, and no significant interaction between diagnosis and gender (F=1.8, p=1/83, p=0.20). The serum oxLDL antibodies showed no significant diagnostic performance for ME/CFS, where the area under the ROC curve was only 62.7%.
Figure 2.
Scatter plot of the oxidized LDL antibodies (in mU/mL and in Box-Cox transformation) in normal controls (1.0) and ME/CFS patients (2.0).
Using LDA, we found that both the residualized plasma peroxide and oxLDL antibodies were significantly discriminating variables (χ2=9.15, F=4.71, df=2/160, p=0.01). There was only one FF symptom that correlated with the oxidative stress markers, i.e. headache. We found significant and positive correlations between headache and the residualized peroxide (r=0.27, p=0.05) and oxLDL antibody (r=0.34, p=0.01) values. There was a weak albeit significant correlation between the residualized plasma peroxide and serum oxLDL antibodies (r=0.27, p=0.01).
Discussion
The major finding of this study is that patients with ME/CFS have significantly increased peroxide levels compared to normal controls. This indicates increased ROS and oxidative stress in ME/CFS. As such the results corroborate earlier findings that ME/CFS is accompanied by induction of O&NS pathways. These data also extend previous studies that show ME/CFS is accompanied by a lowered antioxidant status. A significantly lowered antioxidant capacity may impair the defenses against ROS/RNS and O&NS [31]. Recently, it was reported that ME/CFS is accompanied by lowered levels of serum/plasma zinc, dehydroepiandrosterone, coenzyme-Q10, and vitamin E, which are all strong antioxidants [31–34)]. This may not only cause increased O&NS and the consequent damage to lipids, proteins and DNA, but also a decreased antiinflammatory capacity in the body, because these three antioxidants are known to reduce the production of NFκB, COX-2 and proinflammatory cytokines [31–33]. The results of the present study are also in agreement with animal models of ME/CFS, e.g. the forced swimming test and administration of LPS to mice. Thus, forced swimming (one 6-minute session a day during 7–15 days) induces ROS and RNS and decreases antioxidant defenses [35–37]. Administration of LPS to mice induces a behavior complex, characterized by an increased immobility period, post swim fatigue and thermal hyperalgesia, which is associated with increased O&NS and reduced antioxidant levels [38].
In the present study we were unable to find significant differences in serum oxLDL antibodies between ME/CFS patients and normal controls. At first sight, these findings do not corroborate previous findings on increased IgM-mediated autoimmune responses against neoepitopes formed by oxidative damage to fatty acids [25]. First, the oxLDL antibodies measured here are of the IgG subtype, whereas the reactions against neoepitopes of Pi, and oleic, palmitic and myristic acid are IgM mediated [25]. Secondly, the latter are typical membrane fatty acids/lipids which reside on the inner and outer membrane layers, whereas the oxLDL antibodies are directed against LDL particles in the cardiovascular system. Interestingly, we found a weak albeit significant correlation between plasma peroxide concentrations and serum oxLDL antibodies. This may be explained since lipid peroxidation (as indicated by increased oxLDL antibodies) is induced by increased ROS (as indicated by increased plasma peroxide levels).
We found that only one of the 12 FF symptoms was significantly correlated with the oxidative markers, i.e. headache. Previous research on the other hand observed significant correlations between different key symptoms of ME/CFS and oxidative markers or antioxidant levels. For example, aches and pain, muscular tension and fatigue were significantly correlated to decreased antioxidant defenses [14], and increased serum IgM levels directed against oxidatively modified lipids [25]. Jammes et al. [16] reported that O&NS is a causal factor in fatigue, pain and muscle tension. These authors found that the response to incremental exercise in patients with ME/CFS associates increased O&NS with marked alterations of muscle membrane excitability.
It is interesting to note that there is a strong co-occurrence of ME/CFS and depression and that shared disorders in O&NS pathways may underpin this co-occurrence ([39)]. Thus, lower levels of both zinc and coenzyme Q10, and oxidative damage to fatty acids, proteins and DNA are hallmarks of both ME/CFS and depression ([39)]. Recently we found significantly increased peroxide levels and oxLDL antibody levels in depression ([40)]. This indicates that while there are similarities in O&NS markers between ME/CFS and depression, oxLDL antibodies are more strongly associated with depression that with ME/CFS.
Conclusions
In conclusion, the results of the present study show that plasma peroxide concentrations are significantly increased in ME/CFS, whereas serum oxLDL antibodies showed a trend toward increased levels in ME/CFS. These findings further underscore that O&NS pathways are involved in the pathophysiology of ME/CFS.
Footnotes
Source of support: Self financing
References
- 1.Maes M, Mihaylova I, Bosmans E. Not in the mind of neurasthenic lazybones but in the cell nucleus: patients with chronic fatigue syndrome have increased production of nuclear factor kappa beta. Neuro Endocrinol Lett. 2007;28:456–62. [PubMed] [Google Scholar]
- 2.Maes M, Mihaylova I, Kubera M, Bosmans E. Not in the mind but in the cell: increased production of cyclo-oxygenase-2 and inducible NO synthase in chronic fatigue syndrome. Neuro Endocrinol Lett. 2007;28(4):463–69. [PubMed] [Google Scholar]
- 3.Maes M. Inflammatory and oxidative and nitrosative stress pathways underpinning chronic fatigue, somatization and psychosomatic symptoms. Curr Opin Psychiatry. 2009;22:75–83. doi: 10.1097/yco.0b013e32831a4728. [DOI] [PubMed] [Google Scholar]
- 4.Maes M, Twisk FN. Chronic fatigue syndrome: Harvey and Wessely’s (bio)psychosocial model versus a bio(psychosocial) model based on inflammatory and oxidative and nitrosative stress pathways. BMC Medicine. 2010;8:35. doi: 10.1186/1741-7015-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fletcher MA, Zeng XR, Barnes Z, et al. Plasma cytokines in women with chronic fatigue syndrome. J Transl Med. 2009;7:96. doi: 10.1186/1479-5876-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klimas NG, Salvato FR, Morgan R, Fletcher MA. Immunologic abnormalities in chronic fatigue syndrome. J Clin Microbiol. 1990;28(6):1403–10. doi: 10.1128/jcm.28.6.1403-1410.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maher KJ, Klimas NG, Fletcher MA. Chronic fatigue syndrome is associated with diminished intracellular perforin. Clin Exp Immunol. 2005;142(3):505–11. doi: 10.1111/j.1365-2249.2005.02935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mihaylova I, DeRuyter M, Rummens JL, et al. Decreased expression of CD69 in chronic fatigue syndrome in relation to inflammatory markers: evidence for a severe disorder in the early activation of T lymphocytes and natural killer cells. Neuro Endocrinol Lett. 2007;28(4):477–83. [PubMed] [Google Scholar]
- 9.Nijs J, De Meirleir K. Impairments of the 2-5A synthetase/RNase L pathway in chronic fatigue syndrome. In Vivo. 2005;19(6):1013–21. [PubMed] [Google Scholar]
- 10.Myhill S, Booth NE, McLaren-Howard J. Chronic fatigue syndrome and mitochondrial dysfunction. Int J Clin Exp Med. 2009;2(1):1–16. [PMC free article] [PubMed] [Google Scholar]
- 11.Behan WM, More IA, Behan PO. Mitochondrial abnormalities in the postviral fatigue syndrome. Acta Neuropathol. 1991;83(1):61–65. doi: 10.1007/BF00294431. [DOI] [PubMed] [Google Scholar]
- 12.Gow JW, Hagan S, Herzyk P, et al. A gene signature for post-infectious chronic fatigue syndrome. BMC Medicine Genomics. 2009;2:38. doi: 10.1186/1755-8794-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr JR, Petty R, Burke B, et al. Gene expression subtypes in patients with chronic fatigue syndrome/myalgic encephalomyelitis. J Infect Dis. 2008;197(8):1171–84. doi: 10.1086/533453. [DOI] [PubMed] [Google Scholar]
- 14.Vecchiet J, Cipollone F, Falasca K, et al. Relationship between musculoskeletal symptoms and blood markers of oxidative stress in patients with chronic fatigue syndrome. Neurosci Lett. 2003;335:151–54. doi: 10.1016/s0304-3940(02)01058-3. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy G, Spence VA, McLaren M, et al. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radicals in Biological Medicine. 2005;39:584–89. doi: 10.1016/j.freeradbiomed.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Jammes Y, Steinberg JG, Mambrini O, et al. Chronic fatigue syndrome: assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise. J Intern Med. 2005;257:299–310. doi: 10.1111/j.1365-2796.2005.01452.x. [DOI] [PubMed] [Google Scholar]
- 17.Smirnova IV, Pall ML. Elevated levels of protein carbonyls in sera of chronic fatigue syndrome patients. Mol Cell Biochem. 2003;248:93–95. doi: 10.1023/a:1024176016962. [DOI] [PubMed] [Google Scholar]
- 18.Maes M, Mihaylova I, Kubera M, et al. Increased 8-hydroxy-deoxyguanosine, a marker of oxidative damage to DNA, in major depression and myalgic encephalomyelitis/chronic fatigue syndrome. Neuro Endocrinol Lett. 2009;30:715–22. [PubMed] [Google Scholar]
- 19.Tanaka M, Kovalenko SA, Gong JS, et al. Accumulation of deletions and point mutations in mitochondrial genome in degenerative diseases. Ann NY Acad Sci. 1996;786:102–11. doi: 10.1111/j.1749-6632.1996.tb39055.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Baumer A, Mackay IR, et al. Unusual pattern of mitochondrial DNA deletions in skeletal muscle of an adult human with chronic fatigue syndrome. Hum Mol Genet. 1995;4:751–54. doi: 10.1093/hmg/4.4.751. [DOI] [PubMed] [Google Scholar]
- 21.Hokama Y, Campora CE, Hara C, et al. Anticardiolipin antibodies in the sera of patients with diagnosed chronic fatigue syndrome. J Clin Lab Anal. 2009;23:210–12. doi: 10.1002/jcla.20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vernon SD, Whistler T, Cameron B, et al. Preliminary evidence of mitochondrial dysfunction associated with post-infective fatigue after acute infection with Epstein Barr virus. BMC Infectious Diseases. 2006;31:6–15. doi: 10.1186/1471-2334-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behan WM, More IA, Behan PO. Mitochondrial abnormalities in the postviral fatigue syndrome. Acta Neuropathol. 1991;83(1):61–65. doi: 10.1007/BF00294431. [DOI] [PubMed] [Google Scholar]
- 24.Ohmori H, Kanayama N. Immunogenicity of an inflammation-associated product, tyrosine nitrated self-proteins. Autoimmun Rev. 2005;4:224–29. doi: 10.1016/j.autrev.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Maes M, Mihaylova I, Leunis JC. Chronic fatigue syndrome is accompanied by an IgM-related immune response directed against neopitopes formed by oxidative or nitrosative damage to lipids and proteins. Neuro Endocrinol Lett. 2006;27:615–21. [PubMed] [Google Scholar]
- 26.Maes M, Mihaylova I, Leunis JC. Increased serum IgM antibodies directed against phosphatidyl inositol (Pi) in chronic fatigue syndrome (CFS) and major depression: evidence that an IgM-mediated immune response against Pi is one factor underpinning the comorbidity between both CFS and depression. Neuro Endocrinol Lett. 2007;28:861–67. [PubMed] [Google Scholar]
- 27.Maes M, Mihaylova I, Kubera M, Leunis JC. An IgM-mediated immune response directed against nitro-bovine serum albumin (nitro-BSA) in chronic fatigue syndrome (CFS) and major depression: evidence that nitrosative stress is another factor underpinning the comorbidity between major depression and CFS. Neuro Endocrinol Lett. 2008;29:313–19. [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. Fourth Edition. American Psychiatric Association; Washington, D.C: 2000. (Text Revision) [Google Scholar]
- 29.Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–59. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 30.Zachrisson O, Regland B, Jahreskog M, et al. A rating scale for fibromyalgia and chronic fatigue syndrome (the FibroFatigue scale) J Psychosom Res. 2002;52:501–9. doi: 10.1016/s0022-3999(01)00315-4. [DOI] [PubMed] [Google Scholar]
- 31.Maes M, Mihaylova I, Kubera M, et al. Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol Lett. 2009;30:462–69. [PubMed] [Google Scholar]
- 32.Maes M, Mihaylova I, De Ruyter M. Decreased dehydroepiandrosterone sulfate but normal insulin-like growth factor in chronic fatigue syndrome (CFS): relevance for the inflammatory response in CFS. Neuro Endocrinol Lett. 2005;26:487–92. [PubMed] [Google Scholar]
- 33.Maes M, Mihaylova I, De Ruyter M. Lower serum zinc in Chronic Fatigue Syndrome (CFS): relationships to immune dysfunctions and relevance for the oxidative stress status in CFS. J Affect Disord. 2006;90:141–47. doi: 10.1016/j.jad.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Miwa K, Fujita M. Increased oxidative stress suggested by low serum vitamin E concentrations in patients with chronic fatigue syndrome. Int J Cardiol. 2009;136(2):238–39. doi: 10.1016/j.ijcard.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 35.Singal A, Kaur S, Tirkey N, Chopra K. Green tea extract and catechin ameliorate chronic fatigue-induced oxidative stress in mice. J Med Food. 2005;8:47–52. doi: 10.1089/jmf.2005.8.47. [DOI] [PubMed] [Google Scholar]
- 36.Singh A, Naidu PS, Gupta S, Kulkarni SK. Effect of natural and synthetic antioxidants in a mouse model of chronic fatigue syndrome. J Med Food. 2002;5:211–20. doi: 10.1089/109662002763003366. [DOI] [PubMed] [Google Scholar]
- 37.Singh A, Garg V, Gupta S, Kulkarni SK. Role of antioxidants in chronic fatigue syndrome in mice. Indian Journal of Experimental Biology. 2002;40:1240–44. [PubMed] [Google Scholar]
- 38.Sachdeva AK, Kuhad A, Tiwari V, Chopra K. Epigallocatechin gallate ameliorates chronic fatigue syndrome in mice: behavioral and biochemical evidence. Behavior and Brain Research. 2009;205:414–20. doi: 10.1016/j.bbr.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 39.Maes M. An intriguing and hitherto unexplained co-occurrence: Depression and chronic fatigue syndrome are manifestations of shared inflammatory, oxidative and nitrosative (IO&NS) pathways. Prog Neuropsychopharmacol Biol Psychiatry. 2010 Jul 4; doi: 10.1016/j.pnpbp.2010.06.023. [Epub ahead of print] PubMed. [DOI] [PubMed] [Google Scholar]
- 40.Maes M, Mihaylova I, Kubera M, et al. Increased plasma peroxides and serum oxidized low density lipoprotein antibodies in major depression: markers that further explain the higher incidence of neurodegeneration and coronary artery disease. J Affect Disord. 2010;125(1–3):287–94. doi: 10.1016/j.jad.2009.12.014. Epub 2010 Jan 18. PubMed. [DOI] [PubMed] [Google Scholar]