Summary
Background
Doxorubicin (DOX) is a commonly used chemotherapeutic agent. It is associated with serious dose-limiting cardiotoxicity, which is at least partly caused by generation of reactive oxygen species (ROS). Supplementations with bilberries were effective in reducing oxidative stress in many tissue injuries due their high content of antioxidants. The present study investigated the potential protective effect of bilberry extract against DOX-induced cardiotoxicity in rats.
Material/Methods
Rats were treated orally with a methanolic extract of bilberry for 10 days. DOX was injected intraperitoneally on day 7. Twenty-four hours after the last bilberry administration, rats were subjected to ECG study. Blood was then withdrawn and cardiac tissues were dissected for assessment of oxidative stress and cardiac tissue injury. Cardiac tissues were also subjected to histopathological examination.
Results
Bilberry extract significantly inhibited DOX-provoked reduced glutathione depletion and accumulation of oxidized glutathione, malondialdehyde and protein carbonyls in cardiac tissues. This was accompanied by significant amelioration of reduced cardiac catalase, superoxide dismutase, and glutathione peroxidase activities; and increased cardiac myeloperoxidase activity in response to DOX challenge. Pretreatment with bilberry significantly guarded against DOX-induced increase in serum activities of lactate dehydrogenase, creatine phosphokinase and creatine kinase-MB, as well as the level of troponin I. Bilberry alleviated ECG changes in rats treated with DOX and attenuated its pathological changes.
Conclusions
Bilberry protects against DOX-induced cardiotoxicity in rats. This can be attributed, at least in part, to its antioxidant activity.
Keywords: bilberry, doxorubicin, cardiotoxicity, antioxidant, rats
Background
Doxorubicin (DOX) is an anthracycline antibiotic used widely in treatment of various malignancies, including acute leukemias, malignant lymphomas, and a variety of solid tumors. However, the occurrence of cardiotoxicity as a major side effect limits its use [1,2]. Although numerous mechanisms have been proposed, most studies implicate increased oxidative stress [3–5]. The idea that reactive oxygen species (ROS) plays a role is supported by the findings that treatment of animals with a variety of antioxidants protects against DOX-induced cardiotoxicity [6,7].
Blueberries belong to the genus Vaccinium, a widespread genus with over 200 species. The prominent species are the North-American highbush (Vaccinium corymbosum) and lowbush (Vaccinium angustifolium) blueberries, together with the native European blueberry, also called bilberry (Vaccinium myrtillus). In Northern Europe, bilberry is one of the most important wild berries. Bilberry is known to have a high content of anthocyanins, flavonoids and phenolic acids [7]. Anthocyanins, flavonoids and other phenolic compounds are reported to have multiple biological effects including antioxidant, antimutagenic, anticarcinogenic, anti-inflammatory, antihypertensive, antihyperlipidemic, antiproliferative and antimicrobial activities [8–12]. Further, bilberry fruit was reported to possess strong antiradical activity [13]. Recently, we showed that cranberry (Vaccinium macrocarpon), which contains flavonoids and phenolic acids, protects against DOX-induced cardiotoxicity in rats [14]. Therefore, the present study was designed to explore the potential protective effects of the alcoholic extract of bilberry “Vaccinium myrtillus” against DOX-induced cardiotoxicity in rats.
Material and Methods
DOX was obtained as doxorubicin hydrochloride (2 mg/ml) from EBWE Pharma, A-4866 Unterach, Austria. Bilberry (Vaccinium myrtillus) capsules were purchased from GNC products (bilberry 100 Capsules, USA). Each capsule contained 100 mg of lyophilized powdered fruits. The powdered fruit content of 100 capsules (equivalent to 10 g) was extracted with 90% MeOH (3×150 ml) by using ultra-turrex T25 homogenizer (Janke and Kunkel, IKA Labortechnik, Stauten, Germany) at a temperature not exceeding 25°C, using an ice bath. The extract was evaporated under reduced pressure, lyophilized, and protected from light at −4°C until use. The extract was analyzed by thin layer chromatography as fingerprint using the chromatographic system reported by Camag (http://www.camag.com, last visited 10/8/2010). All other chemicals were purchased from Sigma-Aldrich Chemical Company (St Louis, MO, USA).
Male Wistar rats, weighing 250–300 g, were obtained from the animal facility of King Fahd Research Center, King Abdulaziz University, Jeddah, Saudi Arabia. They were used in the study according to the guidelines of the Biochemical and Research Ethics Committee at King Abdulaziz University. Animals were housed in a well-ventilated, temperature-controlled room at 22±3°C with a 12 h light-dark cycle. They were provided with a balanced commercial diet and tap water. All experimental procedures were performed between 8–10 a.m. and care was taken to avoid stressful conditions.
Rats were randomly divided into 4 groups (12 animals in each group). Group I received carboxymethylcellulose (CMC; 0.5%) (1 ml/200 g body weight/day) orally for 10 consecutive days. Group II received bilberry alone suspended in 0.5% CMC (100 mg/kg; orally once daily for 10 consecutive days). The dose of bilberry was chosen according to pilot experiments conducted by our research group. Group III received CMC orally for 10 consecutive days and a single dose of DOX (15 mg/kg, i.p.) on day 7 [15]. Group IV received both bilberry and DOX in the previously mentioned doses; bilberry was administered for 10 consecutive days and DOX was administered once on day 7.
Twenty-four hours after the last bilberry or CMC treatment (day 11), rats were anesthetized with thiopentone (35 mg/kg; i.p.) and subjected to ECG recording. Blood samples (2–3 ml each) were collected by orbital puncture and were centrifuged for 10 minutes at 3000 ×g at room temperature to separate the sera for biochemical analyses. The abdomen of each rat was opened and the heart were rapidly dissected out, washed in ice-cold isotonic saline and blotted between 2 filter papers. Six hearts from each group were fixed in 10% formalin for histopathological examination, and the remaining hearts from each group were homogenized in ice-cold 0.1 M potassium phosphate puffer (pH 7.4) for subsequent analyses.
ECG was recorded in thiopentone anesthetized rats using the Bioscience ECG recorder (Bioscience, Washington, USA). Needle electrodes were inserted under the skin for the limb lead at position II. Paper speed was 50 mm/sec and the voltage was 1 mV/cm. Noise was minimized by a digital filter.
Reduced glutathione (GSH) content was determined according to the method of Adams et al. [16], and oxidized glutathione (GSSG) level was assessed according to the method of Hissin and Hilf [17] (values are expressed as nmol/mg protein). Lipid peroxidation was determined by measuring thiobarbituric acid reactive substances (TBARS) in tissue homogenates referring to the malondialdehyde (MDA) standard calibration curve according to the method of Uchiyama and Mihara [18] (values are expressed as nmol/g protein). Catalase (CAT) activity was determined according to the method described by Aebi [19] based on determination of the H2O2 decomposition rate at 240 nm (values are expressed as U/mg protein). Superoxide dismutase (SOD) activity was determined according to the method of Sun et al. [20] based on inhibition of nitroblue-tetrazolium reduction by the xanthine-xanthine oxidase system as a superoxide generator (values are expressed as U/mg protein). Glutathione peroxidase (GSH-Px) activity was assessed spectrophotometrically according to the method of Paglia and Valentine [21] (values are expressed as U/mg protein). Myeloperoxidase (MPO) activity was determined by the method of Wei and Frenkel [22] based on using 4-aminoantipyrine/phenol solution as the substrate for MPO-mediated oxidation by H2O2 and recording the changes in absorbance at 510 nm (values are expressed as mU/g protein). Protein carbonyl content (PCC) was determined spectrophotometrically by a method based on the reaction of the carbonyl group with 2,4-dinitrophenylhydrazine to form 2,4-dinitrophenylhydrazone [23] (values are expressed as nanomoles carbonyl/mg protein). The protein content of cardiac tissue homogenates was determined by the Lowry protein assay, using bovine serum albumin as the standard [24].
Creatine phosphokinase (CPK), creatine kinase isoenzyme-MB (CK-MB) and lactate dehydrogenase (LDH) activities were determined according to standard methods, using diagnostic kits from BioSystems S.A. (Barcelona, Spain). Assessment of serum troponin I was carried out by enzyme-linked immunosorbent assay (ELISA) using a kit purchased from DRG International Inc. (Mountainside, NJ, USA).
Hearts were cut at 0.5 μm, mounted on slides, stained with hematoxylin and eosin (H&E) and examined under light microscope (Olympus BX-50 Olympus Corporation, Tokyo, Japan).
Results are expressed as means ±SEM. Assessment of these results was performed using one-way analysis of variance (ANOVA), followed by Tukey-Kramer test for multiple comparisons using GraphPad InStat software, Version 4 (GraphPad Software Inc, La Jolla, CA, USA). The statistical significance was accepted at a level of P<0.05.
Results
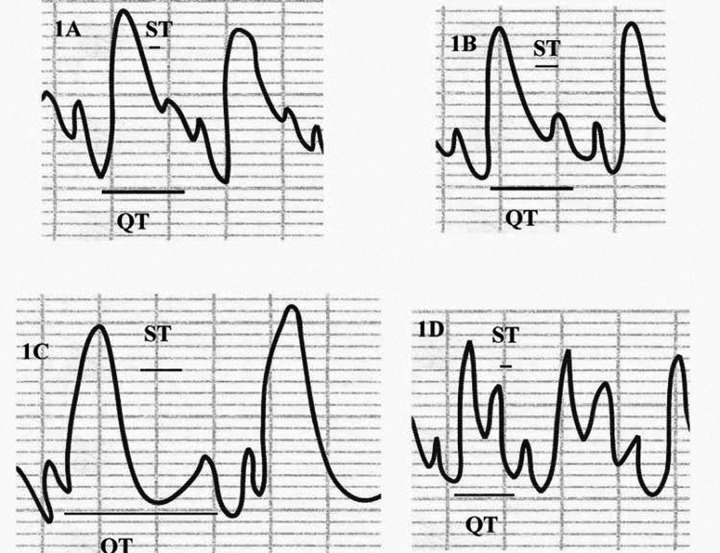
ECG tracing showed normal cardiac activity in all rats in the control and bilberry groups, with a mean heart rate of 366±12 and 360±15 beat/min, respectively. Rats in the DOX-only treated group showed bradycardia (254±14 beat/min), ST segment depression and prolongation of both ST and QT intervals. Such ECG abnormalities were clearly improved in the bilberry+DOX group, as evidenced by normalization of heart rate, ST segment, and both ST and QT intervals (Table 1 and Figure 1).
Table 1.
The effect of bilberry extract on doxorubicin (DOX)-induced alterations in heart rate, ST interval and QT interval.
| Heart rate (beat/min) | ST interval (msec) | QT interval (msec) | |
|---|---|---|---|
| Control | 366±12 | 110±8 | 140±20 |
| Bilberry | 360±15 | 100±10 | 135±18 |
| DOX | 254±14* | 390±9* | 410±35* |
| Bilberry+DOX | 360±12** | 120±6** | 138±21** |
Data are the mean ±SEM of 12 rats.
p<0.05 vs. corresponding control group;
p<0.05 vs. corresponding DOX group.
Figure 1.
The effect of bilberry on doxorubicin (DOX)-induced changes in ECG tracing. Control ECG (A) shows control heart rate, ST segment and interval, and QT interval. Bilberry ECG tracing (B) shows control heart rate, ST segment and interval, and QT interval. DOX-treated group (C) shows bradycardia, depressed long ST segment, and long QT interval. DOX+bilberry group (D) shows nearly normalized heart rate, ST segment and QT interval.
Cardiac content of GSH was significantly reduced in the DOX-only treated group as compared with the control group. Animals in the bilberry+DOX group showed significantly higher levels of GSH as compared to DOX-treated animals. However, GSH level in the bilberry+DOX group was still significantly lower than in the control group. Cardiac levels of GSSG, MDA and protein carbonyl contents were increased significantly in the DOX-only treated group compared with the control group. Bilberry pretreatment significantly guarded against elevations in the latter parameters. Although not normalized, GSSG and MDA levels were significantly lower than the corresponding values in the DOX group. In addition, premedication with bilberry restored the DOX-elevated PCC to the control value (Table 2).
Table 2.
The effect of bilberry extract on doxorubicin (DOX)-induced alterations in the cardiac reduced and oxidized glutathione (GSH and GSSG), malondialdehyde (MDA) and protein carbonyl content (PCC).
| GSH (μmol/g protein) | GSSG (μmol/g protein) | MDA (nmol/g protein) | PCC (nmol/mg protein) | |
|---|---|---|---|---|
| Control | 4.56±0.20 | 0.85±0.11 | 64.6±3.10 | 0.180±0.03 |
| Bilberry | 4.68±0.10 | 0.90±0.09 | 58.2±2.20 | 0.174±0.03 |
| DOX | 2.00±0.09* | 1.58±0.06* | 130.3±6.10* | 0.380±0.04* |
| Bilberry+DOX | 3.29±0.14*,** | 0.92±0.03** | 79.2±3.30*,** | 0.186±0.05** |
Data are the mean ±SEM of 6 rats.
p<0.05 vs. corresponding control group;
p<0.05 vs. corresponding DOX group.
Administration of DOX to rats significantly reduced the cardiac activities of CAT, SOD, and GSH-Px as compared to the control group. The drop in CAT activity caused by DOX treatment was not influenced by bilberry pretreatment, while the activities of SOD and GSH-Px in the bilberry+DOX group were significantly restored to near control values (Table 3). Assessment of MPO activity in cardiac tissues indicated a significant increase in the DOX-only treated group. Pretreatment with bilberry exerted a significant reduction compared to DOX-only treated group, and still significantly higher than the control group (Table 3).
Table 3.
The effect of bilberry extract on doxorubicin (DOX)-induced alterations in the cardiac activities of catalase (CAT), superoxide dismutase (SOD) glutathione peroxidase (GSH-Px) and myeloperoxidase (MPO).
| CAT (U/mg protein) | SOD (U/mg protein) | GSH-Px (U/mg protein) | MPO (mU/mg protein) | |
|---|---|---|---|---|
| Control | 8.05±0.25 | 32.3±1.40 | 5.11±0.13 | 1.10±0.12 |
| Bilberry | 8.12±0.24 | 36.6±1.70 | 5.09±0.16 | 1.26±0.11 |
| DOX | 3.40±0.16* | 17.7±1.20* | 1.80±0.13* | 4.66±0.21* |
| Bilberry+DOX | 3.86±0.28* | 30.1±1.14** | 4.88±0.17** | 2.30±0.10*,** |
Data are the mean ±SEM of 6 rats.
p<0.05 vs. corresponding control group;
p<0.05 vs. corresponding DOX group.
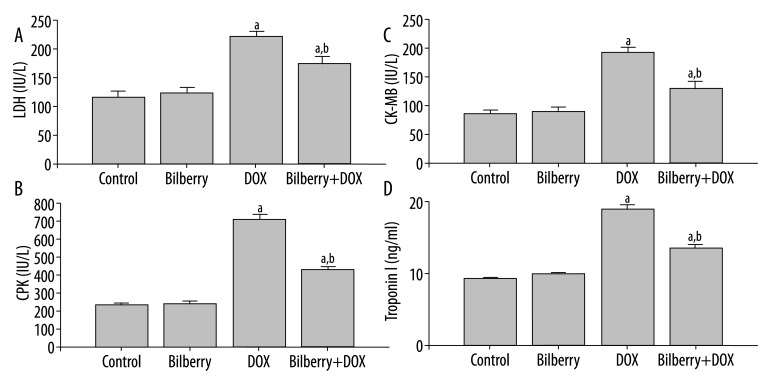
The serum markers indicating myocardial injury (LDH, CPK, CK-MB and troponin I) were significantly elevated in the DOX-only treated group compared with the control and bilberry-only treated group. Pretreatment with bilberry in the bilberry+DOX group significantly reduced their levels as compared with the DOX-only treated group, but were not restored to their corresponding control values (Figure 2).
Figure 2.
The effect of bilberry on doxorubicin (DOX)-induced alterations in serum biomarkers of cardiac injury; (A) lactate dehydrogenase (LDH), (B) creatine phosphokinase (CPK), (C) creatine phosphokinase isoenzyme MB (CK-MB) and (D) troponin I. Data are the mean ±SEM of 12 rats in DOX group and 8 rats in the other groups. ap<0.05 vs. corresponding control group. bp<0.05 vs. corresponding DOX group.
Hearts from control and bilberry groups showed normal cell distribution and myocardium architecture (Figure 3A, B). Hearts from DOX-only treated rats revealed severe vacuolar degeneration and interstitial edema (Figure 3C). These pathological alterations were markedly ameliorated in animals from the bilberry+DOX group, with mild congestion and some interstitial edema (Figure 3D).
Figure 3.
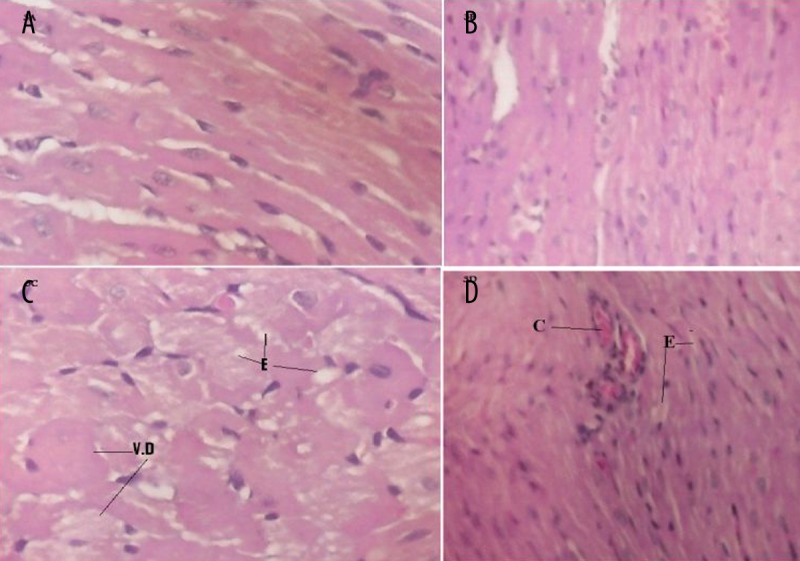
The effect of bilberry on doxorubicin (DOX)-induced alterations in the histology of cardiac muscle. Histopathological effects of cardiac tissues from control and bilberry groups show normal histological pattern (A and B, respectively). Cardiac tissues from doxorubicin (DOX)-treated group show severe vacuolar degeneration (VD) and interstitial edema (E) (C). Cardiac tissues from bilberry+DOX treated rats show some congestion (C) and minimal interstitial edema (E) with no fibrosed bands (D) (H & E ×125).
Discussion
Despite the development of several antitumor drugs, DOX continues to be an effective and widely used broad spectrum chemotherapeutic agent; however, its use is limited because of its serious dose-dependent cardiotoxicity [25]. Previous studies have suggested that the generation of free radicals and depletion of endogenous antioxidants induced by DOX are involved in the mechanism of DOX-induced cardiotoxicity [3,26,27]. Inhibiting generation of ROS by antioxidants, as well as the iron chelating agent dexrazoxane, have been shown to ameliorate DOX cardiotoxicity [28]. The present study was designed to investigate the potential protective effects of bilberry extract against DOX-induced cardiotoxicity in rats.
ECG tracing of rats in the DOX group showed bradycardia, ST segment depression and prolongation of both ST and QT intervals, which reflected arrhythmias, conduction abnormalities and attenuation of left ventricular function. Similar changes have been reported by other investigators [15,29–31]. These ECG changes were ameliorated in the bilberry+DOX group, indicating the protective effect of bilberry.
Our data show that cardiac levels of GSSG, MDA and PCC were significantly elevated, while those of GSH were significantly reduced following DOX administration as compared to the control group. Clearly, these data indicate the occurrence of oxidative stress, which is in accordance with reports of previous studies [32,33]. The reduced GSH and the elevated GSSG levels in the DOX group might be due to GSH consumption in the interactions of DOX-induced free radicals with bio-membrane and the subsequent lipid peroxidation. Pretreatment of rats with bilberry significantly guarded against the oxidative stress observed in the DOX group. Furthermore, the activity of the cardiac antioxidant enzymes CAT, SOD and GSH-Px were significantly reduced, while that of MPO was significantly elevated in response to DOX administration as compared to the control group. These data are in accord with those reported by many investigators [34,35]. Our results also indicate that bilberry alleviated the decrease of the activity of the antioxidant enzymes, and can be explained by its high content of several antioxidants including anthocyanine, flavonoids and phenolic acids [7]. These compounds were reported to inhibit oxidative processes in other tissues [36]. They have been shown to effectively scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) and superoxide [37], improve the ferric-reducing antioxidant power in plasma [38] and suppress the DNA damage induced by DOX [39]. Moreover, Li et al. [31] reported that procyanidins from grape seeds protected rats from DOX-induced myocardial damage and cardiac dysfunction by scavenging the free radical. The increased MPO activity in the DOX group denotes leukocyte accumulation, with subsequent oxidative injury in cardiac tissue [40,41]. Despite the positive effects of flavonoids in experimental models of DOX cardiotoxicity, clinical trials have shown little success [42].
Conclusions
Regarding serum biochemical parameters, DOX significantly elevated serum LDH activity and CPK, CK-MB and troponin I levels; all of which indicate cardiac injury [43]. Similar observations were previously reported by Shi et al. [44] and Iqbal et al. [45]. Bilberry, in the present study, significantly inhibited DOX-induced elevations in serum activity of LDH, CPK and CK-MB, as well as troponin I levels. Histopathological examination showed cardiac injury in the DOX group in the form of cytoplasmic vacuole formation, interstitial edema and vascular degeneration, typically present in acute DOX-induced cardiotoxicity in rats [46,47] and mice [48]. The severity of the histological changes was much less in sections from rats pretreated with bilberry. According to the results of the present study, it can be concluded that bilberry protects against DOX-induced cardiotoxicity in rats, which can be attributed, at least in part, to its antioxidant activity.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
Source of support: This study was supported by grant # 429/004-11 offered by the Deanship of Scientific Research, King Abdulaziz University, Jeddah, Saudi Arabia
References
- 1.Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996;125:47–58. doi: 10.7326/0003-4819-125-1-199607010-00008. [DOI] [PubMed] [Google Scholar]
- 2.Hortobagyi GN. Anthracyclines in the treatment of cancer. An overview. Drugs. 1997;54:1–7. doi: 10.2165/00003495-199700544-00003. [DOI] [PubMed] [Google Scholar]
- 3.Myers C, Bonow R, Palmeri S, et al. A randomized controlled trial assessing the prevention of doxorubicin cardiomyopathy by N-acetylcysteine. Semin Oncol. 1983;10:53–55. [PubMed] [Google Scholar]
- 4.Singal PK, Segstro RJ, Singh RP, Kutryk MJ. Changes in lysosomal morphology and enzyme activities during the development of adriamycin-induced cardiomyopathy. Can J Cardiol. 1985;1:139–47. [PubMed] [Google Scholar]
- 5.Singal PK, Deally CM, Weinberg LE. Subcellular effects of adriamycin in the heart: a concise review. J Mol Cell Cardiol. 1987;19:817–28. doi: 10.1016/s0022-2828(87)80392-9. [DOI] [PubMed] [Google Scholar]
- 6.Nazeyrollas P, Prévost A, Baccard N, et al. Effects of amifostine on perfused isolated rat heart and on acute doxorubicin-induced cardiotoxicity. Cancer Chemother Pharmacol. 1999;43:227–32. doi: 10.1007/s002800050888. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Chen Z, Chua CC, et al. Melatonin as an effective protector against doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol. 2002;283:H254–63. doi: 10.1152/ajpheart.01023.2001. [DOI] [PubMed] [Google Scholar]
- 8.Morton LW, Abu-Amsha Caccetta R, Puddey IB, Croft KD. Chemistry and biological effects of dietary phenolic compounds: Relevance to cardiovascular disease. Clin Exp Pharmacol Physiol. 2000;27(3):152–59. doi: 10.1046/j.1440-1681.2000.03214.x. [DOI] [PubMed] [Google Scholar]
- 9.Tapiero H, Tew KD, Ba GN, Mathé G. Polyphenols: Do they play a role in the prevention of human pathologies? Biomed Pharmacother. 2002;56(4):200–7. doi: 10.1016/s0753-3322(02)00178-6. [DOI] [PubMed] [Google Scholar]
- 10.Devi A, Jolitha AB, Ishii N. Grape seed proanthocyanidin extract (GSPE) and antioxidant defense in the brain of adult rats. Med Sci Monit. 2006;12(4):124–29. [PubMed] [Google Scholar]
- 11.Heinonen M. Antioxidant activity and antimicrobial effect of berry phenolics – a finnish perspective. Mol Nutr Food Res. 2007;51(6):684–91. doi: 10.1002/mnfr.200700006. [DOI] [PubMed] [Google Scholar]
- 12.Broneel M, Kozirog M, Duchnowicz P, et al. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker level in patients with metabolic syndrome. Med Sci Monit. 2010;16(1):CR28–34. [PubMed] [Google Scholar]
- 13.Burdulis D, Sarkinas A, Jasutiené I, et al. Comparative study of anthocyanin composition, antimicrobial and antioxidant activity in bilberry (Vaccinium myrtillus L.) and blueberry (Vaccinium corymbosum L.) fruits. Acta Pol Pharm. 2009;66(4):399–408. [PubMed] [Google Scholar]
- 14.Elberry AA, Abdel-Naim AB, Abdel-Sattar EA, et al. Cranberry (Vaccinium macrocarpon) protects against doxorubicin-induced cardiotoxicity in rats. Food Chem Toxicol. 2010;(48):1178–84. doi: 10.1016/j.fct.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Fadillioglu E, Oztas E, Erdogan H, et al. Protective effects of caffeic acid phenethyl ester on doxorubicin-induced cardiotoxicity in rats. J Appl Toxicol. 2004;24:47–52. doi: 10.1002/jat.945. [DOI] [PubMed] [Google Scholar]
- 16.Adams JD, Jr, Lauterburg BH, Mitchell JR. Plasma glutathione and glutathione disulfide in the rat. Regulation and response to oxidative stress. J Pharmacol Exp Ther. 1983;227:749–54. [PubMed] [Google Scholar]
- 17.Hissin PJ, Hilf RA. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74:214–26. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 18.Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–78. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 19.Aebi H. Catalase in vitro. In: Packer L, Orlando FL, editors. Methods in Enzymology. Vol. 105. Academic Press; New York: 1984. pp. 121–26. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 21.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 22.Wei H, Frenkel K. Relationship of oxidative events and DNA oxidation in SENCAR mice to in vivo promoting activity of phorbol ester-type tumor promoters. Carcinogenesis. 1993;14:1195–201. doi: 10.1093/carcin/14.6.1195. [DOI] [PubMed] [Google Scholar]
- 23.Levine RL, Garland D, Oliver CN, et al. Determination of carbonyl content in oxidatively modified proteins. In: Packer L, Glazer AN, editors. Methods in Enzymology, V 186, Oxygen radicals in Biological Systems. Academic Press; New York: 1990. pp. 464–78. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 25.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–5. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 26.Franco F, Dubois SK, Peshock RM, Shohet RV. Magnetic resonance imaging accurately estimates LV mass in a transgenic mouse model of cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 1998;274:H679–83. doi: 10.1152/ajpheart.1998.274.2.H679. [DOI] [PubMed] [Google Scholar]
- 27.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from Cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330–52. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Šimùnek T, Štìrba M, Popelová O, et al. Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep. 2009;61:154–71. doi: 10.1016/s1734-1140(09)70018-0. [DOI] [PubMed] [Google Scholar]
- 29.Larsen RL, Jakacki RI, Vetter VL, et al. Electrocardiographic changes and arrhythmias after cancer therapy in children and young adults. Am J Cardiol. 1992;70:73–77. doi: 10.1016/0002-9149(92)91393-i. [DOI] [PubMed] [Google Scholar]
- 30.Puri A, Maulik SK, Ray R, Bhatnagar V. Electrocardiographic and biochemical evidence for the cardioprotective effect of vitamin E in doxorubicin-induced acute cardiotoxicity in rats. Eur J Pediatr Surg. 2005;15:387–91. doi: 10.1055/s-2005-872923. [DOI] [PubMed] [Google Scholar]
- 31.Li W, Xu B, Xu J, Wu XL. Procyanidins produce significant attenuation of doxorubicin-induced cardiotoxicity via suppression of oxidative stress. Basic Clin Pharmacol Toxicol. 2009;104:192–97. doi: 10.1111/j.1742-7843.2008.00358.x. [DOI] [PubMed] [Google Scholar]
- 32.Choi EH, Lee N, Kim HJ, et al. Schisandra fructus extract ameliorates doxorubicin-induce cytotoxicity in cardiomyocytes: altered gene expression for detoxification enzymes. Genes Nutr. 2008;2:337–45. doi: 10.1007/s12263-007-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim MA, Ashour OM, Ibrahim YF, et al. Angiotensin-converting enzyme inhibition and angiotensin AT(1)-receptor antagonism equally improve doxorubicin-induced cardiotoxicity and nephrotoxicity. Pharmacol Res. 2009;60(5):373–81. doi: 10.1016/j.phrs.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Dbrowska K, Stuss M, Gromadzińska J, et al. The effects of melatonin on glutathione peroxidase activity in serum and erythrocytes after adriamycin in normal and pinealectomised rats. Endokrynol Pol. 2008;59(3):200–6. [PubMed] [Google Scholar]
- 35.Tatlidede E, Sehirli O, Velioğlu-Oğünc A, et al. Resveratrol treatment protects against doxorubicin-induced cardiotoxicity by alleviating oxidative damage. Free Radic Res. 2009;43:195–205. doi: 10.1080/10715760802673008. [DOI] [PubMed] [Google Scholar]
- 36.Ofek I, Goldhar J, Zafriri D, et al. Anti-Escherichia coli adhesin activity of cranberry and blueberry juices. N Engl J Med. 1991;324(22):1599. doi: 10.1056/NEJM199105303242214. [DOI] [PubMed] [Google Scholar]
- 37.Chang WT, Shao ZH, Yin JJ, et al. Comparative effects of flavonoids on oxidant scavenging and ischemia-reperfusion injury in cardiomyocytes. Eur J Pharmacol. 2007;566:58–66. doi: 10.1016/j.ejphar.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Busserolles J, Gueux E, Balasińska B, et al. In vivo antioxidant activity of procyanidin-rich extracts from grape seed and pine (Pinus maritima) bark in rats. Int J Vitam Nutr Res. 2006;76:22–27. doi: 10.1024/0300-9831.76.1.22. [DOI] [PubMed] [Google Scholar]
- 39.de Rezende AA, Graf U, da Guterres ZR, et al. Protective effects of proanthocyanidins of grape (Vitis vinifera L.) seeds on DNA damage induced by doxorubicin in somatic cells of Drosophila melanogaster. Food Chem Toxicol. 2009;47:1466–72. doi: 10.1016/j.fct.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 40.Riad A, Bien S, Westermann D, et al. Pretreatment with statin attenuates the cardiotoxicity of doxorubicin in mice. Cancer Res. 2009;69(2):695–99. doi: 10.1158/0008-5472.CAN-08-3076. [DOI] [PubMed] [Google Scholar]
- 41.Arnhold J, Osipov AN, Spalteholz H, et al. Effects of hypochlorous acid on unsaturated phosphatidylcholines. Free Radic Biol Med. 2001;31(9):1111–19. doi: 10.1016/s0891-5849(01)00695-5. [DOI] [PubMed] [Google Scholar]
- 42.Bruynzeel AME, Niessen HWM, Bronzwaer JGF, et al. The effect of monohydroxyethylrutoside on doxorubicin-induced cardiotoxicity in patients treated for metastatic cancer in a phase II study. Br J Cancer. 2007;97:1084–89. doi: 10.1038/sj.bjc.6603994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herman E, Mhatre R, Lee IP, et al. Comparison of the cardiovascular actions of daunomycin, adriamycin and N-acetyldaunomycin in hamsters and monkeys. Pharmacology. 1971;6:230–41. doi: 10.1159/000136248. [DOI] [PubMed] [Google Scholar]
- 44.Shi R, Liu L, Huo Y, Cheng YY. Study on protective effects of Panax notoginseng saponins on doxorubicin-induced myocardial damage. Zhongguo Zhong Yao Za Zhi. 2007;32(24):2632–35. [PubMed] [Google Scholar]
- 45.Iqbal M, Dubey K, Anwer T, et al. Protective effects of telmisartan against acute doxorubicin-induced cardiotoxicity in rats. Pharmacol Rep. 2008;60(3):382–90. [PubMed] [Google Scholar]
- 46.Morishima I, Matsui H, Mukawa H, et al. Melatonin, a pineal hormone with antioxidant property, protects against adriamycin cardiomyopathy in rats. Life Sci. 1998;63:511–21. doi: 10.1016/s0024-3205(98)00302-6. [DOI] [PubMed] [Google Scholar]
- 47.Mukherjee S, Banerjee SK, Maulik M, et al. Protection against acute adriamycin-induced cardiotoxicity by garlic: role of endogenous antioxidants and inhibition of TNF-α expression. BMC Pharmacol. 2003;3:16. doi: 10.1186/1471-2210-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xin YF, Zhou GL, Shen M, et al. Angelica sinensis: a novel adjunct to prevent doxorubicin-induced chronic cardiotoxicity. Basic Clin Pharmacol Toxicol. 2007;101:421–26. doi: 10.1111/j.1742-7843.2007.00144.x. [DOI] [PubMed] [Google Scholar]