Summary
Background
To evaluate the relationship between site of infarction (anterior vs. inferior) and circadian variation in patients with ST segment elevation myocardial infarction (STEMI) in a Turkish cohort.
Material/Methods
This restrospective study enrolled 465 patients (407 male, mean age 65±7 years) with STEMI. Patients were then categorised into 4 6-hour increments according to the time of day during which the symptoms began (12:00 AM–06:00 AM, 06:00 AM–12:00 PM; 12:00 PM–06:00 PM and 06:00 PM–12:00 AM hours). Characteristics of patients by site of infarction (anterior vs. inferior) were compared.
Results
The frequency of onset of acute anterior MI as determined by onset of pain demonstrated significant circadian variation among the 4 time periods, demonstrating bimodal peaks (afternoon and morning) and a trough between 06:00 PM to 06:00 AM. The incidence of occurrence of MI between 06:00 AM to 06:00 PM was 4.50 times that of the average frequency of the remaining 12 hours of the day. The frequency of onset of acute inferior MI as determined by onset of pain exhibited significant circadian variation among the 4 time periods, demonstrating bimodal peaks (midnight to 06:00 AM and 06:00 AM to noon) and a trough between noon to midnight. The incidence of occurrence of MI between midnight to noon was 4.25 times that of the average frequency of the remaining 12 hours of the day.
Conclusions
Different circadian periodicity in the time of onset of STEMI was found regarding infarction site in a Turkish cohort. This may be related to genetic and/or demographic characteristics of the Turkish population.
Keywords: acute ST elevation myocardial infarction, circadian rhythm, infarction site
Background
Circadian rhythms have long been known to occur in many biologic phenomena, including secretion of hormones and activities of the nervous system. Circadian rhythms have also been noted to occur in the incidences of certain cardiac and cerebrovascular events, including transient myocardial ischemia, myocardial infarction (MI), sudden cardiac death, and stroke [1].
Acute myocardial infarction (AMI) is one of the leading cause of mortality and mortality worldwide. Heart attacks are more common in the morning hours than at any other time of day. A circadian rhythm with a peak in the early morning has been reported for the frequency of myocardial infarction [2], giving rise to the idea that circadian variation plays a prominent role in the genesis of AMI. This role is commonly attributed to increased sympathetic activation as a result of physiological changes and biochemical parameters [3]. The changes in blood pressure, blood fibrinolytic activity, platelet aggregability, endothelial function and coronary tone during the daily cycle may increase the risk of heart attack, especially in the early morning hours [4–9]. As a result of increased sympathetic activation and changes in hemostatis, atherosclerotic plaque might be disrupted and thrombosis may occur. Autonomic nervous system activity may also play a role [3].
The occurrence of variations in the spectrum of cardiovascular diseases between different regions of the world and ethnic groups is well known [10–12]. Differences in the circadian variation of AMI have also been reported in different regions of the world and in different ethnic groups [10,11,13]. Although several studies have shown the association between acute coronary syndromes and circadian rhythm, studies investigating the association between circadian rhythm and coronary artery involvement in the genesis of AMI are limited [3]. Thus, we sought to evaluate the relationship between site of infarction (anterior vs. inferior) and beginning time of chest pain in patients with ST segment elevation myocardial infarction (STEMI) in a Turkish cohort.
Material and Methods
Patients
This restrospective study enrolled 465 patients (407 male, mean age 65±7 years) with acute ST elevation MI (STEMI) between April 2002 and January 2007. Of 535 patients, timing of the onset of AMI could not be definitely identified in 70 patients, and therefore only the data obtained from 465 patients were analyzed. Of 465 patients, 72 (15%) underwent thrombolytic treatment and 430 (85%) underwent primary percutaneous coronary ntervention (pPCI). The diagnosis and the treatment of AMI was made according to the ACC/AHA/ESC Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction [14]. Characteristics of patients by site of infarction (anterior vs. inferior) were compared. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP)/International Conference on Harmonization (ICH) guidelines. The time of AMI onset was determined by the attending physician on the basis of patients’ self-reports. The time in which chest pain had started was considered as the beginning of AMI, within ±30 min. Patients experiencing MI without chest pain, those who could not exactly remember the time of onset of chest pain, patients with non-ST elevation MI and unstable angina pectoris, patients experiencing MI after coronary bypass grafting or any invasive cardiac procedures, and patients with occupations requiring work during various shifts (changing hours of work) were not included into the current study.
Evaluation of ST segment resolution
Twelve-lead surface ECG (Hewlett Packard M 1700A, Houston, USA) was recorded from all patients before, and an average of 55 (± 12 min) after, primary PCI. The sum of ST elevation was assessed in 3 contiguous leads in the infarct zone, 60 miliseconds (ms) from the J point (anterior: leads I, aVL, V1–V6; nonanterior: leads II, III, aVF, V5–V6). The extent of ST segment resolution was assessed an average of 55 min (±12 min) after primary PCI and is expressed as a percentage of the ST elevation measured on the ECG recorded before primary PCI.
Echocardiography
Transthoracic echocardiography was performed by using EASOTE 2.5 Mhz probe (ESAOTE, Genova, Italy) at the left lateral decubitis position before primary PCI.
Blood chemistry
Venous blood samplings were withdrawn into the tubes containing K3 EDTA and the tubes containing no anticoagulant agent. After all tubes were spun at 5000 rpm for 15 minutes, plasma and serum samplings were stored at –80°C until analyses were made. For the measurement of lipid profile, fasting blood samples were withdrawn from the patients with AMI within 24 hours of admission. Total plasma cholesterol, triglyceride and high-density lipoprotein [HDL] cholesterol concentrations were measured with a spectrophotometric technique by the Olympus AU-2700 autoanalyzer using commercial kits (Olympus, Hamburg, Germany). Low-density lipoprotein [LDL] cholesterol levels were calculated by the Friedwald formula.
Circadian analysis
The standard hourly profile of the onset of AMI was obtained over a 24-h period. Patients were then categorised into 4 6-h increments according to the time that the symptoms began (12:00 AM–06:00 AM, 06:00 AM–12:00 PM; 12:00 PM–06:00 PM and 06:00 PM–12:00 AM hours). The time of onset and the electrocardiographic site of acute myocardial infarction, previous history of coronary artery disease (CAD), age, coronary risk factors (smoking, diabetes mellitus, hypertension, hypercholesterolemia, family history of CAD), coronary culprit lesion, prevalence of male sex, alcohol consumption, marital status, aspirin usage, beta-blocker usage and employment status were recorded.
Statistical analysis
Statistical analysis was performed using the SPSS 15.0 Statistical Package Program for Windows (SPSS Inc., Chicago, Illinois, USA). We used One-Sample Kolmogorov-Smirnov and Levene tests to determine the distribution characteristics of variables and variance homogeneity. Results are expressed as the mean ±SD, median and percentages. The means of groups were compared with each other. The differences between groups were tested by chi-square, independent samples t test and Mann-Whitney U tests. Differences were considered significant at p<0.05.
Results
The baseline demographic and clinical charecteristics are presented in Table 1. The mean age of the study population was 62.9±7.2 years. Of 465 patients, more than half of the total infarctions were inferior, including posterior or right ventricular infarctions (n=243 patients [53.3%]); the remaining were anterior or anterolateral infarctions (n=222 patients [47.7%]). When baseline clinical, demographic and biochemical characteristics of the patients with anterior MI were compared to those of the patients with inferior MI, no statistically significant differences were found (Table 2). The angiographic, electrocardiographic and echocardiographic characteristics of the patients are given in Table 3. The reference diameter of the infarct-related artery in patients with inferior MI was larger than that of the patients with anterior MI.
Table 1.
Baseline demographic and clinical charecteristics of the all study patients.
| Age (yrs) | 62.9±7.2 |
| Sex (M) (%) | 81.1 |
| MI localization (%) | Anterior (47.7); inferior (52.3) |
| Diabetes mellitus (%) | 34.0 |
| Hypertension (%) | 57.6 |
| Smoking (%) | 35.5 |
| Family history of CAD (%) | 18.7 |
| Previous myocardial infarction (%) | 15.1 |
| Aspirin usage (%) | 37.4 |
| Beta-blocker usage (%) | 31.8 |
| Marital status (married) (%) | 91.0 |
| Employment status (%) | 82.4 |
MI – myocardial infarction; CAD – coronary artery disease. Data are given as mean ±SD or% of the patients.
Table 2.
Clinical, demographic and biochemical characteristics and angiographic findings by localization of acute myocardial infarction.
| Anterior MI (n=222) | Inferior MI (n=243) | p | ||
|---|---|---|---|---|
| Age (yrs) | 62.3±7.0 | 63.5±7.4 | 0.12 | |
| Sex (M), n (%) | 182 (82.0) | 195 (80.2) | 0.63 | |
| Marital status (married) n (%) | 203 (91.4) | 201 (90.5) | 0.75 | |
| Alcohol consumption, n (%) | 21 (9.5) | 24 (9.9) | 0.87 | |
| Employment status, n (%) | 185 (83.3) | 198 (81.5) | 0.60 | |
| Diabetes Mellitus, n (%) | 71 (32.0) | 88 (36.2) | 0.33 | |
| Family history of CAD, n (%) | 45 (20.3) | 42 (17.3) | 0.40 | |
| Hypertension, n (%) | 133 (59.9) | 135 (55.6) | 0.34 | |
| Smoking, n (%) | 87 (39.2) | 78 (32.1) | 0.11 | |
| Previous MI, n (%) | 37 (16.7) | 33 (13.6) | 0.35 | |
| Total cholesterol (mg/dl) | 225.9±33.0 | 219.9±30.8 | 0.05 | |
| LDL cholesterol (mg/dl) | 146.2±36.0 | 140.3±33.3 | 0.06 | |
| HDL cholesterol (mg/dl) | 41.3±8.5 | 42.3±8.0 | 0.22 | |
| Triglyceride (mg/dl) | 191.6±54.3 | 186.2±53.8 | 0.28 | |
| Preprocedural medications | Aspirin, n (%) | 86 (38.7) | 88 (36.2) | 0.57 |
| Nitrat, n (%) | 55 (24.8) | 52 (21.4) | 0.38 | |
| BAB, n (%) | 69 (31.1) | 79 (32.5) | 0.74 | |
| ACEI, n (%) | 76 (34.2) | 96 (39.5) | 0.24 | |
MI – myocardial infarction; HR – heart rate; LDL – low density lipoprotein; HDL – high density lipoprotein; BAB – beta adrenergic blocker; ACEI – angiotensin converting enzyme inhibitor. Data are given as mean ±SD or% of the patients.
Table 3.
Angiographic,electrocardiographic and echocardiographic charecteristics of the patients.
| Anterior MI (n=222) | Inferior MI (n=243) | p | ||
|---|---|---|---|---|
| Vessel disease | 1-vessel n (%) | 103 (46.4) | 120 (49.4) | 0.42 |
| 2-vessel n (%) | 77 (34.7) | 88 (36.2) | ||
| 3-vessel n (%) | 42 (18.9) | 35 (14.4) | ||
| IRA n (%) | LMCA n (%) | 2 (0.9) | – | – |
| LAD n (%) | 220 (99.1) | – | ||
| RCA n (%) | – | 222 (91.4) | ||
| CFX n (%) | – | 21 (8.6) | ||
| Tirofiban use n (%) | 58 (26.1) | 76 (31.3) | 0.22 | |
| Reference diameter (mm) | 3.10±0.24 | 3.18±0.54 | 0.02 | |
| Postprocedural percent stenosis (%) | 8.98±2.81 | 9.36±2.96 | 0.16 | |
| Lesion length (mm) | 17.40±4.18 | 16.87±3.02 | 0.11 | |
| ST segment resolution (%) | 82±7 | 77±75 | 0.09 | |
| LVEF (%) | 48.37±6.85 | 52.22±6.76 | 0.001 | |
MI – Myocardial infarction; IRA – infarct related artery; LVEF – left ventricular ejection fraction. Data are given as mean ±SD or% of the patients.
Time of onset of symptoms of myocardial ınfarction
Anterior myocardial infarction
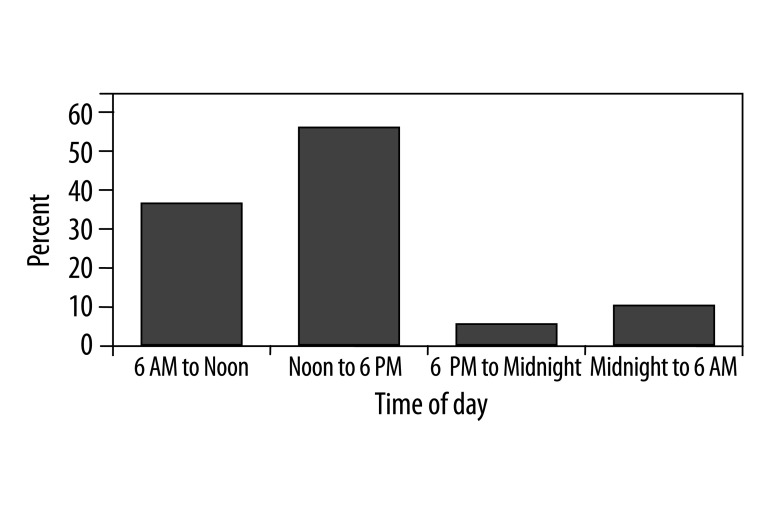
The frequency of onset of acute anterior MI as determined by onset of pain demonstrated significant circadian variation among the 4 time periods, demonstrating bimodal peaks (afternoon and morning) and a trough between 06:00 PM to 06:00 AM (Table 4, Figure 1). The onset of MI was between 12:00 PM and 06:00 PM hours in 119 patients (54.1%); between 06:00 AM and 12:00 PM hours in 79 patients (36%); between 12:00 AM and 06:00 AM hours in 13 patients (5.9%) and between 06:00 PM and 12:00 AM hours in 9 patients (4.1%). The incidences of MI between 06:00 AM and 06:00 PM hours were significantly higher when compared with other 2 6-h periods (p<0.001). The incidence of occurrence of MI between 06:00 AM to 06:00 PM hours was 4.50 times that of the average frequency of the remaining 12 hours of the day.
Table 4.
Culprit lesions in patients with acute ST segment elevation myocardial infarction regarding to the time onset.
| Time onset of MI | LMCA (n=2) | LAD (n=220) | RCA (n=222) | LCX (n=21) |
|---|---|---|---|---|
| 6 AM to noon n (%) | 1 (50.0) | 79 (36.0) | 103 (46.5) | 6 (28.6) |
| Noon to 6 PM n (%) | 1 (50.0) | 119 (54.0) | 24 (10.7) | 12 (57.2) |
| 6 PM to midnight n (%) | 0 | 9 (4.1) | 10 (4.5) | 0 |
| Midnight to 6 AM n (%) | 0 | 13 (5.9) | 85 (38.3) | 3 (14.2) |
MI – Myocardial infarction; LMCA – Left main coronary artery; LAD – Left anterior descending coronary artery; RCA – Right coronary artery; LCX – Left circumflex coronary artery. Data are given as% of the patients.
Figure 1.
Circadian distribution of anterior myocardial infarction. Compared with other times of the day, anterior infarctions were more common from 06:00 to 18:00.
Inferior myocardial infarction
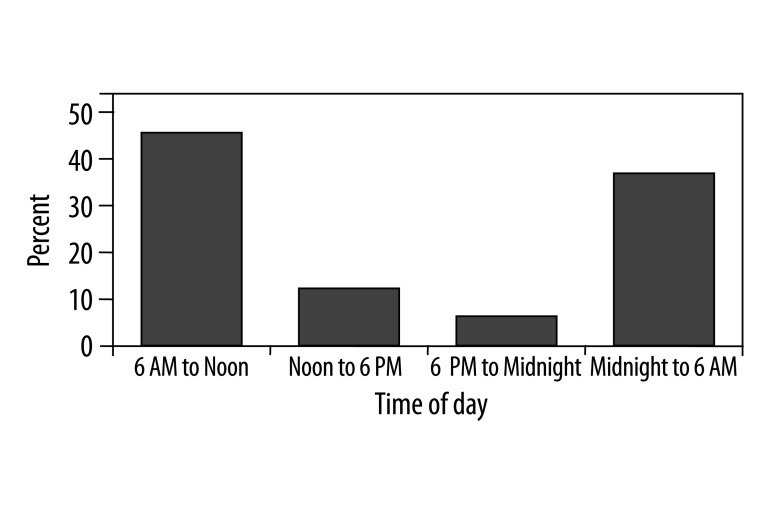
The frequency of onset of acute inferior MI as determined by onset of pain exhibited significant circadian variation among the 4 time periods, demonstrating bimodal peaks (midnight to 06:00 AM and 06:00 AM to noon) and a trough between noon to midnight (Table 4; Figure 2). The onset of MI was between 12:00 AM and 06:00 AM hours in 85 patients (38.3%); 06:00 AM and 12:00 PM hours in 103 patients (46.5%); between 12:00 PM and 06:00 PM hours in 24 patients (10.7%) and between 06:00 PM and 12:00 AM hours in 11 patients (4.5%). The incidences of MI between 12:00 AM and 12:00 PM hours were significantly higher when compared with the 2 other 6-h periods (p<0.001). The incidence of occurrence of MI between midnight to noon was 4.25 times that of the average frequency of the remaining 12 hours of the day.
Figure 2.
Circadian distribution of inferior myocardial infarction. Compared with other times of the day, anterior infarctions were more common from midnight to noon.
Discussion
The current study showed different circadian periodicity in patients with acute anterior MI compared to that of inferior MI in a Turkish cohort. Compared with other times of the day, anterior MIs were found to be more common from noon to 06:00 PM, whereas inferior MIs were found to be more common from midnight to noon in our patient population.
Studies on the circadian rhythm of AMI onset have suggested that the morning peak of incidence is related to other known daily rhythms [2,5,15]. One obvious explanation is the increase in physical and mental stress that occurs after waking. An increase in sympathetic activity occurs after waking and a rise occurs in plasma levels of catecholamine and of cortisol, heart rate, blood pressure, coronary vascular tone, and platelet aggregability [7,16,17]. The reasons for a second, late evening peak are less obvious. The evening peak may reflect altered circadian rhythms due to late working hours or reflect that some individuals are “night people” rather than “morning people” [2]. Some investigators have postulated that the morning and evening peaks are related to sleep-mediated factors, with changes in autonomic regulation, as a bimodal rhythm for the tendency to fall asleep has been described [18].
Circadian variation of the onset of AMI has been noted in many studies and may carry important pathophysiologic implications. A circadian variation in the onset of cardiac events and stroke is well recognised, with prominent clustering of MI events between 06:00 AM and 12:00 PM hours in the studies came from Western populations [2,19]. However, Lopez et al demonstrated a different circadian rhythm in MI onset between ethnic groups, with a significantly higher number of acute MI onsets occurring between midnight and noon in British Caucasians and Indo-Asians [20]. In contrast, Mediterranean Caucasians showed the opposite circadian pattern, with most acute MI events happening between noon and midnight. Although a morning peak of MI onset is very common, some populations studied have demostrated different results. For example, a maximal peak of onset of MI between 04:00 PM and 12:00 AM hours, with a second smaller peak between 06:00 AM and 08:00 AM hours, was found in a Bulgarian cohort [21], and a peak between 01:00 AM and 07:00 AM hours and a trough between 01:00 PM and 07:00 PM hours was demonstrated in a Chinese cohort [22]. On the other hand, a double-peak distribution of MI was reported in a South American patient population, with a lower MI incidence between 03:00 AM and 07:00 AM hours, a first maximum between 08:00 AM and 12:00 PM hours, and a second maximum between 03:00 PM and 10:00 PM hours [23]. The data on circadian variation of acute myocardial infarction in the Turkish population came from the very well-designed study by Sari et al, who examined circadian variation of AMI in a Turkish cohort [24]. Although the authors showed afternoon predominance in circadian variation of AMI in the Turkish cohort, they did not examine circadian distribution of AMI by anatomic location and coronary artery involvement.
Environmental-genetic background, socio-economic factors and customs could possibly underlie ethnic disparities in cardiovascular risk factor profiles [25,26]. However, ethnicity is not only one’s genetic background, but also includes social and cultural habits that might alter the cardiovascular disease spectrum in different populations. Ethnic disparities may be partly responsible for cardiovascular risk prevalence.
The precise mechanisms for these circadian differences remains uncertain. Ethnicity and lifestyle may also be responsible in the discrepancy in circadian variation of MI among different populations. Differences in eating habits, daytime rhythm, sunshine hours, sleep patterns and disparities in cardiovascular risk prevalence might also contribute to this discrepancy. Although controversial, eating a large meal is considered by some to be a trigger of MI [27–29]. There are variations in which the main meal of the day is breakfast in some populations, but lunch or supper is the largest meal in others. Although we did not clearly document that lunch was the main meal of our study population, afternoon peak in patients with anterior MI can be partly explained by eating a large lunch. However, we were not able to explain the morning peak in patients with inferior MI in the the current study population.
The current study had several limitations. First, this study is a single-centre study with a relatively small sample size, making it difficult to generalise the results to the entire Turkish population, and needs to be clarified with large-scale multicentre studies. Secondly, data were gathered retrospectively, and we could not determine whether patients were awake or asleep at the time of symptom onset. We also could not distinguish REM and non-REM sleep. Thirdly, we did not fully evaluate the socioeconomic status, physical activity level, eating and sleep habits that might affect the circadian distribution. Fourthly, we did not perform subgroup analysis. Presence of DM, beta blockers and aspirin usage have been reported to blunt the circadian variation of MI, although the current study sample showed circadian variation in both study groups regarding those clinical charecteristics. More importantly, DM frequency, aspirin and beta blocker usage were not statistically different between the patients with anterior and inferior MI. Lastly, a potential weakness of our study is its subjective reporting of the exact time of onset of symptoms, although we believe that our data are as accurate as such retrospectively gathered information can be.
Conclusions
In conclusion, different circadian periodicity in the time of onset of ST elevation MI was found regarding infarction site in a Turkish cohort. The present study suggests that circadian variation of AMI with morning peak might not be the pattern throughout the world; instead, one can refer to ‘population rhythm’. In fact, the factors known to affect circadian rhythm of MI may not be equally effective in all populations. Further large-scale clinical studies are needed to analyze the underlying pathophysiological mechanisms causing these differences in the chronobiology of MI.
Footnotes
Source of support: Departmental sources
References
- 1.Quyyumi AA. Circadian rhythms in cardiovascular disease. Am Heart J. 1990;120:726–33. doi: 10.1016/0002-8703(90)90044-x. [DOI] [PubMed] [Google Scholar]
- 2.Muller JE, Stone PH, Turi ZG, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–22. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 3.Moruzzi P, Marenzi G, Callegari S, Contini M. Circadian distribution of acute myocardial infarction by anatomic location and coronary artery involvement. Am J Med. 2004;116:24–27. doi: 10.1016/j.amjmed.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Otto ME, Svatikova A, Barretto RB, et al. Early morning attenuation of endothelial function in healthy humans. Circulation. 2004;109:2507–10. doi: 10.1161/01.CIR.0000128207.26863.C4. [DOI] [PubMed] [Google Scholar]
- 5.Rocco MB, Barry J, Campbell S, et al. Circadian variation of transient myocardial ischemia in patients with coronary artery disease. Circulation. 1987;75:395–400. doi: 10.1161/01.cir.75.2.395. [DOI] [PubMed] [Google Scholar]
- 6.Rosing DR, Brakman P, Redwood DR, et al. Blood fibrinolytic activity in man. Diurnal variation and the response to varying intensities of exercise. Circ Res. 1970;27:171–84. doi: 10.1161/01.res.27.2.171. [DOI] [PubMed] [Google Scholar]
- 7.Tofler GH, Brezinski D, Schafer AI, et al. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987;316:1514–18. doi: 10.1056/NEJM198706113162405. [DOI] [PubMed] [Google Scholar]
- 8.Turton MB, Deegan T. Circadian variations of plasma catecholamine, cortisol and immunoreactive insulin concentrations in supine subjects. Clin Chim Acta. 1974;55:389–97. doi: 10.1016/0009-8981(74)90014-x. [DOI] [PubMed] [Google Scholar]
- 9.Knapp M, Baranowski M, Czarnowski D, et al. Plasma sphingosine-1-phosphate concentration is reduced in patients with myocardial infarction. Med Sci Monit. 2009;15(9):CR490–93. [PubMed] [Google Scholar]
- 10.Anand SS, Yusuf S, Vuksan V, et al. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE) Lancet. 2000;356:279–84. doi: 10.1016/s0140-6736(00)02502-2. [DOI] [PubMed] [Google Scholar]
- 11.Ounpuu S, Negassa A, Yusuf S. INTER-HEART: A global study of risk factors for acute myocardial infarction. Am Heart J. 2001;141:711–21. doi: 10.1067/mhj.2001.114974. [DOI] [PubMed] [Google Scholar]
- 12.Kuch M, Janiszewski M, Mamcarz A, et al. Major adverse cardiac event predictors in survivors of myocardial infarction with asymptomatic left ventricular dysfunction or chronic heart failure. Med Sci Monit. 2009;15(6):PH40–48. [PubMed] [Google Scholar]
- 13.Mak KH, Chia KS, Kark JD, et al. Ethnic differences in acute myocardial infarction in Singapore. Eur Heart J. 2003;24:151–60. doi: 10.1016/s0195-668x(02)00423-2. [DOI] [PubMed] [Google Scholar]
- 14.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction) Circulation. 2004;110:e82–292. [PubMed] [Google Scholar]
- 15.Beamer AD, Lee TH, Cook EF, et al. Diagnostic implications for myocardial ischemia of the circadian variation of the onset of chest pain. Am J Cardiol. 1987;60:998–1002. doi: 10.1016/0002-9149(87)90340-7. [DOI] [PubMed] [Google Scholar]
- 16.Kostis JB, Moreyra AE, Amendo MT, et al. The effect of age on heart rate in subjects free of heart disease. Studies by ambulatory electrocardiography and maximal exercise stress test. Circulation. 1982;65:141–45. doi: 10.1161/01.cir.65.1.141. [DOI] [PubMed] [Google Scholar]
- 17.Misra MK, Sarwat M, Bhakuni P, et al. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15(10):RA209–19. [PubMed] [Google Scholar]
- 18.Carskadon MA, van den Hoed J, Dement WC. Sleep and daytime sleepiness in the elderly. J Geriatr Psychiatry. 1980;13:135–51. [PubMed] [Google Scholar]
- 19.Mulcahy D, Keegan J, Cunningham D, et al. Circadian variation of total ischaemic burden and its alteration with anti-anginal agents. Lancet. 1988;2:755–59. doi: 10.1016/s0140-6736(88)92414-2. [DOI] [PubMed] [Google Scholar]
- 20.Lopez F, Lee KW, Marin F, et al. Are there ethnic differences in the circadian variation in onset of acute myocardial infarction? A comparison of 3 ethnic groups in Birmingham, UK and Alicante, Spain. Int J Cardiol. 2005;100:151–54. doi: 10.1016/j.ijcard.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Dimitrov I, Khadzhikhristev A. Dynamics of the incidence of myocardial infarct in Smolyan District 1965–1979. Vutr Boles. 1983;22:40–46. [PubMed] [Google Scholar]
- 22.Zhou RH, Xi B, Gao HQ, et al. Circadian and septadian variation in the occurrence of acute myocardial infarction in a Chinese population. Jpn Circ J. 1998;62:190–92. doi: 10.1253/jcj.62.190. [DOI] [PubMed] [Google Scholar]
- 23.D’Negri CE, Nicola-Siri L, Vigo DE, et al. Circadian analysis of myocardial infarction incidence in an Argentine and Uruguayan population. BMC Cardiovasc Disord. 2006;6:1. doi: 10.1186/1471-2261-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sari I, Davutoglu V, Erer B, et al. Analysis of circadian variation of acute myocardial infarction: afternoon predominance in Turkish population. Int J Clin Pract. 2009;63:82–86. doi: 10.1111/j.1742-1241.2008.01717.x. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer BM, Caracciolo V, Frishman WH, Charney P. Gender, ethnicity and genetics in cardiovascular disease: part 1: Basic principles. Heart Dis. 2003;5:129–43. doi: 10.1097/01.hdx.0000061694.62343.01. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi M, Nakayama T, Fu Z, et al. Relationship between haplotypes of KCNN4 gene and susceptibility to human vascular diseases in Japanese. Med Sci Monit. 2009;15(8):CR389–97. [PubMed] [Google Scholar]
- 27.Lavie P. Do not take a siesta after lunch – fact or myth? Sleep. 2005;28:298–99. [PubMed] [Google Scholar]
- 28.Lipovetzky N, Hod H, Roth A, et al. Heavy meals as a trigger for a first event of the acute coronary syndrome: a case-crossover study. Isr Med Assoc J. 2004;6:728–31. [PubMed] [Google Scholar]
- 29.Peters RW, Zoble RG, Liebson PR, et al. Identification of a secondary peak in myocardial infarction onset 11 to 12 hours after awakening: the Cardiac Arrhythmia Suppression Trial (CAST) experience. J Am Coll Cardiol. 1993;22:998–1003. doi: 10.1016/0735-1097(93)90408-s. [DOI] [PubMed] [Google Scholar]