Summary
Background
To understand the heterogeneity of platelets, we investigated the correlation between von Willebrand factor (vWf) and mean platelet volume (MPV) in subjects with isolated impaired fasting glucose (IFG).
Material/Methods
We selected 48 subjects with isolated IFG and 48 normoglycemic control subjects matched for age, sex, and body mass index. We measured levels of vWf and MPV in all subjects.
Results
The levels of vWf and MPV were significantly higher in the isolated IFG group than the control group (p<0.05) Also, vWf level was positively correlated with MPV level in subjects in the isolated IFG group (r=0.452, p=0.001).
Conclusions
Our results suggest that vWf seems to be profoundly related to platelet volume in subjects with isolated IFG.
Keywords: von Willebrand factor, endothelial dysfunction, mean platelet volume, impaired fasting glucose
Background
Endothelial dysfunction (ED), which is characterized by an imbalance between relaxing and contracting factors, procoagulant and anticoagulant substances, and between pro-inflammatory mediators, may play a particularly significant role in the pathogenesis of atherosclerosis [1]. Under physiological conditions, the vascular endothelium produces many substances that are closely involved in hemostasis, fibrinolysis, growth factor synthesis, and the regulation of vessel tone and permeability. One of these substances is von Willebrand factor (vWf), which is synthesized by and stored in endothelial cells. vWf is a multimeric glycoprotein, and it is essential for platelet aggregation and adhesion [2]. Numerous clinical and experimental reports suggest that a high vWf level reflects endothelial damage or ED. vWf levels have been proposed as an indicator of ED [3–5].
Mean platelet volume (MPV), which is routinely determined by complete blood count analyzers, is a parameter reflecting the platelet size. Large platelets are more thrombogenic and active than normal sized platelets since they have a higher content of granules [6]. MPV, a determinant of platelet activation, is a newly emerging risk factor for atherothrombosis [7]. Elevated MPV levels have been identified as an independent risk factor for myocardial infarction in patients with coronary heart disease [8] and for death or recurrent vascular events after myocardial infarction [9]. Moreover, increased platelet size has been reported in patients with vascular risk factors such as diabetes mellitus [10,11], and in patients with acute ischemic stroke [12], essential hypertension [13], obesity [14].
There have been few studies of the relationship between vWf and MPV [15,16]. To the best of our knowledge, there has been no study on the relationship between levels of vWf and MPV in subjects with isolated IFG. Therefore, we investigated the relationship between von vWf and MPV in subjects with isolated IFG.
Material and Methods
Patients
This study was performed in the population of our previous study (48 subjects with isolated IFG and 48 normoglycemic healthy controls matched for age, sex, and body mass index, who attended our outpatient clinic) [17]. We performed a retrospective analysis of the data of our previous study to determine if there is a relationship between levels of vWf and MPV levels.
Measurement of vWf
Plasma vWf levels were measured quantitatively by STA-LIATEST [(Diagnostica Stago (France)]. All of the sample measurements were performed in the same run.
Measurement of MPV
We measured MPV in blood samples collected in tubes containing citrate (1:4 v/v) in order to avoid the platelet swelling induced by EDTA [18]; samples were analyzed within 1 hour. A Cell-Dyn 3500 (Abbot) was used for all blood counts.
Statistical analysis
SPSS 10.0 for Windows was used for the statistical analysis. For α=0.05 (between each group) and power =80%, a sample size of >36 subjects per group was needed to detect an actual difference. Two-group comparisons (IFG vs. control) were performed with independent t-tests. Correlation between levels of vWf and MPV were assessed using Pearson’s correlation analysis. Data were expressed as the mean ±SD. A value of p<0.05 was considered as statistically significant.
Results
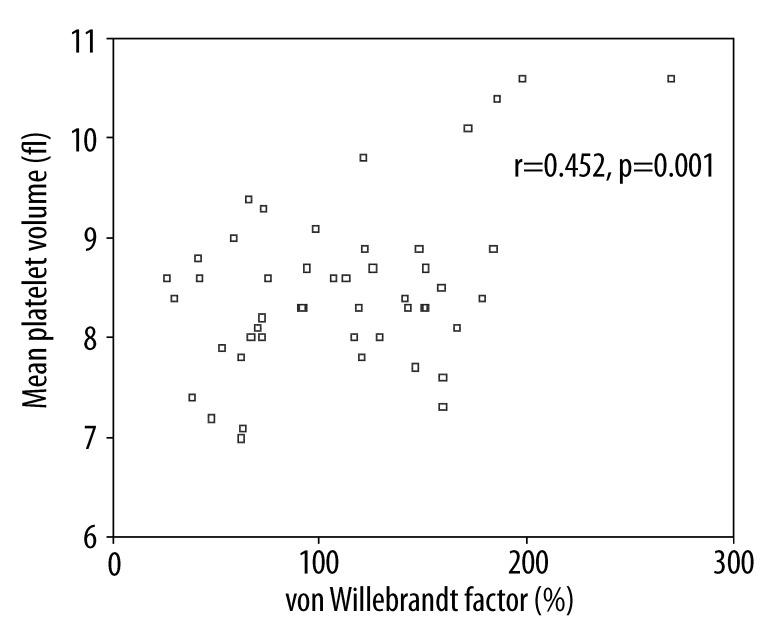
The main characteristics and laboratory results of the study population are reported in Table 1. The level of vWf was significantly higher in the IFG group than in the normoglycemic control group (111.08±52.78% vs. 74.08±48.47%, p=0.001). MPV was significantly higher in the isolated IFG group than in the control group (8.49±0.83 vs. 7.99±0.57, p=0.002). vWf level was positively correlated with MPV level in the IFG group (r=0.452, p=0.001) (Figure 1).
Table 1.
The main characteristics and laboratory results of study groups.
| Parameter | Impaired fasting glucose group | Control group | P values |
|---|---|---|---|
| N (men/women) | 48 (33/15) | 48 (29/19) | NS |
| Age (y) | 48.89±7.94 | 45.79±8.91 | NS |
| Body mass index (kg/m2) | 23.76±1.40 | 23.59±1.72 | NS |
| Systolic blood pressure (mmHg) | 122.81±16.32 | 123.56±15.46 | NS |
| Diastolic blood pressure (mmHg) | 74.18±9.16 | 73.47±10.07 | NS |
| Fasting glucose (mg/dl) | 107.29±5.27 | 87.87±6.86 | 0.001 |
| Alanin amino transferase (U/l) | 27.14±6.83 | 26.38±5.92 | NS |
| Creatinine (mg/dl) | 1.0±0.3 | 0.9±0.2 | NS |
| Total cholesterol (mg/dl) | 181.18±21.21 | 186.64±19.35 | NS |
| LDL-cholesterol (mg/dl) | 103.18±23.05 | 109.12±21.75 | NS |
| HDL-cholesterol (mg/dl) | 52.60±13.22 | 57.41±13.39 | NS |
| Trygliceride (mg/dl) | 120.75±44.10 | 109.62±41.46 | NS |
| Von Willebrand faktor (%) | 111.08±52.78 | 74.08±48.47 | 0.001 |
| Mean platelet volume (fl) | 8.49±0.83 | 7.99±0.57 | 0.002 |
| Platelet counts (×109/l) | 249.7±68.9 | 253.1±66.3 | NS |
NS – not significant.
Figure 1.
Relationship between vWf and mean platelet volume in isolated IFG group.
Discussion
The present study demonstrated that there was significant correlation between vWf and MPV in subjects with isolated IFG. These results demonstrate that there was a relationship between endothelial dysfunction and platelet activation in subjects with isolated IFG. This finding has not been reported previously. Endothelial dysfunction may be triggerring the same mechanisms, and stimulate the production of large platelets in the bone marrow. On the other hand, hyperglycemia activates coagulation by raising concentrations of procoagulant factors such as vWF [19]. There have been few studies of the relationship between vWf and MPV. Ihara et al reported positive correlation between vWf and MPV in patients with ischemic heart disease and in patients with aortic aneurysm [15,16]. However, with the current data it is not possible to say whether high vWf levels are the cause of large platelet volume. Future cohort studies will be helpful in providing an answer.
Multiple mechanisms are involved in platelet activation of patients with diabetes mellitus, which can be categorized as follows: 1) hyperglycemia, 2) insulin deficiency and resistance, 3) associated metabolic conditions, and 4) other cellular abnormalities, and there may be similar mechanisms for IFG [20]. Several mechanisms have been proposed to contribute to the increased platelet activity caused by hyperglycemia, including the following: 1) non-enzymatic glycation of platelet membrane proteins that decrease membrane fluidity, which may increase platelet adhesion [21,22]; 2) osmotic effect of glucose that activates platelet GP IIb/IIIa and P-selectin expression [23]; 3) activation of protein kinase C, a mediator of platelet activation [24], and 4) glycation of circulating low-density lipoproteins (LDL), which increases intracellular calcium concentration and nitric oxide (NO) production [25].
The present study has some limitations. First, the study population was rather small, thus large-scale studies would be helpful in order to make further comments on the relation between MPV and vWf. Second, unfortunately, we could not perform the measurement of other ED and platelet activation markers. The third limitation is that our analyses were based on a single baseline determination that may not reflect the patients’ status over long periods.
Conclusions
In conclusion, our results suggest that VWF seems to be related to platelet volume in subjects with isolated IFG.
Footnotes
Source of support: Self financing
References
- 1.Poredos P. Endothelial dysfunction in the pathogenesis of atherosclerosis. Int Angiol. 2002;21:109–16. [PubMed] [Google Scholar]
- 2.Lip GY, Blann AD. Von Willebrand factor: a marker of endothelial dysfunction in vascular disorders? Cardiovasc Res. 1997;34:255–65. doi: 10.1016/s0008-6363(97)00039-4. [DOI] [PubMed] [Google Scholar]
- 3.Wagner DD. Cell biology of von Willebrand factor. Annu Rev Cell Biol. 1990;6:217–46. doi: 10.1146/annurev.cb.06.110190.001245. [DOI] [PubMed] [Google Scholar]
- 4.Pearson JD. Markers of endothelial cell perturbation and damage. Br J Rheumatol. 1993;32:651–52. doi: 10.1093/rheumatology/32.8.651. [DOI] [PubMed] [Google Scholar]
- 5.Blann AD, Taberner DA. A reliable marker of endothelial cell dysfunction: does it exist? Br J Haematol. 1995;90:244–48. doi: 10.1111/j.1365-2141.1995.tb05143.x. [DOI] [PubMed] [Google Scholar]
- 6.Bath PM, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis. 1996;7:157–61. [PubMed] [Google Scholar]
- 7.Banrroft AJ, Abel EW, Mclaren M, Belch JJ. Mean platelet volume is a useful parameter: a reproducible routine method using a modified Coulter thrombocytometer. Platelets. 2000;11:379–87. doi: 10.1080/09537100020008311. [DOI] [PubMed] [Google Scholar]
- 8.Endler G, Klimesch A, Sunder-Plassmann H, et al. Mean platelet volume is an independent risk factor for myocardial infarction but not for coronary artery disease. Br J Haematol. 2002;117:399–404. doi: 10.1046/j.1365-2141.2002.03441.x. [DOI] [PubMed] [Google Scholar]
- 9.Martin JF, Bath PM, Burr ML. Influence of platelet size on outcome after myocardial infarction. Lancet. 1991;338:1409–11. doi: 10.1016/0140-6736(91)92719-i. [DOI] [PubMed] [Google Scholar]
- 10.Tschoepe D, Esser J, Schwippert B, et al. Large platelets circulate in an activated state in diabetes mellitus. Semin Thromb Hemost. 1991;17:433–39. doi: 10.1055/s-2007-1002650. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim HA, El-Meligi AA, Abdel Hamid M, Elhendy A. Relations between von Willebrand factor, markers of oxidative stress and microalbuminuria in patients with type 2 diabetes mellitus. Med Sci Monit. 2004;10(3):CR85–89. [PubMed] [Google Scholar]
- 12.Greisenegger S, Endler G, Hsieh K, et al. Is elevated mean platelet volume associated with a worse outcome in patients with acute ischemic cerebrovascular events? Stroke. 2004;35:1688–91. doi: 10.1161/01.STR.0000130512.81212.a2. [DOI] [PubMed] [Google Scholar]
- 13.Coban E, Yazicioglu G, Berkant Avci A, Akcit F. The mean platelet volume in patients with essential and white coat hypertension. Platelets. 2005;16:435–38. doi: 10.1080/09537100500163572. [DOI] [PubMed] [Google Scholar]
- 14.Coban E, Ozdogan M, Yazicioglu G, Akcit F. The mean platelet volume in patients with obesity. Int J Clin Pract. 2005;59:981–82. doi: 10.1111/j.1742-1241.2005.00500.x. [DOI] [PubMed] [Google Scholar]
- 15.Ihara A, Kawamoto T, Matsumoto K, et al. Relationship between hemostatic factors and the platelet index in patients with ischemic heart disease. Pathophysiol Haemost Thromb. 2006;35:388–91. doi: 10.1159/000097694. [DOI] [PubMed] [Google Scholar]
- 16.Ihara A, Matsumoto K, Kawamoto T, et al. Relationship between hemostatic markers and platelet indices in patients with aortic aneurysm. Pathophysiol Haemost Thromb. 2006;35:451–56. doi: 10.1159/000102053. [DOI] [PubMed] [Google Scholar]
- 17.Coban E, Bostan F. Plasma levels of von Willebrand factor in subjects with isolated impaired fasting glucose. Med Sci Monit. 2009;15(4):CR194–97. [PubMed] [Google Scholar]
- 18.Bath PM. The routine measurement of platelet size using sodium citrate alone as the anticoagulant. Thromb Haemost. 1993;70:687–90. [PubMed] [Google Scholar]
- 19.Kessler L, Wiesel ML, Attali P, et al. Von Willebrand factor in diabetic angiopathy. Diabetes Metab. 1998;24:327–36. [PubMed] [Google Scholar]
- 20.Ferreiro JL, Gomez-Hospital JA, Angiolillo DJ. Platelet abnormalities in diabetes mellitus. Diab Vasc Dis Res. 2010;7(4):251–59. doi: 10.1177/1479164110383994. [DOI] [PubMed] [Google Scholar]
- 21.Winocour PD, Watala C, Perry DW. Kinlough-Rathbone RL. Decreased platelet membrane fluidity due to glycation or acetylation of membrane proteins. Thromb Haemost. 1992;68:577–82. [PubMed] [Google Scholar]
- 22.Watala C, Golański J, Boncler MA, et al. Membrane lipid fluidity of blood platelets: a common denominator that underlies the opposing actions of various agents that affect platelet activation in whole blood. Platelets. 1998;9:315–27. doi: 10.1080/09537109876564. [DOI] [PubMed] [Google Scholar]
- 23.Keating FK, Sobel BE, Schneider DJ. Effects of increased concentrations of glucose on platelet reactivity in healthy subjects and in patients with and without diabetes mellitus. Am J Cardiol. 2003;92:1362–65. doi: 10.1016/j.amjcard.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 24.Assert R, Scherk G, Bumbure A, et al. Regulation of protein kinase C by short-term hyperglycaemia in human platelets in vivo and in vitro. Diabetologia. 2001;44:188–95. doi: 10.1007/s001250051598. [DOI] [PubMed] [Google Scholar]
- 25.Ferretti G, Rabini RA, Bacchetti T, et al. Glycated low-density lipoproteins modify platelet properties: a compositional and functional study. J Clin Endocrinol Metab. 2002;87:2180–84. doi: 10.1210/jcem.87.5.8466. [DOI] [PubMed] [Google Scholar]