Summary
Background
The broad spectrum of oxidative damage DNA biomarkers: urinary excretion of 8-oxodG (8-oxo-7,8-dihydro-2′-deoxyguanosine), 8-oxoGua (8-oxo-7,8-dihydroguanine) as well as the level of oxidative damage DNA in leukocytes, was analyzed in cancer patients and healthy subjects.
Material/Methods
222 cancer patients and 134 healthy volunteers were included in the analysis, using methodologies which involve HPLC (high-performance liquid chromatography) prepurification followed by gas chromatography with isotope dilution mass spectrometry detection and HPLC/EC.
Results
For the whole patient population (n=222) the median values of 8-oxoGua and 8-oxodG in urine samples were 12.44 (interquartile range: 8.14–20.33) [nmol/24 hr] and 6.05 (3.12–15.38) [nmol/24 hr], respectively. The median values of 8-oxoGua and 8-oxodG in urine samples of the control group (n=85) were 7.7 (4.65–10.15) [nmol/24 hr] and 2.2 (1.7–2.8) [nmol/24 hr], respectively. The level of 8-oxodG in DNA isolated from leukocytes of the patient population (n=179) and of the control group (n=134) was 4.93 (3.46–9.27) per 10’6 dG and 4.46 (3.82–5.31) per 10’6 dG, respectively.
Conclusions
The results suggest that oxidative stress in cancer patients, demonstrated by augmented amounts of these modifications in urine, could be typical not only for affected tissue but also for other tissues and even the whole organism. An assay that enables the determination of levels of basic markers of oxidative stress might be applied in clinical practice as an additional, helpful marker to diagnose cancer.
Keywords: damage DNA, molecular markers, cancer patients, 8-oxoGua, 8-oxodG
Background
Oxidatively modified bases
Hydroxyl radical attack on DNA most frequently leads to base damages that result in generating a range of derivatives [1–3]. Aerobic organisms, within the course of evolution, developed a range of adaptive mechanisms inducing synthesis of anti-oxidative enzymes and/or enzymes repairing oxidative damages of DNA [5–7].
Oxidative damage to nucleic acid has been associated with a number of pathologies including cancer and neurodegenerative and cardiovascular diseases [8–10].
To date, more than 20 different types of oxidative modifications of bases have been identified [4].
Of all the modified bases, the processes which repair 8-oxoGua are perhaps best understood, and may be regarded as a template for the processes which repair other lesions. To combat the deleterious biological effect of the presence of 8-oxoGua, cells have developed specific mechanisms to remove this lesion from cellular DNA [11].
In mammalian cells, 3 enzymes form the equivalent of the bacterial “GO” system. The first level of this protection is human Mut T homologue (hMTH1) which hydrolyses 8-oxodGTP (a potential substrate for DNA polymerase), thereby eliminating it from the nucleotide pool. The second level of defence is specific glycosylases that initiate base excision repair (BER). Finally, human Mut Y homologue (hMYH) removes adenine that is mis-paired with 8-oxoGua. Most recently, we proposed that nucleotide excision repair (NER), which involves the removal of a lesion-containing oligonucleotide, may compliment the “GO” system [12,13], based upon evidence that oxidative DNA damage may be repaired by this route [14,15].
However, there is very little evidence that 8-oxodG is a direct product of DNA repair itself (ie, released as the deoxynucleoside, rather than the base, from DNA) [16].
It is generally accepted that products of cellular repair of oxidatively damaged DNA, such as modified bases and nucleosides: 8-oxo-7,8-dihydroguanine (8-oxoGua) and 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) are excreted in urine.
Diet as a potential source of 8-oxoGua and 8-oxodG in urine
An assay was performed to analyze levels of 8-oxoGua and 8-xoxdG in urine samples with regard to diet. In case of the examined group, a conclusion was drawn that diet does not determine excretion of these biomolecules in urine [17]. In another study, different amounts (up to 25 mg) of 15N labeled oxidatively modified DNA were absorbed orally by volunteers. Throughout 2 weeks, blood and urine samples were collected.
No 15N 8-oxoGua or 8-oxodG were detected either in urine and or in DNA of monoclonal cells in venous blood obtained from the same subjects taking part in the study [18], demonstrating that diet has no influence on the level of these damages.
Cell death as a potential source of 8-oxoGua and 8-oxodG in urine
In both known works by Faure et al. [19] and Erhola et al. [20], no rise of levels in 8-oxodG excretion in urine was observed despite unequivocal evidence for mass reduction of treated tumors. In certain reports, a clear rise of 8-oxodG in urine excretion was observed after radio-chemotherapy or radiotherapy itself [21–23].
However, measuring excretion of repair product in urine exclusively may be misleading since it provides no information on the oxidative state of the organism (damage rate vs. repair rate) in cellular DNA and reports only the mean value of damage repair in the past.
Purpose
The aim of the work was to investigate whether the levels of markers of oxidative damages of DNA: excreted in urine 8-oxo-7,8-dyhydro-2′-deoxyguanosine (8-oxodG) and 8-oxo-7,8-dyhydroguanine (8-oxoGua) as well as the level of 8-oxodG in DNA of venous blood leukocytes differ in a population of healthy subjects when compared with cancer patients.
Material and Methods
Patients
Analysis of daily excretion of 8-oxoGua and 8-oxodG with urine was done in a study group consisting of 222 patients with malignant cancer (III and IV degree of clinical stage). Leukocytes from peripheral blood samples for analysis of 8-oxodG level were obtained from 179 patients from among the study group. Control peripheral blood samples for analysis of 8-oxodG in leukocytes were obtained from 134 healthy volunteers. From this group, 85 urine samples were taken for the measurement of daily excretion of 8-oxoGua and 8-oxodG in urine. The patients had various malignant tumors:, head and neck cancer (n=45), breast cancer (n=32), colon cancer (n=25), lung cancer (n=37), uterine cancer (n=15), ovarian cancer (n=39), testicular cancer (n=7), prostate cancer (n=11), gastrointestinal cancer (n=11).
Spot urine samples and blood were collected before the treatment. The patients were asked to abstain from vitamin supplementation for at least 1 month before the chemotherapy started and during the course of the treatment, and only these patients qualified. The control group was chosen in such a way that the following criteria matched the patient group: eating habits, age, body weight, sex, and smoking status.
The study was approved by the medical ethics committee of The Collegium Medicum Nicolaus Copernicus University Bydgoszcz, Poland, (in accordance with Good Clinical Practice, Warsaw 1998), and all the patients gave informed consent.
Isolation of leukocytes from venous blood
Venous blood samples (18 ml) from the patient and volunteer groups were collected. The blood was carefully applied on top of Histopaque 1119 solution (Sigma-Aldrich Inc.; St. Louis, MO) and leukocytes were isolated by centrifugation according to the procedure specified by the manufacturer.
DNA isolation and 8-oxodG determination in DNA isolates
DNA from leukocytes was isolated using the method as described earlier [24]. Determination of 8-oxodG by the mean of HPLC/EC technique was as described previously [24,25].
Urine analysis
Urine sample preparation, HPLC purification and GC/MS analysis were conducted as described earlier [24].
Statistical analysis
All results are expressed as median (interquartile range). STATISTICA (data analysis software system), version 9.0. www.statsoft.com. (lic. no: JXVP002E256522AR-E) was used for the statistical analysis. Mann-Whitney testing for independent groups with abnormal distribution was performed. For normal distribution, variables were analyzed by the Kolmogorov-Smirnov test with Lillefor’s correction. Statistical significance was considered at P<0.05.
Results
Significantly elevated levels of 8-oxoGua and 8-oxodG excreted in urine daily as well as 8-oxodG in DNA of leucocytes in venous blood were observed in cancer patients as compared with healthy subjects, with considerable statistic significance (Table 1).
Table 1.
The level of 8-oxogua, 8-oxodg in the urine, and 8-oxodg in the leukocytes’ DNA.
| Median (interquartile range) | Mean (± SD) | |
|---|---|---|
| Healthy group (n=134) | Cancer group (n=222) | |
| Urinary 8-oxoGua (nmol/24h) | ||
| Median | 7.7 (4.65–10.15) | 12.44 (8.14–20.33) |
| Mean | 7.8 (±4.21) | 15.25 (±10.91) |
| Urinary 8-oxodG (nmol/24h) | ||
| Median | 2.2 (1.7–2.8) | 6.05 (3.12–15.38) |
| Mean | 2.3 (±0.84) | 13.05 (±20.46) |
| Leukocytes’ 8-oxodG/106dG | ||
| Median | 4.46 (3.82–5.31) | 4.93 (3.46–9.27) |
| Mean | 4.73 (±1.11) | 7.24 (±5,59) |
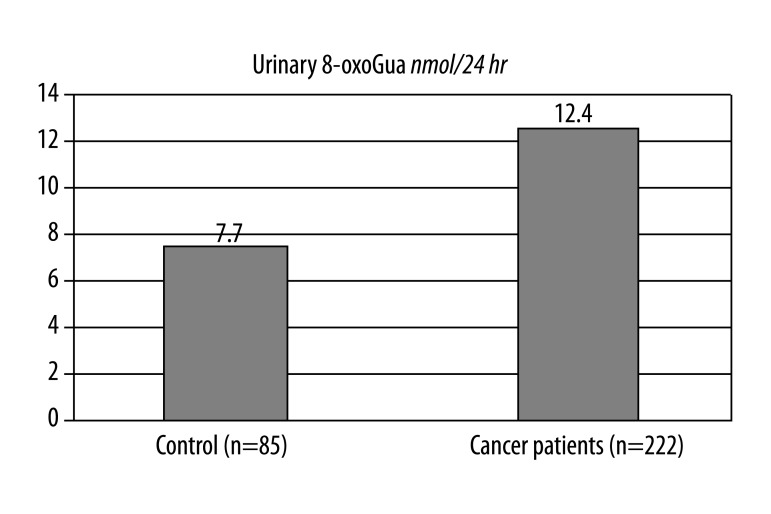
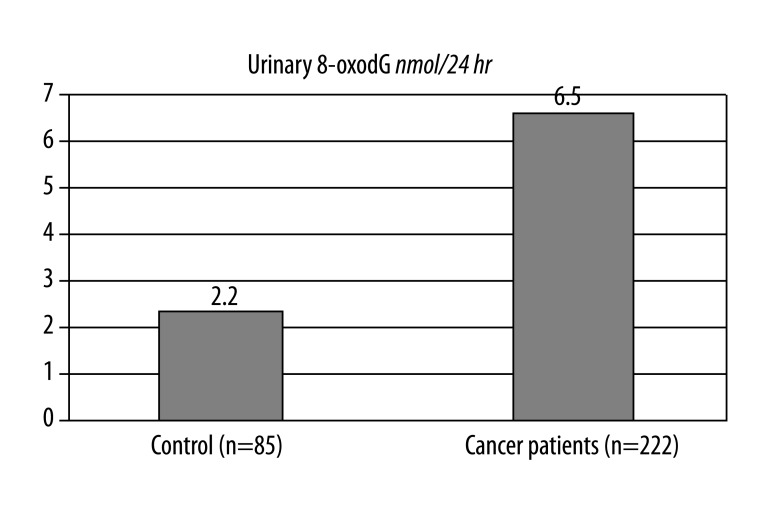
For the whole patient population (n=222), the median values of 8-oxoGua and 8-oxodG in urine samples were 12.44 (interquartile range: 8.14–20.33) [nmol/24 hr] and 6.05 (3.12–15.38) [nmol/24 hr], respectively.
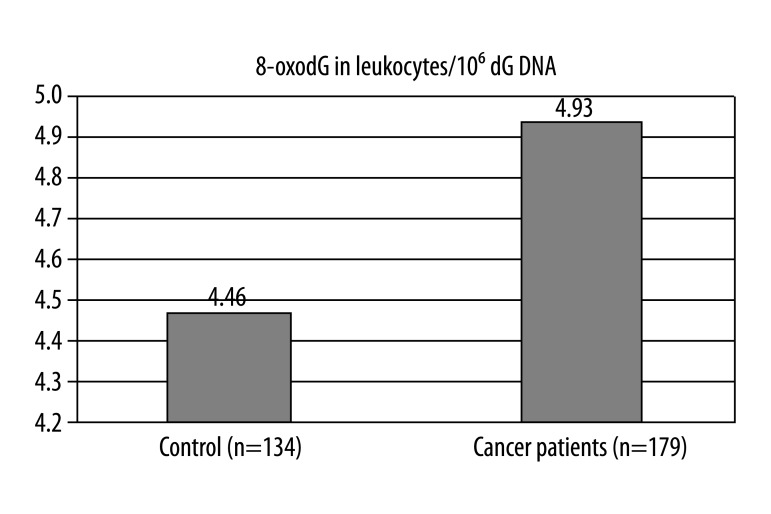
The median values of 8-oxoGua and 8-oxodG in urine samples of the control group (n=85) were 7.7 (4.65–10.15) [nmol/24 hr] and 2.2 (1.7–2.8) [nmol/24 hr], respectively. The level of 8-oxodG in DNA isolated from leukocytes in the patient group (n=179) and of the control group (n=134) was 4.93 (3.46–9.27) per 106 dG and 4.46 (3.82–5.31) per 106 dG, respectively.
Discussion
Certain amounts of oxidatively modified bases are present in every cell, reflecting the balance between ROS attacking DNA in the course of many metabolic processes and damage repair of these molecules by specific enzymes repairing DNA. It is not known at present what is the endogenous level of these potentially mutagenic damages. According to the reports of authors applying different analytical techniques, the values range from 0.2 to several modifications/106 pairs of bases for healthy cells [26,27]. It seems, however, that the level differs considerably among subjects [28,29]. They are removed in the process of repair and excreted in urine in unchanged state. A balance between producing ROS which induce oxidative DNA damages and removing these damages was observed in a cell (background level) [30]. According to some authors, the levels of these modifications do not depend on the type of cancer and histopathologic diagnosis [23,31–33].
It is possible to estimate the extent of repair on the level of the whole organism while analyzing the amount of oxidative DNA damages in urine. High values of oxidative damages of DNA excreted in urine indicate an intensified level of oxidative stress, but they may also reflect high efficiency of repair systems of these damages (oxidative stress may be high, yet repair mechanisms remove its effects). However, combining the background level specific for every patient and analyzing 8-oxoGua and 8-oxodG in excreted urine may clearly reflect information about DNA repair mechanisms.
The results presented in the present study comparing amounts of 8-oxoGua and 8-oxodG excreted in urine daily as well as amounts of 8-oxodG in venous blood DNA leucocytes in patients with diagnosed cancer when compared with healthy subjects demonstrate higher levels of these modifications in cancer patients (Figures 1–3). Significantly elevated levels of analyzed markers of oxidative damage DNA may reflect the oxidative stress situation which accompanies cancer [34,35].
Figure 1.
Daily urine excretion of 8-oxoGua in control group of healthy and all patients before treatment (median values). Mann-Whitney Test p=0.004.
Figure 3.
Levels of 8-oxodG in DNA isolated from venous blood leucocytes in control group of healthy subjects and cancer patients before treatment (median values). Mann-Whitney Test p=0.0017.
Several reasons account for this phenomenon. Several studies [19,20,23,31] have analyzed 8-oxoGua and 8-oxodG in urine in different types of cancer patients and control groups, reporting elevated level of 8-oxoGua in urine of cancer patients and in a control group of smoking subjects, and the level of 8-oxodG in DNA isolated from venous blood leucocytes was higher in the patient group.
These findings suggest that in cancer patients repair mechanisms of oxidative damage to DNA are less efficient (the concentration of modified nucleoside/base in urine reflects DNA damages of the whole organism, and the level of 8-oxodG in cellular DNA reflects balance between processes generating damages and the ability of their repair).
Rozalski et al. [34] compared a group of cancer patients and healthy volunteers with regard to the amount of 8-oxoGua and 8-oxodG excreted in urine, demonstrating elevated levels of oxidatively modified base in patients when compared with healthy subjects by 50%.
Conclusions
Our work indicates the levels of these derivatives elevated to such an extent cannot be related to its increase in cancer cells exclusively. The results suggest that oxidative stress in patients with cancer, demonstrated by elevated levels of these modifications in urine, may be typical not only of affected tissue but also of other tissues and even the whole organism.
From practical point of view, a test that would enable determination of background levels of basic markers of oxidative stress (8-oxoGua and 8-oxodG in urine and 8-oxodG in DNA in leukocytes), might be applied as an additional and helpful marker for early detection of the development of cancer.
Figure 2.
Daily urine excretion of 8-oxodG in control group of healthy and all patients before treatment (median values) Mann-Whitney Test p=0.0001.
Footnotes
Source of support: Departmental sources
References
- 1.Floyd RA, West MS, Eneff KL, Schneider JE. Methylene blue plus light mediates 8-hydroxyguanine formation in DNA. Arch Biochem Biophys. 1989;273:106–11. doi: 10.1016/0003-9861(89)90167-7. [DOI] [PubMed] [Google Scholar]
- 2.Dizdaroglu M. Characterization of free radical-induced damage to DNA by the combined use of enzymatic hydrolysis and gas chromatography-mass spectrometry. J Chromatogr. 1986;367:357–66. doi: 10.1016/s0021-9673(00)94856-8. [DOI] [PubMed] [Google Scholar]
- 3.Oliński R. DNA damage induced by active oxygen species and its role in the carcinogenesis process. Postepy Hig Med Dosw. 1993;47:463–74. [PubMed] [Google Scholar]
- 4.Dizdaroglu M. Quantitative determination of oxidative base damage in DNA by stable isotope-dilution mass spectrometry. FEBS Lett. 1993;315:1–6. doi: 10.1016/0014-5793(93)81120-o. [DOI] [PubMed] [Google Scholar]
- 5.Demple B, Herman T, Chcn DS. Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: definition of a family of DNA repair enzymes. Proc Natl Acad Sci USA. 1991;88:11450–54. doi: 10.1073/pnas.88.24.11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bessho T, Roy R, Yamamoto K, et al. Repair of 8-hydroxyguanine in DNA by mammalian N-methylpurine-DNA glycosylase. Proc Natl Acad Sci USA. 1993;90:8901–4. doi: 10.1073/pnas.90.19.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nash M, Bruner SD, Scharer GD, et al. Cloning of a yeast 8-oxyguanine DNA glycosylase reveals the existence of a base-excision DNA-repair protein superfamily. Curr Biol. 1996;6:968–80. doi: 10.1016/s0960-9822(02)00641-3. [DOI] [PubMed] [Google Scholar]
- 8.Bilinska M, Wolszakiewicz J, Duda M, et al. Antioxidative activity of sulodexide, a glycosaminoglycan, in patients with stable coronary artery disease: A pilot study. Med Sci Monit. 2009;15(12):CR618–23. [PubMed] [Google Scholar]
- 9.Misra M, Sarwat M, Bhakuni P, et al. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15(10):RA209–19. [PubMed] [Google Scholar]
- 10.Ueno T, Watanabe H, Fukuda N, et al. Influence of genetic polymorphisms in oxidative stress related genes and smoking on plasma MDA-LDL, soluble CD40 ligand, E-selectin and soluble ICAM1 levels in patients with coronary artery disease. Med Sci Monit. 2009;15(7):CR341–48. [PubMed] [Google Scholar]
- 11.Cooke MS, Olinski R, Evans MD. Does measurement of oxidative damage to DNA have clinical significance? Clinica Chimica Acta. 2006;365:30–49. doi: 10.1016/j.cca.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Cooke MS, Evans MD, Herbert KE, Lunec J. Urinary 8-oxo-2′deoxyguanosine- source, significance and supplements. Free Radic Res. 2000;32:381–97. doi: 10.1080/10715760000300391. [DOI] [PubMed] [Google Scholar]
- 13.Olinski R, Rozalski R, Gackowski D, et al. Urinary measurement of 8-OksodG, 8-OksoGua, and 5HMUra: a noninvasive assessment of oxidative damage to DNA. Antioxid Redox Signal. 2006;8:1011–19. doi: 10.1089/ars.2006.8.1011. [DOI] [PubMed] [Google Scholar]
- 14.Kuraoka I, Bender C, Romieu A, et al. Removal of oxygen freeradical- induced 5′,8-purine cyclodeoxynucleosides from DNA by the nucleotide excision-repair pathway in human cells. Proc Natl Acad Sci USA. 2000;97:3832–37. doi: 10.1073/pnas.070471597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks PJ, Wise DS, Berry DA, et al. The oxidative DNA lesion 8,5′-(S)-cyclo-2′-deoxyadenosine is repaired by the nucleotide excision repair pathway and blocks gene expression in mammalian cells. J Biol Chem. 2000;275:22355–62. doi: 10.1074/jbc.M002259200. [DOI] [PubMed] [Google Scholar]
- 16.Mundt JM, Hah SS, Sumbad RA, et al. Incorporation of extracellular 8-oxodG into DNA and RNA requires purine nucleoside phosphorylase in MCF-7 cells. Nucleic Acids Res. 2008;36(1):228–36. doi: 10.1093/nar/gkm1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gackowski D, Rozalski R, Roszkowski K, et al. 8-Okso-7,8-dihydroguanine and 8-okso-7,8-dihydro-2-deoxyguanosine levels in human urine do not depend on diet. Free Radic Res. 2001;35:825–32. doi: 10.1080/10715760100301321. [DOI] [PubMed] [Google Scholar]
- 18.Cooke MS, Evans MD, Dove R, et al. DNA repair is responsible for the presence of oxidatively damaged DNA lesions in urine. Mutat Res. 2005;574:58–66. doi: 10.1016/j.mrfmmm.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Faure H, Mousseau M, Cadet J, et al. Urine 8-okso-7,8-dihydro-2-deoxyguanosine vs. 5-(hydroxymethyl) uracil as DNA oxidation marker in adriamycin-treated patients. Free Radic Res. 1998;28:377–82. doi: 10.3109/10715769809070806. [DOI] [PubMed] [Google Scholar]
- 20.Erhola M, Toyokuni S, Okada K, et al. Biomarker evidence of DNA oxidation in lung cancer patients: association of urinary 8-hydroxy-2-deoxyguanosine excretion with radiotherapy, chemotherapy, and response to treatment. FEBS Lett. 1997;409:287–91. doi: 10.1016/s0014-5793(97)00523-1. [DOI] [PubMed] [Google Scholar]
- 21.Bergman V, Leanderson P, Starkhammar H, Tagesson C. Urinary excretion of 8-hydroxydeoxyguanosine and malondialdehyde after high dose radiochemotherapy preceding stem cell transplantation. Free Radic Biol Med. 2004;36:300–6. doi: 10.1016/j.freeradbiomed.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Haghdoost S, Svoboda P, Naslund I, et al. Can 8-okso-dG be used as a predictor for individual radiosensitivity? Int J Radiat Oncol Biol Phys. 2001;50:405–10. doi: 10.1016/s0360-3016(00)01580-7. [DOI] [PubMed] [Google Scholar]
- 23.Roszkowski K, Gackowski D, Rozalski R, et al. Small field radiotherapy of head and neck cancer patients is responsible for oxidatively damaged DNA/oxidative stress on the level of a whole organism. Int J Cancer. 2008;123:1964–67. doi: 10.1002/ijc.23700. [DOI] [PubMed] [Google Scholar]
- 24.Siomek A, Gackowski D, Rozalski R, et al. Higher leukocyte 8-oxo-7,8-dihydro-2′-deoxyguanosine and lower plasma ascorbate in aging humans? Antioxid Redox Signal. 2007;9:143–50. doi: 10.1089/ars.2007.9.143. [DOI] [PubMed] [Google Scholar]
- 25.Foksinski M, Bialkowski K, Skiba M, et al. Evaluation of 8-oxodeoxyguanosine, typical oxidative DNA damage, in lymphocytes of ozone-treated arteriosclerotic patients. Mutat Res. 1999;438:23–27. doi: 10.1016/s1383-5718(98)00155-7. [DOI] [PubMed] [Google Scholar]
- 26.Jaruga P, Speina E, Gackowski D, et al. Endogenous oxidative DNA base modifications analysed with repair enzymes and GC/MS technique. Nucleic Acids Res. 2000;28:e16. doi: 10.1093/nar/28.6.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gedik CM, Collins A ESCODD. Establishing the background level of base oxidation in human lymphocyte DNA: results of an interlaboratory validation study. FASEB J. 2005;19:82–84. doi: 10.1096/fj.04-1767fje. [DOI] [PubMed] [Google Scholar]
- 28.Collins A, Gedik CM, Olmedilla B, et al. Oxidative DNA damage measured in human lymphocytes: large differences between sexes and between countries, and correlations with heart disease mortality rates. FASEB J. 1998;12:1397–400. [PubMed] [Google Scholar]
- 29.Maynard S, Schurman SH, Harboe C, et al. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2009;30(1):2–10. doi: 10.1093/carcin/bgn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooke MS, Olinski R, Loft S. Measurement and Meaning of Oxidatively Modified DNA Lesions in Urine. Cancer Epidemiol Biomarkers Prev. 2008;17(1):3–14. doi: 10.1158/1055-9965.EPI-07-0751. [DOI] [PubMed] [Google Scholar]
- 31.Siomek A, Tujakowski J, Gackowski D, et al. Severe oxidatively damaged DNA after cisplatin treatment of cancer patients. Int J Cancer. 2006;119:2228–30. doi: 10.1002/ijc.22088. [DOI] [PubMed] [Google Scholar]
- 32.Gackowski D, Speina E, Zielinska M, et al. Products of oxidative DNA damage and repair as possible biomarkers of susceptibility to lung cancer. Cancer Res. 2003;63:4899–902. [PubMed] [Google Scholar]
- 33.Inoue M, Osaki T, Noguchi M, et al. Lung Cancer Patients Have Increased 8-Hydroxydeoxyguanosine Levels in Peripheral Lung Tissue DNA. Jpn J Cancer Res. 1998;89:691–95. doi: 10.1111/j.1349-7006.1998.tb03272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rozalski R, Gackowski D, Roszkowski K, et al. The level of 8-hydroxyguanine, a possible repair product of oxidative DNA damage, is higher in urine of cancer patients than in control subjects. Cancer Epid Biom Prev. 2002;11:1072–75. [PubMed] [Google Scholar]
- 35.Cooke MS, Rozalski R, Dove R, et al. Evidence for attenuated cellular 8-oxo-7,8-dihydro-29-deoxyguanosine removal in cancer patients. Biol Chem. 2006;387(4):393–400. doi: 10.1515/BC.2006.053. [DOI] [PubMed] [Google Scholar]