Summary
Background
Syncope accounts for about 1–2% of emergency department visits, but the etiology in many patients with syncope is unclear. Recently, with the use of the head-up tilt test (HUT), the number of patients with unexplained syncope (UPS) has been decreasing; however, the spectrum of underlying diseases of syncope in children is unclear. This retrospective study aimed to analyze the spectrum of underlying diseases in children with syncope.
Material/Methods
This multi-center clinical study consisted of 888 children (417 males, 471 females, aged 5–18 yrs, median age 12.0±3.0 yrs) with syncope who came from Beijing city, Hunan province, Hubei province and Shanghai from August 1999 to March 2009. The clinical and laboratory data of children were studied and the spectrum of underlying diseases in children with syncope was analyzed.
Results
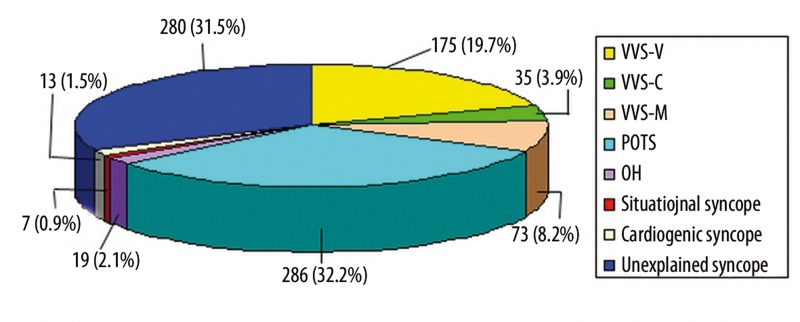
In 888 children with syncope, 175 (19.7%) had vasovagal syncope (VVS) with vasoinhibitory response, 35 (3.9%) had VVS with cardioinhibitory response, 73 (8.2%) had VVS with mixed response, 286 (32.2%) had postural orthostatic tachycardia syndrome (POTS), 19 (2.1%) had orthostatic hypotension, 7 (0.9%) had situational syncope, 13 (1.5%) had cardiogenic syncope, and 280 (31.5%) had unexplained syncope.
Conclusions
The data suggest that neurally-mediated syncope was the most common cause in children with syncope. POTS and VVS were the most common hemodynamic patterns of neurally-mediated syncope.
Keywords: children, syncope, neurally-mediated syncope
Background
Syncope accounts for about 1–2% of emergency department visits [1,2], but the etiology of many patients with syncope is unclear [3–7]. Recently, with the use of the head-up tilt test (HUT), the number of patients with unexplained syncope (UPS) has been decreasing. In the elderly with syncope, the most common cause of syncope is organic heart disease [8,9]. In non-elderly adults with syncope, 50–66% of cases are neurally-mediated syncope (NMS) [9], and less than 5% of cases are attributed to supraventricular tachycardia [10]. Some studies have concluded that the etiology of syncope is correlated with age [11]. There are marked differences in pathophysiology between children and adults; however, up to now, the spectrum of underlying diseases of syncope in children has been unclear. We analyzed the distribution of etiologies of syncope in children in China through a multicenter, large sample-based study.
Material and Methods
1. Patients
The study population consisted of 888 children (417 boys, 471 girls) aged 5 to 18 years (mean 12.0±3.0 years) with syncope, consecutively recruited from August 1999 to March 2009 in a multi-center network study of childhood syncope in Beijing, Shanghai, Hunan province, and Hubei province in China.
2. Clinical diagnostic protocol
All patients underwent an initial evaluation consisting of carefully taken history and physical examination including orthostatic and supine heart rate, blood pressure measurements and standard electrocardiogram (ECG). Through the initial evaluation: ➀ some common diseases could be diagnosed definitively, for example, postural orthostatic tachycardia syndrome (POTS), orthostatic hypotension (OH), pharmacal syncope, some severe arrhythmias, etc; ➁ several disorders that resemble syncope in different ways could be tentatively diagnosed (e.g., myocardiopathy, pulmonary hypertension, cyanotic congenital heart disease, some arrhythmias and carotid sinus hypersensitivity) on the basis of some of the following examinations when indicated – echocardiogram, 24-hour ambulatory electrocardiography monitoring, electroencephalogram (EEG), cranial or cervical computed tomography (CT), cardiac catheterization or cardiac electrophysiology examination to diagnose the aforementioned diseases; ➂ for those whose diagnosis still could not be made exactly or suggestively through aforesaid examinations in ➀ and ➁, the head-up tilt test (HUT) was indicated to diagnose vasovagal syncope (VVS), POTS, OH, etc. If some patients still could not be diagnosed exactly through all these examinations, the work-up was re-appraised.
3. Baseline head-up tilt test (BHUT)
Patients fasted for 12 hours before testing, and for at least 3 days before testing were not allowed to have any drugs that could possibly influence the autonomic nervous system. Baseline recordings of heart rate (HR), electrocardiogram and blood pressure (BP) with a Dash 2000 Multileads Physiological Monitor (GE, USA) in the supine position were obtained near the end of a 10-minute resting period. Then, the patient was tilted to 60 degrees on a tilt table. The monitoring of the variation of electrocardiogram, HR and BP was carried out along with clinical monitoring of symptoms. The BHUT was continued until positive response appeared, or for 45 minutes, whichever came first. If a positive response was elicited, the test was terminated by rapidly lowering the tilt table.
4. Sublingual nitroglycerin head-up tilt test (SNHUT)
SNHUT was conducted in patients with negative response in BHUT using nitroglycerin (4–6 μg/kg, the maximum dose ≤300 μg). The monitoring of electrocardiogram, HR and BP with a Dash 2000 Multileads Physiological Monitor (GE, USA) was carried out along with clinical monitoring of symptoms. The tests were terminated when positive responses appeared or for 20 minutes, whichever came first. If a positive response was elicited, the test was terminated by a rapid lowering of the tilt table. Informed consent was obtained from each child or their parents, and the study protocol was approved by the Ethics Committee of Peking University First Hospital.
5. Diagnostic criteria
A detailed history and physical examination are central to the diagnosis, which requires ruling out cardiovascular or neurologic diseases. VVS is diagnosed if precipitating events such as fear, severe pain, emotional distress, instrumentation or prolonged standing are associated with typical prodromal symptoms. The positive standard of HUT for VVS was that patients had syncope or presyncope in association with any of the following data in HUT: ➀ BP decreased; ➁ HR decreased; ➂ sinus arrest appeared; ➃ temple ➃°or larger than ➃° atrioventricular block and asystole for 3 seconds [12]. The standard of BP decrease was ≤80 mmHg (10.7 kPa, 1 mmHg=0.133kPa) in systolic BP or ≤50 mmHg in diastolic BP or a ≥25% decrease in mean BP [12]. If the child didn’t achieve the standard, but the symptom of syncope or presyncope appeared, the response was also defined as positive. If only the BP decreased, a positive response couldn’t be defined. Decreased HR included bradycardia. Vasoinhibitory response was defined as an obvious decrease in BP without HR reduction and cardioinhibitory response as an abrupt decrease in HR without systolic BP decrease. The mixed pattern response was characterized by a decrease in both HR and BP [13].
When HR increased ≥30 bpm or the maximum of HR ≥120 bpm during the head-up tilt test of 10 minutes, it was defined as postural orthostatic tachycardia syndrome (POTS), which is often accompanied by dizziness, chest distress, headache, palpitation, pallor, etc [14].
For the diagnosis of OH, orthostatic blood pressure measurements are recommended after 5 min of lying supine, followed by measurements taken each minute, or more often, after standing for 3 min. Measurements may continue longer if blood pressure is still falling at 3 min. If the patient does not tolerate standing for this long, the lowest systolic blood pressure during the upright posture should be recorded. A decrease in systolic blood pressure ≥20 mmHg, or a decrease in systolic blood pressure to <90 mmHg, is defined as OH, regardless of whether or not symptoms occur. The positive standard of standing test or HUT was as follows: ➀ BP was normal in prostration; ➁ BP decreased within 3 minutes when the patient’s position changed from prostration to orthostatism; and BP decrease was ≥20 mmHg (10.7 kPa, 1 mmHg=0.133 kPa) in systolic BP, or a decrease in systolic blood pressure to <90 mmHg [15].
A situational syncope is suggested if the event occurred with defecation, urination, coughing, or swallowing [16].
The diagnosis of cardiogenic syncope mainly relies on electrocardiogram, echocardiogram and 24-hour Holter monitoring, and consists primarily of cardiac ischemia and arrhythmia. Cardiac ischemia-related syncope is diagnosed when symptoms are present with ECG evidence of acute ischaemia with or without myocardial infarction, independent of its mechanism. Arrhythmia-related syncope is diagnosed by ECG when there is sinus bradycardia <40 beats/min or repetitive sinoatrial blocks or sinus pauses >3 s in the absence of negatively chronotropic medications, Mobitz II 2nd or 3rd degree atrioventricular block, alternating left and right bundle branch block, rapid paroxysmal supraventricular tachycardia or ventricular tachycardia and pacemaker malfunction with cardiac pauses [8].
Statistical analysis
All data were analyzed using SPSS version 10.0 for windows. Results are expressed as mean ± standard deviation. The scale variables are expressed as percentages.
Results
1. The underlying diseases of children with syncope
Among the 888 patients aged 5 to 18 years (mean 12.0±3.0 years) with syncope, 471 (53.0%) were female and 417 (47.0%) male. In patients with syncope, 175 (19.7%) were of VVS-vasoinhibitory pattern, 35 (3.9%) VVS-cardioinhibitory pattern, 73 (8.2%) VVS-mixed pattern, 286 (32.2%) POTS, 19 (2.1%) OH, 7 (0.9%) situational syncope, 13 (1.5%) cardiogenic syncope, and 280 (31.5%) were unexplained syncope (UPS) (Table 1).
Table 1.
The disease spectrum of syncope in children.
| Disgnosis | Nn(%) | Sex | Age (years) | |
|---|---|---|---|---|
| Male | Female | |||
| Neurally mediated syncope | 595 (67.0%) | 271 (45.5%) | 324 (54.5%) | 12.3±2.9 |
| VVS-V | 175 (19.7%) | 77 (44.0%) | 98 (56.0%) | 13.0±3.0 |
| VVS-C | 35 (3.9%) | 17 (48.6%) | 18 (51.4%) | 12.3±2.7 |
| VVS-M | 73 (8.2%) | 26 (35.6%) | 47 (64.4%) | 12.7±2.6 |
| POTS | 286 (32.2%) | 133 (46.5%) | 153 (53.5%) | 11.8±2.7 |
| OH | 19 (2.1%) | 12 (63.2%) | 7 (36.8%) | 10.0±2.9 |
| Situational syncope | 7 (0.9%) | 6 (85.7%) | 1 (14.3%) | 15.1±3.6 |
| Cardiogenic syncope | 13 (1.5%) | 4 (30.8%) | 9 (69.2%) | 12.5±3.2 |
| Unexplained syncope | 280 (31.5%) | 142 (50.7%) | 138 (49.3%) | 11.5±3.3 |
| Total | 888 (100.0%) | 417 (47.0%) | 471 (53.0%) | 12.0±3.0 |
VVS – stands for vasovagal syncope; POTS – stands for postural orthostatic tachycardia syndrome; OH – stands for orthostatic hypotension; VVS-V – stands for VVS-vasoinhibitory pattern; VVS-C – stands for VVS-cardioinhibitory pattern; VVS-M – stands for VVS-mixed pattern.
2. The baseline characteristics of children with syncope
In 888 children (417 male and 471 female) with syncope, their age ranged from 5 years to 18 years, and mean age was 12.0±3.0 years. In 595 children aged 5 to 18 years (mean 12.3±2.9 years) with NMS, there were 271 (45.5%) males and 324 (54.5%) females.
VVS-vasoinhibitory pattern accounted for 175 cases (19.7%), whose ages ranged from 5 to 18 years (mean 13.0±3.0 years); 77 (44.0%) were male and 98 (56.0%) were female. There were 35 (3.9%) cases with VVS-cardioinhibitory pattern, with an age range from 6 to 18 years (mean 12.3±2.7 years old), of whom 17 (48.6%) were males and 18 (51.4%) were females; 73 (8.2%) cases had VVS-mixed pattern, including 26 (35.6%) males and 47 (64.4%) females, age range from 5 to 17 years, with a mean of 12.7±2.6 years (Table 1, Figure 1).
Figure 1.
In this figure, we could see that the most common cause of syncope in children was postural orthostatic tachycardia syndrome (POTS) (32.2%). Unexplained syncope, vasovagal syncope-vasoinhibitory pattern (VVS-V), vasovagal syncope-mixed pattern (VVS-M), vasovagal syncope-cardioinhibitory pattern (VVS-C), orthostatic hypotension (OH), cardiogenic syncope and situational syncope accounted for 31.5%, 19.7%, 8.2%, 3.9%, 2.1%, 1.5% and 0.9%, respectively.
There were 286 (32.2%) (133 males, 153 females) children with POTS, with an age range from 5 to 18 years (mean 11.8±2.7 years old) (Table 1, Figure 1).
Nineteen (2.1%) children had OH, including 12 (63.2%) males and 7 (36.8%) females. Their age range was from 5 to 15 years, with a mean of 10.0±2.9 years old (Table 1, Figure 1).
There were 7 (0.9%) children with situational syncope, with an age range of 8 to 18 years (mean 15.1±3.6 years). Six cases were males and one was female (Table 1, Figure 1).
There were 13 children (4 male and 9 female) aged 7 to 18 years (mean 12.5±3.2 years) with cardiogenic syncope (Table 1, Figure 1).
In 280 children aged 5 to 18 years (mean 11.5±3.3 years) with UPS, 142 (50.7%) were males and 138 (49.3%) females (Table 1, Figure 1).
Discussion
Syncope is common in pediatric clinical practice. It is defined as “a sudden and brief loss of consciousness associated with a loss of postural tone, from which recovery is spontaneous” with a variety of causes [17]. Conditions causing syncope include NMS, followed by arrhythmia and organic heart diseases [8,18]. It usually accounts for about 1–2% [1,2] of emergency department visits. In clinical practice, more and more costly investigations typically yield fewer and fewer diagnoses [19], and the causes of syncope in many patients are unclear [3–7]. Children with syncope always are diagnosed as UPS in clinical service [20]. Recently, with the use of HUT [21], most children with UPS can be precisely diagnosed; however, there are still some cases of UPS.
There have been some studies on the disease spectrum in adults with syncope. Some reported that in elderly people with syncope, the most common cause of disease was organic heart disease, and the next was pulmonary artery thrombus and syncope subsequent to taking medicine [8,9]. In 1986, Lipsitz reported that in adults with syncope, 50–66% of patients had neurally-mediated syncope (NMS), 10–20% had psychogenic disease [9], and less than 5% of patients had syncope attributed to supraventricular tachycardia [10]. Other studies have discovered that various of etiologies of syncope were correlated with age [11]. However, there are many differences in physiological functions between children and adults.
However, there have been few recent studies on the disease spectrum of children with syncope. In 2004, Liu et al. [22] analyzed the cause of syncope in 30 children, finding that in 20 cases (66.7%), etiology of syncope was as follows: neurological disorders in 12 cases (40.0%), including ischemic of intracranialis artery in 7 cases, seizure in 1 case, hysteria in 2 cases, concussion of the brain in 1 case, and episodic dizziness accompanied by elbow pain in 1 case. Cardiovascular disorders were found in 7 cases (23.3%), including supraventricular tachycardia in 3 cases, long Q-T syndrome leading to ventricular tachycardia in 1 case, and tetralogy of Fallot in 3 cases. Anemia due to menorrhagia in found in 1 case (3.3%). The other 10 cases (33.3%) were found to be of uncertain etiologies, and 2 of them occurred post-exercise or following a long period of standing. However, because the diagnostic measures were limited and the number of patients was quite small, the outcome of the study could not reveal the constituent ratio of syncope in children. In 2008, we analyzed the etiology of syncope in 474 children [23]; however, this was a single center study instead of a multi-center study in China and the number of patients evaluated was small. There have not been any multi-center-based studies on the spectrum of underlying disease in children with syncope. Therefore, it would be very important to conduct a multi-center study with a large sample size on the disease spectrum in children with syncope. In 2009, Noizet-Yverneau, et al reported the frequency of syncope and pre-syncope in a pediatric emergency care unit. In their study, 159 children (mean age, 11±4 years) were included, accounting for 0.8% of the PECU’s visits. It was shown that 48% of patients had syncope and 52% had pre-syncope. The most common cause was neurally-mediated syncope. Neither cardiac arrhythmia nor obstructive cardiomyopathy was diagnosed [24].
In our multicenter, large sample study, we found that in 888 children with syncope, 175 (19.7%) were VVS-vasoinhibitory pattern, 35 (3.9%) were VVS-cardioinhibitory pattern, 73 (8.2%) were VVS-mixed pattern, 286 (32.2%) were POTS, 19 (2.1%) were OH, 7 (0.9%) were situational syncope and 13 (1.5%) were cardiogenic syncope, and there were 280 (31.5%) remaining unexplained cases of syncope.
Conclusions
We discovered that NMS was the most common cause of syncope in children, accounting for 67.0% of cases. NMS is a functional self-limited disease. Identifying the disease spectrum in children with syncope would be of great help in clinical practice. We should pay attention to the association of straddling subjects.
Our study is limited by its relatively small sample size. In spite of this limitation, we report that NMS was the most common cause of syncope in children. In the future, we will investigate the syncope spectrum in children through a larger multi-center study.
Acknowledgements
The work was done in Beijing, Hunan province, Hubei province, Shanghai, P. R. China.
Abbreviations
- VVS
vasovagal syncope
- POTS
postural orthostatic tachycardia syndrome
- HUT
head-up tilt test
- UPS
unexplained syncope
- NMS
neurally-mediated syncope
- ECG
electrocardiogram
- OH
orthostatic hypotension
- EEG
electroencephalogram
- CT
cranial or cervical computed tomography
- BHUT
baseline head-up tilt test
- HR
heart rate
- BP
blood pressure
- SNHUT
sublingual nitroglycerin head-up tilt test
Footnotes
Source of support: This work was supported by a grant from the Capital Medical Development Scientific Project (2007–2003), Science and Technology Program of Beijing (D10100050010059) and Changjiang Scholar Program
References
- 1.Colman N, Nahm K, Ganzeboom KS, et al. Epidemiology of reflex syncope. Clin Auton Res. 2004;14:9–17. doi: 10.1007/s10286-004-1003-3. [DOI] [PubMed] [Google Scholar]
- 2.Mathias CJ, Deguchi K, Schatz I. Observations on recurrent syncope and presyncope in 641 patients. Lancet. 2001;357:348–53. doi: 10.1016/S0140-6736(00)03642-4. [DOI] [PubMed] [Google Scholar]
- 3.Day SC, Cook EF, Funkenstein H, et al. Evaluation and outcome of emergency room patients with transient loss of consciousness. Am J Med. 1982;73:15–23. doi: 10.1016/0002-9343(82)90913-5. [DOI] [PubMed] [Google Scholar]
- 4.Kapoor WN. Evaluation and outcome of patients with syncope. Medicine (Baltimore) 1990;69:160–75. doi: 10.1097/00005792-199005000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Silverstein MD, Singer DE, Mulley AG, et al. Patients with syncope admitted to medical intensive care units. JAMA. 1982;248:1185–89. [PubMed] [Google Scholar]
- 6.Martin GJ, Adams SL, Martin HG, et al. Prospective evaluation of syncope. Ann Emerg Med. 1984;13:499–504. doi: 10.1016/s0196-0644(84)80511-9. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Chetrit E, Flugelman M, Eliakim M. Syncope: a retrospective study of 101 hospitalized patients. Isr J Med Sci. 1985;21:950–53. [PubMed] [Google Scholar]
- 8.Brignole M, Alboni P, Benditt D, et al. Guidelines on management (diagnosis and treatment) of syncope-update 2004. Eur Heart J. 2004;25:2054–72. doi: 10.1016/j.ehj.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Lipsitz LA, Pluchino FC, Wei JY, et al. Syncope in institutionalized elderly: the impact of multiple pathological conditions and situational stress. J Chronic Dis. 1986;39:619–30. doi: 10.1016/0021-9681(86)90187-6. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor WN, Smith MA, Miller NL. Upright tilt testing in evaluating syncope: a comprehensive literature review. Am J Med. 1994;97:78–88. doi: 10.1016/0002-9343(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 11.Strickberger SA, Benson DW, Biaggioni I, et al. AHA/ACCF Scientific Statement on the evaluation of syncope. Circulation. 2006;113:316–27. doi: 10.1161/CIRCULATIONAHA.105.170274. [DOI] [PubMed] [Google Scholar]
- 12.Zhang QY, Du JB, Tang C. The efficacy of midodrine hydrochloride in the treatment of children with vasovagal syncope. J Pediatr. 2006;149:777–80. doi: 10.1016/j.jpeds.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Tan MP, Duncan GW, Parry SW. Head-up Tilt Table Testing: a state-of-the-art review. Minerva Med. 2009;100:329–38. [PubMed] [Google Scholar]
- 14.Low PA, Opfer-Gehrking TL, Textor SC, et al. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–25. [PubMed] [Google Scholar]
- 15.The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470. doi: 10.1212/wnl.46.5.1470. [DOI] [PubMed] [Google Scholar]
- 16.Grubb BP. Clinical pratice. Neurocardiogenic Syncope. N Engl J Med. 2005;352:1004–10. doi: 10.1056/NEJMcp042601. [DOI] [PubMed] [Google Scholar]
- 17.Kapoor WN. Syncope. N Engl J Med. 2000;343:1856–62. doi: 10.1056/NEJM200012213432507. [DOI] [PubMed] [Google Scholar]
- 18.Piorecka-Makula A, Werner B. Prolonged QT dispersion in children with congenital valvular aortic stenosis. Med Sci Monit. 2009;15(10):CR534–38. [PubMed] [Google Scholar]
- 19.Kapoor WN, Karpf M, Weant S, et al. A prospective evaluation and follow-up of patients with syncope. N Engl J Med. 1983;309:197–204. doi: 10.1056/NEJM198307283090401. [DOI] [PubMed] [Google Scholar]
- 20.Stewart JM. Orthostatic intolerance in pediatrics. J Pediatr. 2002;140:404–11. doi: 10.1067/mpd.2002.122727. [DOI] [PubMed] [Google Scholar]
- 21.Rassaf T, Muehlsteff J, Such O, et al. The pulse arrival time approach for non-invasive hemodynamic monitoring in low-acuity settings. Med Sci Monit. 2010;16(11):MT83–87. [PubMed] [Google Scholar]
- 22.Liu F, Wang ZG, Huang GY, et al. Etiologic analysis of 30 cases of syncope in children. Zhong Guo Shi Yong Erke Za Zhi. 2004;19:660–62. [Google Scholar]
- 23.Zhang QY, Du JB, Wang C, et al. The application of a diagnostic protocol to children and adolescents with syncope. Zhong Guo Shi Yong Er ke Za Zhi. 2008;23:118–21. [Google Scholar]
- 24.Noizet-Yverneau O, Hue V, Vaksmann G, et al. Syncope and pre-syncope in children and adolescents: a prospective study in a pediatric emergency care unit. Arch Pediatr. 2009;16:1111–17. doi: 10.1016/j.arcped.2009.04.009. [DOI] [PubMed] [Google Scholar]