Summary
Background
Perioperative optic neuropathy is a disease which can lead to serious, irreversible damage of vision. This complication could be the result of non-ocular surgery, for example, cardiac or spinal procedures.
We present a case of anterior ischemic neuropathy (AION) which occurred following a conventional coronary artery bypass graft procedure.
Case Report
A 57-year-old man, 4 days after Conventional Coronary Artery Bypass Graft surgery as result of multi-vessel stabile coronary artery disease and history of anterolateral wall myocardial infarction, was admitted to the Eye Clinic due to significant loss of vision in his right eye. The patient had hypertension and was a heavy smoker. On admission, the slit lamp examination revealed a relative afferent pupillary defect in the right eye. The fundus examination showed optic disc edema with the presence of flame hemorrhages. Best corrected visual acuity (BCVA) was 0.02. The results of eye examination and fluorescein angiography confirmed the diagnosis of AION. Anti-aggregation and antithrombotic treatment was continued with steroids and vasodilators. After 7 days of this treatment we noticed the improvement of BCVA to 0.2. At 6-month follow-up, the vision was stable, and fundus examination revealed optic disc atrophy.
Conclusions
After cardiac surgical operations, such as coronary artery bypass graft procedures, anterior ischemic optic neuropathy may occur. In those cases, close cooperation between the various specialists is necessary.
Keywords: coronary artery bypass graft, off-pump coronary artery bypass, perioperative ischemic neuropathy, anterior ischemic optic neuropathy
Background
Perioperative optic neuropathy (PON) is a disease which can lead to serious, irreversible damage of vision. This complication could be the result of non-ocular surgery, for example cardiac [1,2] or spinal procedures [3,4]. The frequency of PON occurrence among all of surgical procedures ranges from 0.002% to 0.1% [5–7], and after coronary artery bypass graft (CABG) ranges from 0.06% to 0.113% [2,8].
Coronary Artery Bypass Graft (CABG) is a cardio-surgical procedure of by-pass implantation, passing around the place of restriction in coronary arteries. This procedure is performed in some cases of cardiac infraction and advanced coronary disease. Arteries and veins from the patient’s body are grafted to the coronary arteries to bypass atherosclerotic narrowing and improve the blood supply to the ischemic part of the heart muscle. CABG can be performed with extracorporeal circulation (ECC) with cardiopulmonary bypass (CBP) – conventional coronary artery bypass graft (CCABG) or without extracorporeal circulation – operation on beating heart or off-pump coronary artery bypass (OPCAB). The first operation on a beating heart was performed in 1964 by Kolesov [9].
Visual loss due to CABG is a rare complication. The mechanisms of this disorder are not completely understood. It may be the result of optic nerve ischemic neuropathy, although other cortical mechanisms for visual loss are considered [7,10].
We present a case of anterior ischemic optic neuropathy (AION) occurring as a consequence of a conventional coronary artery bypass graft procedure (CCABG).
Case Report
A 57-year-old man was admitted to the Eye Clinic in April 2010 due to significant loss of vision in his right eye. Because of multi-vessel stabile coronary artery disease and history of anterolateral wall myocardial infarction (2008), he had CCABG in extracorporeal circulation performed 4 days before. Two saphenous vein grafts and 1 arterial vein grafts were implanted, with good intraoperative graft flow and preserved left ventricular function, without any perioperative complications. After regaining consciousness following surgery, the patient complained of significant visual loss in his right eye. The consulting ophthalmologist diagnosed AION. Nothing unusual happened during the procedure, and the cardiac surgeon couldn’t indicate any cause of the eye disorder. The patient had been treated for hypertension over the past 10 years and was a heavy smoker.
On admission, the slit lamp examination revealed relative afferent pupillary defect (RAPD) in the right eye. The fundus examination showed swelling of the optic nerve head, with pale and blurred margins. Flame-shaped hemorrhages on the disc margin were visible (Figure 1). BCVA was 0.02, intraocular pressure was 17 mmHg.
Figure 1.
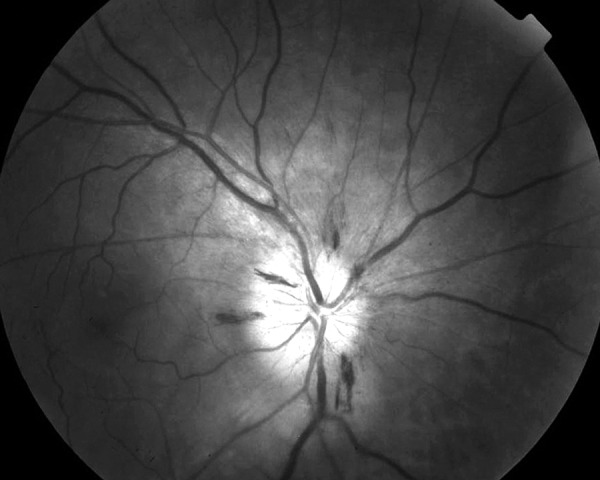
Dilatated fundus photograph in red-free illumination of the right eye (AION) – shows swelling of the optic nerve head with blurred margins. Flame-shaped hemorrhages on the disc margin are visible.
We performed fluorescein angiography (Figures 2–5). These results confirmed the diagnosis of AION.
Figure 2.
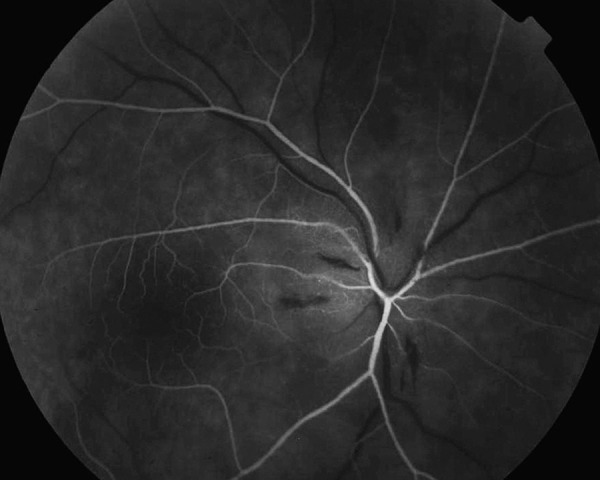
Fluorescein angiography of the right eye arterial phase (AION) – margins of the disc nonvisible. Lack of perfusion in all sectors except temporal part of the disc.
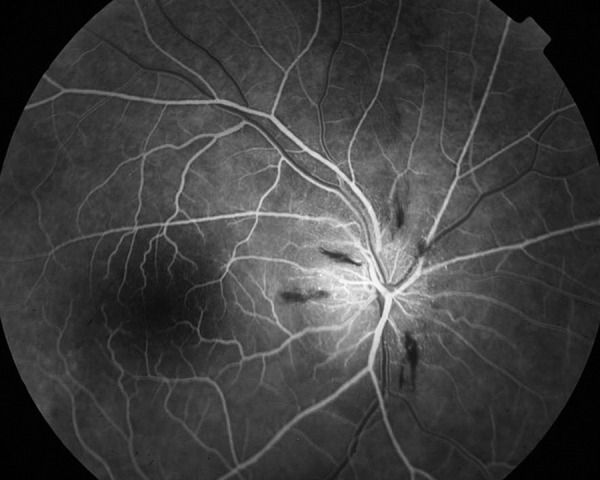
Figure 5.
Fluorescein angiography of the right eye late stage (AION) – the dye leaks only from the affected part of the optic disc head.
After consultation with the cardiac surgeon, anti-aggregation and antithrombotic treatments were continued with steroids and vasodilators.
After 7 days of these treatments we obtained improvement of BCVA to 0.2. In 6 months of follow-up the vision was stable, and fundus examination revealed optic disc atrophy which appeared as optic disc pallor.
Discussion
Anterior ischemic optic neuropathy is the result of oxygen delivery disturbance to the optic nerve head, anterior to the lamina cribrosa. These disturbances are the result of unsatisfactory blood flow in short posterior ciliary arteries (PCA) in this area [8]. Many systemic factors, included surgical procedures, may cause blood flow reduction in the PCA. Systemic, non-correlated-to-surgery risk factors of AION are: high serum cholesterol, triglycerides, hyperlipidemia, hyperfibrinogenemia, prolonged smoking history, hypertension and diabetes mellitus [11].
In spite of the fact that the patient had many risk factors contributing to AION (including hypertension, coronary disease and smoking history), time correlation with the cardiac surgical procedure and appearance of symptoms suggests that this procedure itself increased the risk for AION.
Spectacular developments in cardiac surgery have opened-up new treatment possibilities for patients with coronary disease. At present, many coronary artery bypass grafts are performed. Due to the continuously rising number of procedures, the frequency of complications concomitantly increases – including eye complications.
After cardiac surgery, patients may complain about transient loss of vision, poor reading ability and altered perception of colors. Some of these symptoms are transient, but in other cases visual loss may occur [12].
Ischemic optic neuropathy is one of the serious ocular complications after cardiac surgery [13], and is the result of optic disc ischemia. The risk factors of ION due to CABG are: postoperative decrease of hemoglobin level, history of clinically severe vascular diseases, coronary angiogram within 48 hours of surgery, long duration of the CABG procedure [8], hypotension, arrhythmia and tissue edema [2].
Coronary artery bypass grafting opened-up new possibilities for widespread coronary disease treatment. At present, conventional coronary artery bypass grafts (CCABG) are performed in extracorporeal circulation with cardiopulmonary bypass. In spite of complications due to extracorporeal circulation, new methods without using cardiopulmonary bypasses are being introduced.
Beginning in the mid-1990’s, off-pump coronary artery bypasses (OPCAB) without extracorporeal circulation were elaborated, referred to as “beating heart” [9]. This method has a lower risk of complications in comparison to techniques with heart-stopped procedures, which require the use of cardiopulmonary bypass [14–16]. As OPCAB cannot be performed in all cases, it cannot replace CCABG. The decision on the type of procedure is made individually by cardiac surgeons. In the patient in question, the cardiac surgeons decided to perform CCABG in extracorporeal circulation.
During operations with extracorporeal circulation, such as the present case, the aorta is cannulated and cross-clamped, and, to protect heart tissues, hypothermia is employed. Additional cardioplegic fluid is injected into coronary vessels. This causes general heart ischemia, with metabolic and water-electrolyte balance disturbance [17]. Blood contact with cardiopulmonary bypass elements during extracorporeal circulation is the cause of general inflammation, leading to activation of the complement system, monocytes, neutrophils and many proinflammatory cytokines. This process causes cellular edema, including heart muscle cells and blood vessels, which is detrimental for heart contractility and vascular tension [17,18].
These changes may lead to many postoperative complications such as early morbidity [19,20] central nervous system [17,19,21,22], kidneys [17,23], coagulation and respiratory damages [14,24].
In published papers, author’s noted decreased morbidity after operations on a beating heart in comparison to extracorporeal circulation operations [19,20].
The greater number of complications after CCABG is an effect of forming of micro embolies due to creation of cardiopulmonary bypasses, manipulation in the region of the ascending aorta [17,19,21,22]. General inflammation and mechanical blood cells damage due to extracorporeal circulation, higher doses of heparin and hemodilution are contributing to significantly greater disturbances of postoperative coagulation damage in patients operated in extracorporeal circulation. Because of bleeding, patients after CCABG need more reoperations and blood transfusions [14,24].
Patients after extracorporeal circulation procedure have lower concentration of hemoglobin in comparison to patients operated on a beating heart. This is caused by hemodilution due to pump and greater blood loss [2,8], and contributes to reduction of oxygen supply to retina and brain tissues, leading to ischemia. Other factors, such as hypotension, arrhythmia or tissue edema connected to CBP, may lead to optic disc ischemia and anterior ischemic optic neuropathy. Hypothermia, which is used in those patients, also contributes to blood flow restrictions and may be the factor leading to optic disc ischemia. Some studies have revealed that hypothermia leads to a 6–7% reduction of brain blood flow per every degree the temperature decrease [25].
Another vascular complication after CABG is embolization of small vessels. Both embolization changes and circulatory disturbances may lead to intraoperative ischemia of multiples tissues, including brain and eye tissues. Emmich et al. [26] revealed the presence of infracts due to emboli, macro- and micro-hemorrhages, subarachnoidal hemorrhages and disorders caused by brain tissue ischemia in 49% of patients who died after cardiac surgical procedures.
The embolization in retinal circulation due to cardiac surgical operations was presented for the first time in the 1970’s by Williams [27]. Ascione et al. [28] noticed abnormalities in retinal angiography in patients with previous CCABG. At the examination they revealed vessel-wall injuries leading to abnormalities of fluorescein angiography. Color images of eye fundus demonstrated the presence of multiple, shiny emboli. These changes were observed in patients with previous extracorporeal circulation in contrast to patients operated on a beating heart. The authors concluded that OPCAB operations decreased the risk of retina micro vessels damage as opposed to CCABG.
Among other vascular complications, despite emboli, thrombosis of eye vessels due to cardiac surgical operations are presented in many publications. Fosnot et al. [29] described a case of central retinal vein occlusion (CRVO) after a coronary artery bypass graft.
Occurrence of perioperative optic neuropathy due to cardiac operations is a rare complication. Nevertheless, because of the still rising number of cardiac surgical procedures, cardiac surgeons and ophthalmologists should consider the possibilities of serious complications leading to significant vision damage. Patients with severe vascular disorders seem to be at greater risk of these complications.
Conclusions
Based on the presented case and published data, we conclude that cardiac surgical operations, such as coronary artery bypass graft procedures (hypotony, hypovolemy and hypothermy) may lead to anterior ischemic optic neuropathy. In those cases, close cooperation among the various specialists is necessary.
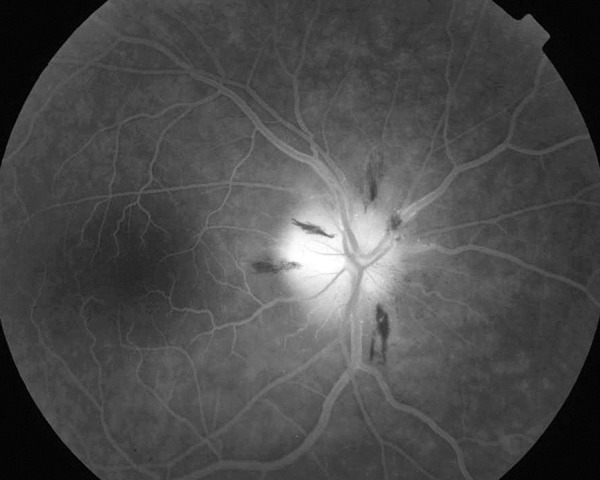
Figure 3.
Fluorescein angiography of the right eye capillary phase (AION) – fluorescein leaks from dilated disc capillaries. Focal hypofluorescent areas in places of disc hemorrhages, down sector of the disc is hypofluorescent.
Figure 4.
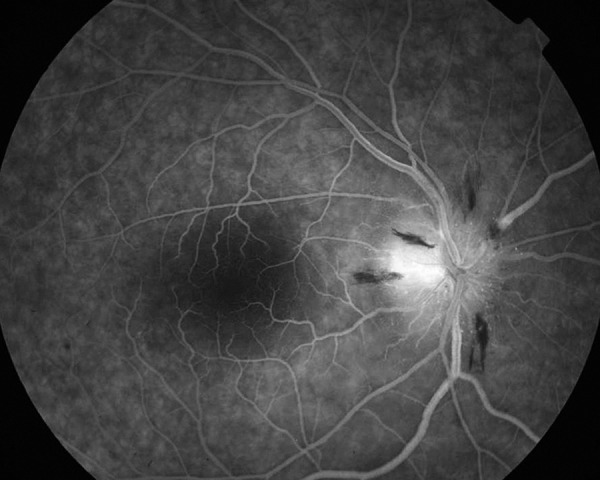
Fluorescein angiography of the right eye venous phase (AION) – leakage from disc capillaries and optic nerve hyperfluorescence is more visible in and beyond margins of the disc.
Abbreviations
- CCABG
conventional coronary artery bypass grafting
- CABG
coronary artery bypass graft
- ECC
extracorporeal circulation
- CBP
cardiopulamonary bypass
- OPCAB
off-pump coronary artery bypass
- PON
perioperative optic neuropathy
- AION
anterior ischemic neuropathy
- RAPD
relative afferent pupillary defect
- BCVA
best corrected visual acuity
- CRVO
central retinal vein occlusion
Footnotes
Source of support: Departmental sources
References
- 1.Busch T, Sirbu H, Aleksic I, et al. Anterior ischemic optic neuropathy: a complication after extracorporal circulation. Ann Thorac Cardiovasc Surg. 1998;4:354–58. [PubMed] [Google Scholar]
- 2.Kalyani SD, Miller NR, Dong LM, Baumgartner WA, Alejo DE, Gilbert TB. Incidence of and risk factors for perioperative optic neuropathy after cardiac surgery. Ann Thorac Surg. 2004;78:34–37. doi: 10.1016/j.athoracsur.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Alexandrakis G, Lam BL. Bilateral posterior ischemic optic neuropathy after spinal surgery. Am J Ophthalmol. 1999;127:354–55. doi: 10.1016/s0002-9394(98)00343-2. [DOI] [PubMed] [Google Scholar]
- 4.Dilger JA, Tetzlaff JE, Bell RG, et al. Ischaemic optic neuropathy after spinal fusion. Can J Anaesth. 1998;45:63–66. doi: 10.1007/BF03011996. [DOI] [PubMed] [Google Scholar]
- 5.Brown RH, Schauble JF, Miller NR. Anemia and hypotension as contributors to perioperative loss of vision. Anesthesiology. 1994;80:222–26. doi: 10.1097/00000542-199401000-00033. [DOI] [PubMed] [Google Scholar]
- 6.Roth S, Thisted RA, Erickson JP, et al. Eye injuries after nonocular surgery. A study of 60,965 anesthetics from 1988 to 1992. Anesthesiology. 1996;85:1020–27. doi: 10.1097/00000542-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Williams EL, Hart WM, Jr, Tempelhoff R. Postoperative ischemic optic neuropathy. Anesth Analg. 1995;80:1018–29. doi: 10.1097/00000539-199505000-00029. [DOI] [PubMed] [Google Scholar]
- 8.Nuttall GA, Garrity JA, Dearani JA, et al. Risk factors for ischemic optic neuropathy after cardiopulmonary bypass: a matched case/control study. Anesth Analg. 2001;93:1410–16. doi: 10.1097/00000539-200112000-00012. table of contents. [DOI] [PubMed] [Google Scholar]
- 9.Kolessov VI. Mammary artery-coronary artery anastomosis as method of treatment for angina pectoris. J Thorac Cardiovasc Surg. 1967;54:535–44. [PubMed] [Google Scholar]
- 10.Shapira OM, Kimmel WA, Lindsey PS, Shahian DM. Anterior ischemic optic neuropathy after open heart operations. Ann Thorac Surg. 1996;61:660–66. doi: 10.1016/0003-4975(95)01108-0. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson DM, Vierkant RA, Belongia EA. Nonarteritic anterior ischemic optic neuropathy. A case-control study of potential risk factors. Arch Ophthalmol. 1997;115:1403–7. doi: 10.1001/archopht.1997.01100160573008. [DOI] [PubMed] [Google Scholar]
- 12.Machida S, Gotoh Y, Tanaka M, Tazawa Y. Predominant loss of the photopic negative response in central retinal artery occlusion. Am J Ophthalmol. 2004;137:938–40. doi: 10.1016/j.ajo.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Sha’aban RI, Asfour WM. Visual loss after coronary artery bypass surgery. Saudi Med J. 2000;21:90–92. [PubMed] [Google Scholar]
- 14.Angelini GD, Taylor FC, Reeves BC, Ascione R. Early and midterm outcome after off-pump and on-pump surgery in Beating Heart Against Cardioplegic Arrest Studies (BHACAS 1 and 2): a pooled analysis of two randomised controlled trials. Lancet. 2002;359:1194–99. doi: 10.1016/S0140-6736(02)08216-8. [DOI] [PubMed] [Google Scholar]
- 15.Magee MJ, Jablonski KA, Stamou SC, et al. Elimination of cardiopulmonary bypass improves early survival for multivessel coronary artery bypass patients. Ann Thorac Surg. 2002;73:1196–202. doi: 10.1016/s0003-4975(01)03587-1. discussion 1202–3. [DOI] [PubMed] [Google Scholar]
- 16.Sergeant P, Wouters P, Meyns B, et al. OPCAB versus early mortality and morbidity: an issue between clinical relevance and statistical significance. Eur J Cardiothorac Surg. 2004;25:779–85. doi: 10.1016/j.ejcts.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Chojecki HP, Gryszko L, Szalański P, et al. Pomostowanie aortalnowieńcowe bez użycia krążenia pozaustrojowego. Pol. Merk. Lek., XXII. 2007;132:560–65. [in Polish] [PubMed] [Google Scholar]
- 18.Edmunds LH., Jr Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1998;66:S12–16. doi: 10.1016/s0003-4975(98)00967-9. discussion S25–28. [DOI] [PubMed] [Google Scholar]
- 19.Mack MJ, Pfister A, Bachand D, et al. Comparison of coronary bypass surgery with and without cardiopulmonary bypass in patients with multivessel disease. J Thorac Cardiovasc Surg. 2004;127:167–73. doi: 10.1016/j.jtcvs.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 20.Reston JT, Tregear SJ, Turkelson CM. Meta-analysis of short-term and mid-term outcomes following off-pump coronary artery bypass grafting. Ann Thorac Surg. 2003;76:1510–15. doi: 10.1016/s0003-4975(03)01195-0. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez F, Cohn WE, Baribeau RY, et al. In-hospital outcomes of off-pump versus on-pump coronary artery bypass procedures: a multicenter experience. Ann Thorac Surg. 2001;72:1528–33. doi: 10.1016/s0003-4975(01)03202-7. discussion 1533–34. [DOI] [PubMed] [Google Scholar]
- 22.Magee MJ, Coombs LP, Peterson ED, Mack MJ. Patient selection and current practice strategy for off-pump coronary artery bypass surgery. Circulation. 2003;108(Suppl 1):II9–14. doi: 10.1161/01.cir.0000089187.51855.77. [DOI] [PubMed] [Google Scholar]
- 23.Gerritsen WB, van Boven WJ, Driessen AH, et al. Aarts, Off-pump versus on-pump coronary artery bypass grafting: oxidative stress and renal function. Eur J Cardiothorac Surg. 2001;20:923–29. doi: 10.1016/s1010-7940(01)00941-1. [DOI] [PubMed] [Google Scholar]
- 24.Puskas JD, Williams WH, Duke PG, et al. Off-pump coronary artery bypass grafting provides complete revascularization with reduced myocardial injury, transfusion requirements, and length of stay: a prospective randomized comparison of two hundred unselected patients undergoing off-pump versus conventional coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:797–808. doi: 10.1067/mtc.2003.324. [DOI] [PubMed] [Google Scholar]
- 25.Reuler JB. Hypothermia: pathophysiology, clinical settings, and management. Ann Intern Med. 1978;89:519–27. doi: 10.7326/0003-4819-89-4-519. [DOI] [PubMed] [Google Scholar]
- 26.Emmrich P, Hahn J, Ogunlade V, et al. Neuropathological findings after cardiac surgery-retrospective study over 6 years. Z Kardiol. 2003;92:925–37. doi: 10.1007/s00392-003-1007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams IM. Intravascular changes in the retina during open-heart surgery. Lancet. 1971;2:688–91. doi: 10.1016/s0140-6736(71)92252-5. [DOI] [PubMed] [Google Scholar]
- 28.Ascione R, Ghosh A, Reeves BC, et al. Retinal and cerebral microembolization during coronary artery bypass surgery: a randomized, controlled trial. Circulation. 2005;112:3833–38. doi: 10.1161/CIRCULATIONAHA.105.557462. [DOI] [PubMed] [Google Scholar]
- 29.Fosnot J, Glazer-Hockstein C, Tolentino MJ. Central retinal vein occlusion immediately following cardiac surgery. Ophthalmic Surg Lasers Imaging. 2003;34:215–16. [PubMed] [Google Scholar]


