Summary
Background
L-carnitine was proposed as a potential treatment for patients diagnosed with an autism spectrum disorder to improve mitochondrial dysfunction, but no prior randomized controlled trials have been conducted.
Material/Methods
Thirty subjects diagnosed with an ASD were randomly assigned to receive a standardized regimen (50 mg L-carnitine/kg bodyweight/day) of liquid L-carnitine (n=19) or placebo (n=11) for 3-months. Measures included changes in professionally completed Childhood Autism Rating Scale (CARS), hand muscle testing, and modified clinical global impression (CGI) forms; parent completed Autism Treatment Evaluation Checklist (ATEC), treatment adherence measurement (TAM), frequency and intensity of side effect rating (FISER)/global rating of side effect burden (GRSEB)/patient report of incidence of side effects (PRISE) forms; and lab testing.
Results
Significant improvements were observed in CARS (−2.03, 95% CI=−3.7 to −0.31), CGI (−0.69, 95% CI=−1.1 to −0.06), and ATEC scores. Significant correlations between changes in serum free-carnitine levels and positive clinical changes were observed for hand muscle strength (R2=0.23, P=0.046), cognitive scores (R2=0.27, P=0.019), and CARS scores (R2=0.20, P=0.047). Study subjects were protocol-compliant (average adherence was >85%) and generally well-tolerated the L-carnitine therapy given.
Conclusions
L-carnitine therapy (50 mg/kilogram-bodyweight/day) administered for 3-months significantly improved several clinical measurements of ASD severity, but subsequent studies are recommended.
Keywords: autism spectrum disorder, carnitine, mitochondrial dysfunction
Background
The U.S. Centers for Disease Control and Prevention (CDC) recently reported that about 1% of U.S. children are diagnosed with an autism spectrum disorder (ASD) [1]. Investigators have described that an ASD is a devastating disease with impairments in social relatedness and communication, repetitive behaviors, abnormal movement patterns, and sensory dysfunction that places an enormous burden on the society in general and the relatives and caregivers of patients diagnosed with an ASD in particular [2]. The diagnosis of ASD can be made as early as 2 years of age; and patients diagnosed with an ASD often have a normal life span. Thus, in terms of the number of “patient years”, ASD patients represent a patient population that is as large as that of Alzheimer’s disease, the current biggest neurological disorder. Despite the clear unmet medical need, currently, there is no recognized effective comprehensive treatment [2].
Some children diagnosed with an ASD demonstrate evidence of impaired energy metabolism. Carnitine deficiency has been observed in some patients diagnosed with an ASD [3]. Carnitine is essential for the utilization of fatty acids by the mitochondria. Thus, a deficiency in carnitine leads to impaired ATP production and decreased availability of high-energy phosphate compounds. Mitochondrial dysfunction in patients diagnosed with an ASD is suggested by neuroimaging procedures, including positron emission tomography (PET) scanning and nuclear magnetic resonance (NMR) spectroscopy [4,5]. Further, several clinical studies found evidence of disturbances of mild mitochondrial energy production among individuals diagnosed with an ASD [6,7].
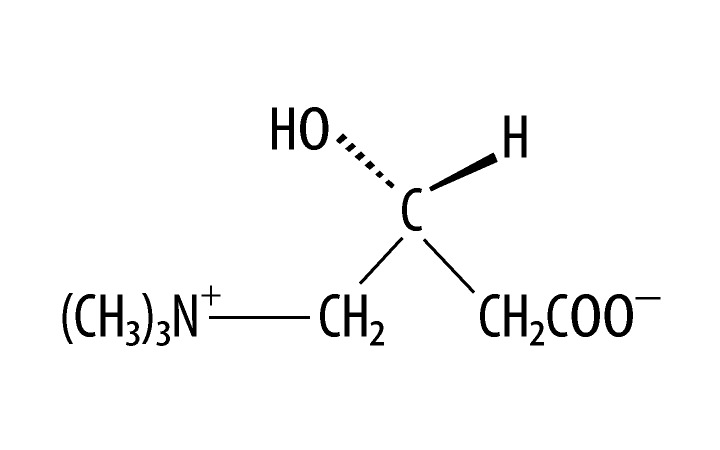
As a result of the aforementioned observations in patients diagnosed with an ASD, one suggested approach to treating patients diagnosed with an ASD entails the administration of mitochondrial respiratory chain co-factors to enhance mitochondrial function. Such a strategy would employ stimulation of enzyme activity by supplying precursors or co-enzyme and alternative substrates. Levocarnitine (L-carnitine) was among the suggested options for treatment [3,8]. L-carnitine is a carrier molecule in the transport of long-chain fatty acids across the inner mitochondrial membrane. The chemical name of L-carnitine is 3-carboxy-2(R)-hydroxy-N,N,N-trimethyl-1-propanaminium, inner salt. L-carnitine is a white crystalline, hygroscopic powder. It is readily soluble in water, hot alcohol, and insoluble in acetone. Its chemical structure is shown in Figure 1. L-carnitine is a naturally occurring substance required in mammalian energy metabolism. The highest concentrations of carnitine are found in red meat and dairy products. In skeletal and cardiac muscle, where fatty acids are the main substrate for energy production, it has been shown to facilitate long-chain fatty acid entry into cellular mitochondria, thereby delivering substrate for oxidation and subsequent energy production.
Figure 1.
The chemical structure of Levocarnitine. Empirical Formula: C7H15NO3; Molecular Weight: 161.20.
The hypothesis tested in the present study was that blood carnitine levels in patients diagnosed with an ASD have a significant impact on behavior, cognition, socialization, and health/physical traits associated with an ASD diagnosis. The present prospective, double-blind, placebo controlled trial evaluated whether a standardized treatment regimen of liquid L-carnitine administered to patients diagnosed with an ASD on a daily basis for 3-months would result in improved behavior, cognition, socialization, and health/physical traits associated with an ASD diagnosis.
Materials and Methods
Subjects
A total of 34 subjects diagnosed with an ASD, aged from 3 to 10 yrs-old (30 males, 4 females) were recruited to the study. The study subjects had bodyweights between 13.2 Kg to 40.4 Kg. None of the study subjects had previously received carnitine-based therapy or previous methionine or lysine supplementation. None of the study subjects had any change in therapy or treatment (including medications) within 1 month prior to the study. The study protocol received Institutional Review Board (IRB) approval from Liberty IRB, Inc. (Deland, Florida). All parents signed a consent and Health Insurance Portability and Accountability Act (HIPAA) form and all received a copy.
Clinical measures
Childhood autism rating scale (CARS)
Study participants were evaluated using a CARS test conducted only by a single study investigator (JKK) who observed the subjects and interviewed the parent(s), and was unaware as to the treatment status of the subject. The CARS test is a 15-item behavioral rating scale developed to identify autism as well as to quantitatively describe the severity of the disorder. The CARS test is a well-established measure of autism severity [9]. The internal consistency reliability alpha coefficient is 0.94; the inter-rater reliability correlation coefficient is 0.71; and the test-retest correlation coefficient is 0.88 [10]. CARS scores have high criterion-related validity when compared to clinical ratings during the same diagnostic sessions, with a significant correlation of 0.84 [10].
Autism treatment evaluation checklist (ATEC)
Each study subject was evaluated by their parents using an ATEC form. Parents were unaware as to the treatment status of their child. The ATEC, designed by the Autism Research Institute (San Diego, CA, USA), is a one-page form [11]. It consists of four subtests designed to measure the effects of treatment in persons with autism. The items are: (1) Speech/Language/Communication (14 items); (2) Sociability (20 items); (3) Sensory/Cognitive Awareness (18 items); and (4) Health/Physical/Behavior (25 items). The internal consistency reliability of the measure is high (0.94 for the Total score). The ATEC has been successfully used to measure treatment effects in autism [12–14].
Modified clinical global impression (CGI)
An overall CGI score was collected by a single study investigator (JKK) unaware of the treatment status of the study subject using a 3 point scoring system defined as follows: subject improved =1, subject the same =2, and subject worse =3.
Hand muscle testing
Each subject had their hand muscle strength tested using a pneumatic, adjustable squeeze pinch-gauge/dynamometer (Baseline Evaluation Instruments; White Plains, NY, USA) by a study investigator unaware of the treatment status of the subject. This instrument is a reliable and valid method for obtaining muscle force or torque measurements in children [15,16]. Subjects were tested using the smallest hand grasp bulb, and were given as many tries as needed to register their maximum grasp reading measured in kilopascals (kPa) for each hand. Special emphasis was placed to ensure that the subject positioned the bulb in the palm of the hand and held the bulb in space to ensure that pressure was not applied by the study subject against a fixed surface. In addition, each study subject was strongly encouraged by a study investigator to give maximum effort.
Treatment adherence measure (TAM) form
A treatment adherence measure (TAM) form was completed by the parents of each study subject. Parents were unaware as to the treatment status of their child. The TAM is a ten-item self-report on treatment adherence that asks specific questions regarding the dose and frequency of use. The TAM was used to calculate the level of adherence to the treatment. It is a Morisky-type self-report adherence measure, designed to measure treatment adherence. Morisky-type adherence measures have been used widely, demonstrating good reliability as a self-report measure [17].
Frequency and intensity of side effect rating (FISER)/global rating of side effect burden (GRSEB)/patient report of incidence of side effects (PRISE)
The FISER/GRSEB/PRISE forms were completed by the parents of study subjects that were unaware of the treatment status of their children. The FISER/GRSEB/PRISE forms include global measures, each using a 7-point Likert-type scale rated 0–6, with one rating anchored for frequency, another rating the intensity of side effects encountered in the prior week that the study subject parents believed were due to the treatment, and the third asking the parents of study subjects to estimate the overall burden or degree of interference in day-to-day activities and function due to the side effects attributable specifically to the treatment [18]. Frequency of side effects is rated as a percent time present: 0= no side effects; 1= present 10% of the time; 2=25% of the time; 3=50% of the time; 4=75%; 5=90%; and 6= present all of the time. Intensity of side effects ranges from 0= no side effects to 6= intolerable side-effects. Impairment due to side effects ranges from 0= no side effects to 6= unable to function at all due to side-effects. The PRISE lists a variety of possible side effects from which to choose and a scale to rate the specific side effect. The measure also has a place to list any side effects not previously listed.
Lab testing
Study subjects had lab testing collected at a Laboratory Corporation of American (LabCorp) draw station in the morning following an overnight fast. The lab was not made aware of the treatment status of the study subjects. The procedures for collection and analysis were defined by LabCorp standard protocols (CLIA-approved). The following blood tests were collected and evaluated on each study subject, including: whole blood white blood cell count (WBC), whole blood red blood cell count (RBC), whole blood platelet count, serum creatinine, serum blood urine nitrogen (BUN), serum alkaline phosphatase, serum aspartate aminotransferase (AST/SGOT), serum alanine aminotransferase (ALT/SGPT), serum glucose, and serum carnitine (total and free).
Study drugs
L-carnitine was supplied in a liquid preparation by the Wellness Pharmacy (Birmingham, AL, USA) using a specific formula containing: 100 mg L-Carntine/mL with the inactive ingredients of methylcellulose, stevioside (stevia), tangerine flavor, and preserved water (containing methylparaben and propylparaben). The placebos were identical in appearance and taste to the active preparation, containing a 1% methylcellulose suspension with the inactive ingredients of stevioside (stevia), tangerine flavor, and preserved water (containing methylparaben and propylparaben). The recommended childhood starting dose of 50 mg L-carnitine per Kg bodyweight per day (half the total dose administered in the morning and half the total dose administered in the evening) described in the package insert for CARNITOR® (Sigma-Tau Pharmaceuticals, Inc., Gaithersburg, MD, USA) was utilized in the present study with dosing calculated based on each participant’s initial weight. The dosing regimen of the liquid preparation was identical in both the L-carnitine and placebo groups, so that each study subject received a total of 0.5 mL per Kg of bodyweight per day (administered as 0.25 mL per Kg of bodyweight in the morning and 0.25 mL per Kg of bodyweight in the evening). Study subject-specific dosing instructions were placed on each liquid preparation provided to study subjects.
Study design
This was a randomized, double-blind, placebo-controlled study. The study was conducted between 2008 and 2010. The study subjects were recruited through community contacts. The study protocol called for 20 subjects to receive L-carnitine and 10 study subjects to receive placebo. Figure 2 summarizes the overall design of the present study. A total of 34 subjects were recruited for the present study. Four subjects withdrew prior to randomization into L-carnitine or placebo groups. A total of 30 subjects were randomly assigned to receive L-carnitine or placebo, and of these a total of 7 subjects (4 in the L-carnitine group and 3 in the placebo group) withdrew prior to successful completion of 3-months of therapy. Among the 7 subjects withdrawing from the study prior to successful completion of 3-months of therapy, 4 subjects withdrew because of adverse reactions (1 in the L-carnitine group and 3 in the placebo group), 2 subjects did not comply with the study protocol, and 1 was lost to follow-up with no known adverse reaction. Further, among the 4 subjects that withdrew because of adverse reactions, these patients were assessed using CARS and CGI scoring at the time of their withdrawal from the study.
Figure 2.
Trial profile of L-carnitine.
Study subjects were shipped a 1 month supply of L-carnitine or placebo by Wellness Pharmacy, which automatically supplied subsequent refills on a monthly basis until each study subject received treatment for a total of 3-months. Laboratory, efficacy, and toleration evaluations were conducted on study subjects at baseline and following 3-months of therapy. In addition, study investigators monitored study subjects to ensure compliance and to monitor for potential adverse reactions.
Prerandomization phase
Study subjects were seen for an initial screening where study investigators obtained information regarding demographics, formal diagnosis, age at diagnosis, age of apparent onset, information regarding delay or regression, any current medical issues, medications, bodyweight, and allergies on each study subject. A baseline CARS evaluation was performed by a single study investigator (JKK), an ATEC form was completed by the parents, hand muscle testing was performed by study investigators, and PRISE form was completed by the parents of study subjects. In addition, blood samples were collected on each study subject at a LabCorp draw station.
Randomization phase
Following the initial screening and collection of labs, all study subjects started therapy within 30 days of baseline measurements. A study investigator (DAG), who did not perform any clinical measurements on study subjects, used a coin-flip to randomly assign study subjects to either the L-carnitine or placebo groups. Since there was a difference in sample size between the L-carnitine and placebo groups, the placebo group was filled with study subjects before the treatment group, so that the latter study subjects were all assigned to the L-carnitine group. Study investigators in contact with the study subjects and the parents of study subjects were not informed of the treatment status (L-carnitine/placebo) of each study participant until all study subjects had completed the trial, and hence the assignment (L-carnitine/placebo) strategy employed should not have revealed any information regarding the treatment status of any study participant to study investigators in contact with study subjects and the parents of study subjects. Subsequently, a study investigator (DAG) arranged with Wellness Pharmacy for shipment of appropriate study medication. For the duration of the trial any concomitant use of drugs/supplements were not changed as far as possible.
Efficacy assessment
The primary lab efficacy measures were changes in total and free carnitine levels. Data were collected at baseline and at the end of the 3-months of treatment. In order to evaluate the clinical efficacy of treatment, CARS (determined by JKK) and ATEC (determined by the parents of the study subject) scores were generated at baseline and at the end of 3-months of treatment. In addition, an overall CGI score at the end of the 3-months of treatment was collected by a single study investigator (JKK). Finally, hand muscle strength (determined by a study investigator) was determined at baseline and at the end of the 3-months of treatment.
Tolerability assessment
The primary lab tolerability measures were changes in WBC, RBC, whole blood platelet count, serum creatinine, serum BUN, serum alkaline phosphatase, serum AST/SGOT, serum alanine aminotransferase ALT/SGPT, and serum glucose. Data were collected at baseline and at the end of the 3-months of treatment.
A TAM form was completed by the parents of each study subject at the end of treatment. In addition, information was collected using a PRISE form completed by the parents of study subjects at baseline and at the end of 3-months of therapy, and a FISER/GRSEB form was completed by the parents of study subjects at the end of 3-months of therapy.
Statistical analysis
The statistical package contained in StatsDirect (Version 2.7.8) was utilized in the present study. All continuous variables were compared with the use of the parametric t-test statistic. Categorical variables were compared with the use of the Fisher’s exact test statistic. Outcomes measurements in the areas of efficacy and tolerability were evaluated as the relative change in tests results following 3-months of treatment in comparison to baseline measurements for each study subject. The null hypothesis was that there would be no difference in the data distributions of the relative change in test results following 3-months of treatment in comparison to baseline measurements between study subjects receiving L-carnitine in comparison to placebo. In addition, the simple linear-regression statistic was used to evaluate: for each study subject, regardless of his or her treatment status, the relationship between the change in serum free- carnitine levels following 3-months of treatment in comparison to his or her baseline measurements, and the changes in specific outcome measurements (hand muscle strength scores, cognitive scores measured using the ATEC, and CARS scores) 3-months of treatment in comparison to his or her baseline measurements. Further, 95% confidence intervals were determined for each linear regression line. The null hypothesis for each statistical test was that the slope of the line should be equal to zero. For all statistical tests performed in the present study, a two-tailed p-value ≤ 0.05 was considered to be statistically significant.
Results
Table 1 summarizes the overall baseline characteristics of the study subjects assigned to the L-carnitine and placebo treatment groups. No significant differences were observed between the L-carnitine and placebo groups with respect to age, gender, race, bodyweight or ASD diagnostic status.
Table 1.
Baseline characteristics of L-carnitine and placebo cohorts at randomization (excludes the 3 study subjects that either were lost to follow-up or not compliant with the study protocol)*.
| L-carnitine (n=16) | Placebo (n=11) | |
|---|---|---|
| Age (y) | 6.3±2.4** | 6.7±1.6 |
| Gender (n) | ||
| Male | 14 | 9 |
| Female | 2 | 2 |
| Race (n) | ||
| Caucasian | 12 | 9 |
| Minorities*** | 4 | 2 |
| Bodyweight (Kg) | 22.3±5.6 | 23.5±3.7 |
| Autism Spectrum Disorder (n) | ||
| Autism | 10 | 8 |
| PDD-NOS/Asperger’s Disorder | 6 | 3 |
Kg – Kilogram; PDD-NOS – Pervasive Developmental Disorder – Not Otherwise Specified. Age, Gender, Race, Bodyweight, or autism spectrum disorder status did not significantly differ between the 2 treatment groups.
mean ±SD;
includes: Blacks, Asians, and Hispanics.
Table 2 compares the change in clinical characteristics between the L-carnitine and placebo groups following 3-months of therapy. There were significant improvements in the clinical outcomes of CARS scores and CGI scores measured by trained professionals among those study subjects receiving L-carnitine in comparison to the placebo group. In addition, for the ATEC completed by parents of study subjects, there were significant improvements in the cognition scores in the L-carnitine group in comparison to the placebo group. Finally, a result approaching a statistically significant (p=0.09) improvement was also observed in the L-carnitine treatment group’s speech scores in comparison to the placebo group.
Table 2.
Comparison of clinical characteristics between treatment groups*.
| L-carntine baseline (n) | L-carnitine end (n) | Total Δ (%) | Placebo baseline (n) | Placebo end (n) | Total Δ (%) | Contrast between groups(95% CI) | P-value for contrast between groups | |
|---|---|---|---|---|---|---|---|---|
| CARS** (professional) | 35.7±5.3*** (16) | 33.8±5.8 (16) | −1.94±2.5 (−5.3) | 38.2±6.0 (11) | 38.4±6.3 (11) | 0.09±1.4 (0.5) | −2.03#(−3.7 to −0.31) | 0.02 |
| Modified** CGI (professional) | 2.0 (16) | 1.5±0.63 (16) | −0.5±0.63 (−25) | 2.0 (11) | 2.09±0.7 (11) | 0.09±0.7 (4.3) | −0.69 (−1.1 to −0.06) | 0.03 |
| Hand Muscle## Testing (professional) | 32.7±13.9 (14) | 34.3±16.7 (14) | 1.6±8.9 (4.7) | 35.3±13.2 (7) | 35.1±7.5 (7) | −0.2±11.0 (−0.6) | 1.8 (−7.5 to 11) | 0.69 |
| ATEC: ** (parent) | ||||||||
| Total | 55.1±23.3 (15) | 40.1±22.8 (15) | −14.3±16.5 (−25.2) | 62.8±31.7 (8) | 56±27.6 (8) | −6.8±9.8 (−10.8) | −7.5 (−21 to 5.8) | 0.25 |
| Speech | 9.9±6.3 (15) | 7.8±5.9 (15) | −2.0±3.3 (−21.2) | 10.5±6.4 (8) | 10.9±7.2 (8) | 0.38±2.6 (3.7) | −2.38 (−5.2 to 0.43) | 0.09 |
| Sociability | 12±7.1 (15) | 8.3±5.9 (15) | −3.7±6.2 (−30.8) | 11.9±7.7 (8) | 10.8±8.4 (8) | −1.1±1.9 (−9.2) | −2.6 (−6.2 to 1.0) | 0.15 |
| Cognitive | 12.7±6.1 (15) | 9.2±5.5 (15) | −3.5±3.4 (−27.6) | 14.4±7.6 (8) | 14.9±7.3 (8) | 0.5±2.6 (3.4) | −4 (−6.9 to −1.1) | 0.009 |
| Health/Behavior | 20.5±8.1 (15) | 14.8±7.9 (15) | −5.0±7.4 (−27.8) | 26±14.8 (8) | 19.5±10.9 (8) | −6.5±7.3 (−25) | 1.5 (−5.2 to 8.2) | 0.65 |
ATEC – Autism Treatment Evaluation Checklist; CARS – Childhood Autism Rating Scale; Modified CGI – Global Impression Score; CI – confidence interval;
lower scores are associated with improvement;
mean ±SD;
t-test statistic;
Higher scores are associated with improvement.
Table 3 summarizes the change in lab characteristics between the L-carnitine and placebo groups following 3-months of therapy. Changes in serum total-carnitine and serum free-carnitine levels significantly increased among those study subjects receiving L-carnitine in comparison to the placebo group. In contrast, no similar changes were observed for measurements of whole blood WBC, whole blood RBC, whole blood platelet count, serum creatinine, serum BUN, serum alkaline phophatase, serum AST/SGOT, serum ALT/SGPT, and serum glucose among those study subjects receiving L-carnitine in comparison to the placebo group.
Table 3.
Comparison of lab characteristics between treatment groups*.
| L-carntine baseline (n) | L-carnitine end (n) | Total Δ (%) | Placebo baseline (n) | Placebo end (n) | Total Δ (%) | Contrast between groups (95% CI) | P-value for contrast between groups | |
|---|---|---|---|---|---|---|---|---|
| Serum Total-Carnitine (μmol/L) | 48.8±18.8 (12) | 82.7±22.0** (12) | 33.9±21.1 (41) | 47.1±17.4 (7) | 55.7±21.4 (7) | 8.6±10.1 (15.4) | 25.3***(7.2 to 43) | 0.009 |
| Serum Free-Carnitine (μmol/L) | 34.6±12.6 (12) | 61.4±22.7 (12) | 26.8±18.3 (43.6) | 35.7±13.0 (7) | 35.7±16.1 (7) | 0.0±13.6 (0.0) | 26.8 (9.9 to 44) | 0.004 |
| Whole Blood WBC (×103/μL) | 6.8±2.4 (12) | 7.0±2.2 (12) | 0.2±1.6 (2.9) | 7.5±1.7 (8) | 7.7±4.7 (8) | 0.2±5.7 (2.6) | 0 (−4.3 to 4.3) | 0.99 |
| Whole Blood RBC (×103/μL) | 4.6±0.30 (12) | 4.7±0.28 (12) | 0.1±0.25 (2.1) | 4.6±0.25 (8) | 4.5±0.30 (8) | −0.01±0.20 (−2.2) | 0.11 (−0.11 to 0.33) | 0.31 |
| Whole Blood Platelet Count (×103/μL) | 297±87.8 (12) | 272±103 (12) | −25±76 (−8.4) | 324±61.3 (8) | 283±50.8 (8) | −40±51.3 (−12.6) | 15 (−50 to 80) | 0.63 |
| Serum Creatinine (mg/dL) | 0.43±0.10 (12) | 0.49±0.16 (12) | 0.06±0.19 (12.2) | 0.43±0.07 (8) | 0.44±0.06 (8) | 0.01±0.06 (2.3) | 0.05 (−0.07 to 0.17) | 0.41 |
| Serum BUN (mg/dL) | 10.8±2.9 (12) | 12.3±3.6 (12) | 1.5±3.1 (12.2) | 12.5±2.7 (8) | 15.3±3.2 (8) | 2.8±4.0 (18.3) | −1.3 (−4.6 to 2.0) | 0.42 |
| Serum Alkaline Phophatase (IU/L) | 209±52.2 (12) | 235±55.7 (12) | 26±55.7 (11.1) | 227±59.2 (8) | 241±50.8 (8) | 14±33.9 (5.8) | 12 (−34 to 58) | 0.60 |
| Serum AST/SGOT (IU/L) | 30.6±5.3 (12) | 30.8±4.8 (12) | 0.20±4.3 (0.65) | 32.9±6.1 (8) | 31.6±4.0 (8) | −1.3±4.8 (−3.9) | 1.5 (−2.8 to 5.8) | 0.48 |
| Serum ALT/SGPT (IU/L) | 16.4±3.8 (12) | 18.0±3.6 (12) | 1.6±2.6 (8.9) | 15.9±3.9 (8) | 16.5±4.4 (8) | 0.6±3.8 (3.6) | 1.0 (−2.0 to 4.0) | 0.49 |
| Serum Glucose (mg/dL) | 86.3±6.1 (12) | 83.5±8.1 (12) | −2.8±9.5 (−3.2) | 87.6±8.2 (8) | 78.9±14.7 (8) | −8.7±11.6 (−9.9) | 5.9 (−4.04 to 16) | 0.23 |
ALT/SGPT – alanine aminotransferase; AST/SGOT – aspartate aminotransferase; BUN – blood urine nitrogen; CI – confidence interval; RBC – red blood cell count; WBC – white blood cell count;
mean ±SD;
t-test statistic.
Table 4 reports on the treatment monitoring characteristics between the L-carnitine and placebo groups following 3-months of therapy. There were similar changes in the scores derived from the parent completed FISER/GRSEB and PRISE forms between the L-carnitine and placebo groups, indicating a similar profile of potential side effects in both groups. Finally, TAM forms completed by the parents of study subjects at the end of 3-months of therapy showed good adherence to the prescribed dosing regimen (average adherence was >85%), and TAM scores were similar in the L-carnitine and placebo groups.
Table 4.
Comparison of monitoring characteristics between treatment groups*.
| L-carntine baseline (n) | L-carnitine end (n) | Total Δ (%) | Placebo baseline (n) | Placebo end (n) | Total Δ (%) | Contrast between groups (95% CI) | P-value for contrast between groups | |
|---|---|---|---|---|---|---|---|---|
| Total PRISE** (Parent) | 4.9±3.7*** (15) | 2.3±1.8 (15) | −2.7±4.0 (−53) | 6.9±4.6 (8) | 4.0±2.0 (8) | −2.9±3.2 (−42) | 0.2# (−3.2 to 3.6) | 0.90 |
| TAM (Parent) | – | 87.3±15.1 (15) | – | – | 93.1±8.8 (8) | – | −5.8 (−18 to 16.3) | 0.33 |
| FISER/GRSEB** | – | 0.73±2.8 (15) | – | – | 0.0±0.0 (8) | – | 0.73 (−0.69 to 2.1) | 0.33 |
CI – confidence interval; FISER/GRSEB – frequency and intensity of side effect rating/global rating of side effect burden; PRISE – patient report of incidence of side effects; TAM – treatment adherence measurement;
higher values are associated with worse clinically outcomes;
mean ±SD;
t-test statistic.
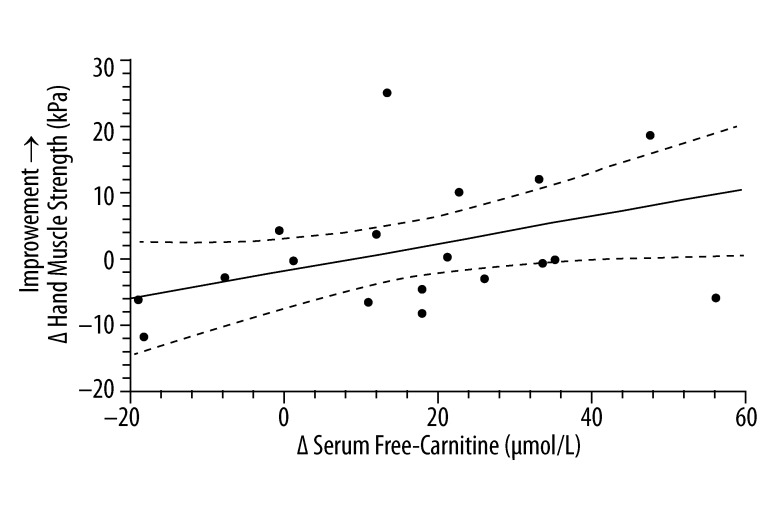
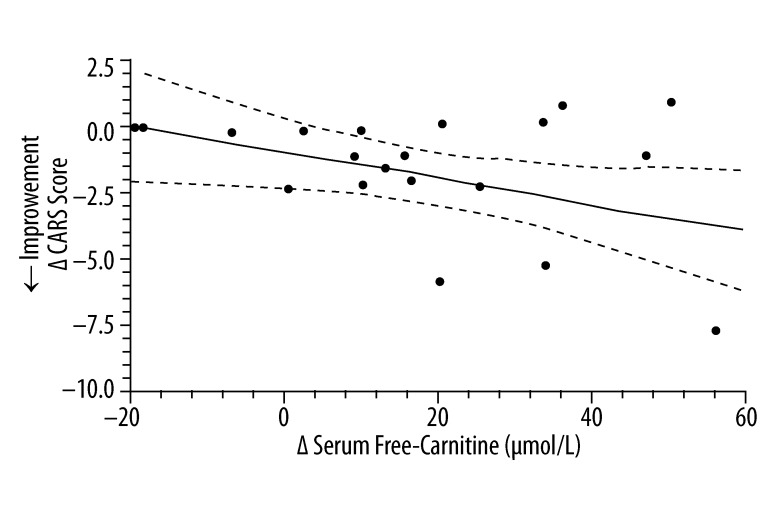
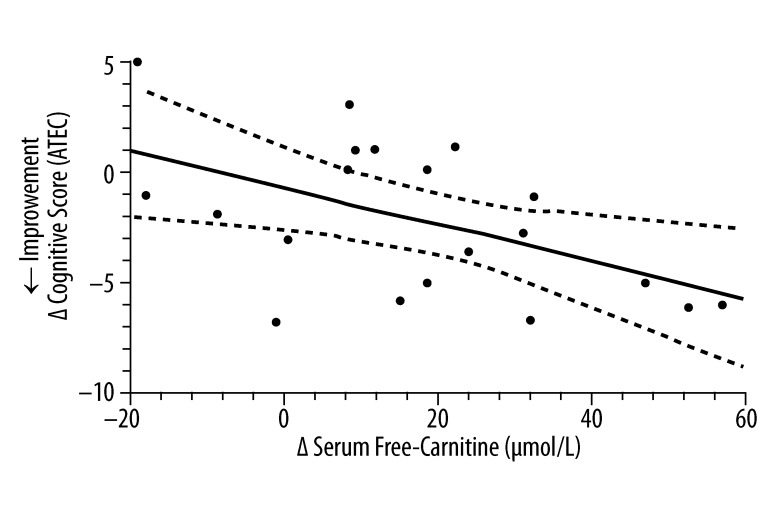
Figures 3–5 evaluate the correlation between changes at the end of 3-months therapy in serum free carnitine and specific clinical outcomes measurements regardless of the treatment status of the study subjects. There was a significant positive correlation between increasing hand muscle strength and increasing serum free-carnitine levels. There were also significant correlations between increasing serum free-carnitine levels and decreasing cognitive scores (from ATEC testing) and CARS scores (decreasing scores on the ATEC and CARS indicate improvement).
Figure 3.
The correlation* between the change in serum free-carnitine and the change in hand muscle testing score (n=18)**. * simple linear-regression statistic; ** L-carnitine group (n=11) and placebo group (n=7). ____ – linear regression estimate; - - - - – 95% confidence interval for linear regression estimate; Δ Hand Muscle Strength (kPa) =0.22 Δ Serum Free Carnitine (μmol/L) − 1.6; R2=0.23, P=0.046.
Figure 5.
The correlation* between the change in serum free-carnitine and the change in CARS score (n=20)**. * simple linear-regression statistic; ** L-carnitine group (n=12) and placebo group (n=8); ____ – linear regression estimate; - - - - – 95% confidence interval for linear regression estimate; Δ CARS Score =−0.047 Δ Serum Free Carnitine (μmol/L) − 0.81; R2=0.20, P=0.047.
Discussion
The present study is the first prospective, double-blind placebo controlled trial to evaluate the effects of L-carnitine therapy among subjects diagnosed with an ASD. In the present study, L-carnitine therapy for 3-months among subjects diagnosed with an ASD significantly improved clinical measurements recorded by trained professionals and parents of study subjects. Further, L-carnitine therapy significantly increased blood levels of total- and free-carnitine among patients diagnosed with an ASD, and there were significant correlations between increasing serum free-carnitine levels and positive outcomes on clinical measurements recorded by trained professionals and parents of study subjects. Finally, L-carnitine therapy was generally well-tolerated with minimal adverse effects. The side effects in the children that did not tolerate the treatment well were irritability and/or stomach discomfort.
The present results are consistent with previous controlled treatment trials of L-carnitine therapy on different populations. For example, investigators evaluated the efficacy of 2 grams L-carnitine administered orally once daily on physical and mental fatigue and on cognitive functions of centenarians in a placebo-controlled, randomized, double-blind treatment trial [19]. Significant increases in plasma total and free carnitine were observed among centenarians receiving L-carnitine in comparison to placebo. In addition, in comparison to the placebo group, L-carnitine recipients were observed to have significant reductions in total fat mass, and increases in total muscle mass. Moreover, this treatment regimen generated an increased capacity for physical and cognitive activity by reducing fatigue and improving cognitive functions. Other investigators evaluated the acute effects of intravenously administered L-carnitine in patients diagnosed with dementia using single-photon emission computed tomography (SPECT) brain scans [20]. In studies comparing SPECT brain scans following intravenously administered L-carnitine at doses of 1 gram and above to the test patients’ basal SPECT brain scans, significantly increased tracer activity was observed in cortical regions, particularly in the parietal lobe.
Investigators have described that the beneficial effects of carnitine are believed to be due to its stimulatory effect on acetylcholine synthesis, increasing the export of acetyl moieties from mitochondria to cytosol [21]. Moreover, administration of carnitine to neural cells was observed to affect their differentiation; for instance, carnitine promotes expression and transfer to the membranes of B-50 protein (named also GAP-43 or neuromodulin), a protein known to be involved in neuronal development, neurogenesis, neuroplasticity and neurotransmission. Finally, the reports on inhibition of protein kinase C activity by palmitoylcarnitine, as well as correlation of its level with the process of ceramide synthesis point to an involvement of carnitine derivatives in the regulation of signal transduction pathways [21].
Strengths/limitations
The main strength of the present study is the design as a prospective, double-blind placebo-controlled trial. Every effort was made to ensure that the present study was truly double-blind so that those evaluating study subjects, both trained professionals and parents, had no knowledge as to the treatment status of any particular study subject. Based on the results of the study, the placebo effects observed were minimal for the outcomes measured. Further, the present study also attempted to minimize the effects of study drop-out for potential adverse reactions in the data, especially for CGI and CARS scores. These particular scoring measurements were conducted by the study investigators on each child regardless of whether or not they dropped-out from the study for potential adverse reactions. As a result, for both of these measurements, there was virtually no change in scores for the study subjects in the placebo group after 3-months of ‘placebo’ therapy. In addition, the technique employed to evaluate outcome measurements is an additional strength of the present study. For each outcome measurement evaluated, the relative change for the parameter following 3-months of therapy in comparison to baseline was examined. As a result, potential variation between study subjects was minimized because each study subject served as his or her own control.
One of the potential limitations of the present study is the small sample size examined. The small sample size in the present study may have resulted in specific effects of L-carnitine therapy being missed because of lack of statistical power to detect significant changes between the L-carnitine and placebo groups. As a result, the observation of so many significant positive effects of L-carnitine therapy in the present study tends to argue that the observed effects represent genuine phenomena. Similarly, it is possible that because of the small sample size examined, there may be differential randomization of potential subgroups of study subjects between the treatment and placebo groups, although this potential possibility seems to not be able to fully explain the biological phenomena observed because significant correlations were observed between changes in serum free carnitine levels and clinical outcome measurements regardless of treatment status. Another potential limitation of the present study is that significant observations may be the result of statistical chance due to multiple statistical comparisons. As a result of the aforementioned potential limitations of the present small study, L-carnitine use in subjects diagnosed with an ASD may lead to improvements in some measures. The data from the present study provide the basis for a larger, more focused study on the promising elements.
A further potential limitation of the present study is the exact mechanism of action of L-carnitine was not elucidated from the present study. In addition, it is also possible that other mitochondrial co-factors such as coenzyme Q10 supplementation may also yield significant improvements among subjects diagnosed with an ASD. Finally, an additional potential limitation of the present study is the fact that the dose of L-carnitine used may not have been optimal. The dosing regimen of L-carnitine used in the present study was derived from the package insert for L-carnitine, as the recommended starting dose for children. The package insert itself acknowledges that it may be necessary to increase the dose of L-carnitine upward as clinically necessary. Fortunately, the dose used was able to significantly increase the levels of serum total and free carnitine measured in study subjects receiving L-carnitine for 3-months in comparison to subjects receiving placebo, but it was observed that there was not a uniform increase in the levels of serum total- and free-carnitine despite the dose of L-carnitine being given by a uniform dosing regimen that adjusted for the initial bodyweight of the study participant. From the Figures 3–5, it is possible to evaluate the relationship between increasing serum free carnitine levels in comparison to specific clinical outcome measurements. These figures suggest the average increase of serum-free carnitine by 27 μmol/L observed in the present study with L-carnitine therapy for 3-months should induce clinical improvements in subjects, but the 95% confidence intervals of the linear regression estimates from these figures suggest that a higher dose of L-carnitine therapy, which would induce significantly higher levels of serum free-carnitine, should be associated with more clinical improvements. Specifically, the figures suggest that an average increase of serum free-carnitine by 40 to 60 μmol/L should ensure that the lower bounds of the 95% confidence intervals from the linear regression estimates would be in the clinically positive range, but it is unknown whether higher dosing will be associated with an increased rate of adverse outcomes.
Conclusions
In conclusion, the results of the present study suggest that L-carnitine therapy at a dose of 50 mg/kilogram bodyweight/day over the course of three months of therapy significantly improved several clinical measurements of ASD severity. Further, there were significant correlations between increasing levels of serum free carnitine and several positive clinical outcomes among the study subjects examined. Overall, the L-carnitine therapy was well-tolerated. It is suggested that future studies further explore additional dosing regimens to identify the potential optimal dosing level or range of L-carnitine for subjects diagnosed with an ASD, to help to further elucidate the biological basis for L-carnitine’s mode of action at the cellular level, and also to identify mitochondrial dysfunction biomarkers for those patients diagnosed with an ASD that would most benefit from L-carnitine therapy.
Figure 4.
The correlation* between the change in serum free-carnitine and the change in cognitive score derived from the ATEC (n=20)**. * simple linear-regression statistic; ** L-carnitine group (n=12) and placebo group (n=8); ____ – linear regression estimate; - - - - – 95% confidence interval for linear regression estimate; Δ Cognitive Score (ATEC) =−0.086 Δ Serum Free Carnitine (μmol/L) − 0.72; R2=0.27, P=0.019.
Acknowledgment
The authors wish to acknowledge the help of the parents and children who participated in the study; without their participation this type of investigation would not be possible. The authors wish to acknowledge Dr. Thomas Carmody for his review of the methods employed in the present study and Melissa Troutman for her involvement in the present study.
Footnotes
Source of support: This research was funded by a grant from the Autism Research Institute, non-profit CoMeD, Inc., and by the non-profit Institute of Chronic Illnesses, Inc from grants received from the Brenen Hornstein Autism Research and Education (BHARE) Foundation, the Yates Foundation for Autism, and the Sandra Gray Memorial Fund. None of the authors owned a financial interest in the production of L-carnitine prior to conducting the present study. Since, the completion of the study Mark Geier and David Geier have a patent pending for the use of L-carnitine for the treatment of autism spectrum disorders.
References
- 1.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2006 Principal Investigators; Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorders – Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2009;58:1–20. [PubMed] [Google Scholar]
- 2.Gerlai J, Gerlai R. Autism: a large unmet medical need and a complex research problem. Physiol Behav. 2003;79:461–70. doi: 10.1016/s0031-9384(03)00165-3. [DOI] [PubMed] [Google Scholar]
- 3.Lombard JL. Autism: a mitochondrial disorder? Med Hypotheses. 1998;50:407–500. doi: 10.1016/s0306-9877(98)90270-5. [DOI] [PubMed] [Google Scholar]
- 4.Schifter T, Hoffman J, Hattten HP, Jr, et al. Neuroimaging in infantile autism. J Child Neurol. 1994;9:155–61. doi: 10.1177/088307389400900210. [DOI] [PubMed] [Google Scholar]
- 5.Minshew NJ, Goldstein G, Dombrowski SM, et al. A preliminary 31PMSR study of autism-evidence for under-synthesis and increased degradation of brain membranes. Biol Psychiatry. 1993;33:762–73. doi: 10.1016/0006-3223(93)90017-8. [DOI] [PubMed] [Google Scholar]
- 6.Filipek PA, Juranek J, Nguyen MT, et al. Relative carnitine deficiency in autism. J Autism Dev Disord. 2004;34:615–23. doi: 10.1007/s10803-004-5283-1. [DOI] [PubMed] [Google Scholar]
- 7.Weissman JR, Kelley RI, Bauman ML, et al. Mitochondrial disease in autism spectrum disorder patients: a cohort analysis. PLoS One. 2008;3:e3815. doi: 10.1371/journal.pone.0003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geier DA, Geier MR. Autism spectrum disorder-associated biomarkers for case evaluation and management by clinical geneticists. Expert Rev Mol Diagn. 2008;8:671–74. doi: 10.1586/14737159.8.6.671. [DOI] [PubMed] [Google Scholar]
- 9.Chlebowski C, Green JA, Barton ML, Fein D. Using the Childhood Autism Rating Scale to diagnose autism spectrum disorders. J Autism Dev Disord. 2010;40:787–99. doi: 10.1007/s10803-009-0926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schopler E, Reichler RJ, Renner BR. The Childhood Autism Rating Scale. Western Psychological Services; 12031 Wilshire Boulevard, Los Angeles, California, 90025-1251: 1994. [Google Scholar]
- 11.Rimland B, Edelson M. Autism Treatment Evaluation Checklist. Autism Research Institute; 4812 Adams Ave, San Diego, CA 92116: ( www.ARI-ATEC.com and https://www.autismeval.com/ari-atec/report1.html) [Google Scholar]
- 12.Jarusiewicz B. Efficacy of neurofeedback for children in the autism spectrum: A pilot study. J Neurotherapy. 2002;6:39–49. [Google Scholar]
- 13.Lonsdale D, Shamberger RJ, Audhya T. Treatment of autism spectrum children with thiamine tetrahydrofurfuryl disulfide: A pilot study. Neuro Endocrinol Lett. 2002;23:303–8. [PubMed] [Google Scholar]
- 14.Geier DA, Geier MR. A clinical trial of combined anti-androgen and anti-heavy metal therapy in autistic disorders. Neuro Endocrinol Lett. 2006;27:833–38. [PubMed] [Google Scholar]
- 15.Berry ET, Giuliani CA, Damiano D. Intrasession and intersession reliability of handheld dynamometry in children with cerebral palsy. Pediatr Phys Ther. 2004;16:191–98. doi: 10.1097/01.PEP.0000145932.21460.61. [DOI] [PubMed] [Google Scholar]
- 16.Merlini L, Domenico D, Granata C. Reliability of dynamic strength knee muscle testing in children. J Orthop Sports Phys Ther. 1995;22:73–76. doi: 10.2519/jospt.1995.22.2.73. [DOI] [PubMed] [Google Scholar]
- 17.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self reported measure of medication adherence. Medical Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH. Self-rated global measure of the frequency, intensity and burden of medication side-effects. J Psychiatr Prac. 2006;12:71–79. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Malaguarnera M, Cammalleri L, Gargante MP, et al. L-carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: a randomized and controlled clinical trial. Am J Clin Nutr. 2007;86:1738–44. doi: 10.1093/ajcn/86.5.1738. [DOI] [PubMed] [Google Scholar]
- 20.Battistin L, Pizzolato G, Dam M, et al. Single-photon emission computed tomography studies with 99mTc-hexamethylpropyleneamine oxime in dementia: effects of acute administration of L-acetylcarnitine. Eur Neurol. 1989;29:261–65. doi: 10.1159/000116423. [DOI] [PubMed] [Google Scholar]
- 21.Wawremczul A, Sacher A, Mac M, et al. Transport of L-carnitine in isolated cerebral cortex neurons. Eur J Biochem. 2001;268:2091–98. doi: 10.1046/j.1432-1327.2001.02087.x. [DOI] [PubMed] [Google Scholar]