Summary
Background
The aim of the paper is the differential diagnosis of various types of Fronto-Temporal Dementia (FTD), with the focus on its behavioural variant (bvFTD).
Material/Method
Material and Method. Screening was done in order to assess the depth of dementia with the short version of MMSE, while evaluation of various variants of FTD was performed with the use of such neuropsychological tests as Newcomb and Chicago Fluency Tests, Wechsler Memory Scale - III (WMS-III), Western Aphasia Battery (WAB-R), and the Boston Naming Test (BNT). Behaviour was evaluated with a Polish version of the Frontal Behavioral Inventory (FBInv). The inventory consists of 24 questions which enable an evaluation of social behaviour disorders. The study included 112 patients - 68 men and 46 women treated in the Reintegrative -Training Centre of the Foundation for Persons with Brain Dysfunctions in Kraków and in the Clinic for Developmental Psychiatry, Psychotic Disorders and Old Age Psychiatry, of the Medical University at Gdańsk, who were suffering from various types of dementia.
Results
It was found that FTD patients scored the highest, while the VAD patients scored somewhat lower in the FBInv. At the same time the scores obtained by PPA patients were higher in comparison to the control groups, but not as high as in the case of patients with FTD. In the process of the neurotherapy of FTD patients we found a reduction of the behavioral disturbances, despite the progression of the illness.
Conclusions
The results obtained in the present study confirmed the diagnostic value of FBInv in the differential diagnosis of various types of FTD and in the evaluation of neurotherapy efficacy.
Keywords: frontal cortex dysfunctions, impairments of cognitive functions, executive functions disorders, disinhibition
Background
Diagnosis of Fronto-temporal dementia (FTD) requires – beside brain-imagining – a careful evaluation of genetic and neuropathological, cognitive processes disorders, and behaviour problems. The complexity of the symptoms observed after frontal lobe damage creates the necessity for a very careful neuropsychological examination [1,2]. Gustafson and Nielsen [3] already in the last century proposed a way of evaluating the behaviour of patients with frontal dementia enabling a differentiation of Pick’s and Alzheimer Disease. The Manchester group evaluated also the symptoms of FTD in retrospective studies in order to compare the clinical diagnosis with post mortem examination [4,5]. This made possible a differential diagnosis of Dementia and of Alzheimer Type (DAT) [6]. Lopez et al. [7] stated more symptoms of depression, anxiety, irritability, mood lability, disinhibition, inertia as well as social withdrawal in FTD patients in comparison to DAT patients, in whom paranoic symptoms were more often observed. Gregory and Hodges [8] conducted a review of psychotic symptoms in FTD patients with at least 5 basic diagnostic features. In a half of the patients a diagnosis of FTD already established, 1/3 had been diagnosed with schizophrenia, psychosis, depression with obsessive-compulsive traits, alcohol addiction, and psychogenic memory disorders.
The course of illness in FTD is similar to DAT and it includes three stages. The nature of the stages including disorders of cognitive, emotional processes as well as disorders of behaviour were described in many works [9]. Lebert [10] applied an Inventory of Symptoms to discriminate FTD, Alzheimer disease, and vascular dementia. The inventory took into account the following disorders:
lack of self-control,
self neglect,
egocentric behaviours,
mood disorders.
Miller et al. [11] performed a retrospective evaluation of occurrence and the lack of symptoms of the Lund-Manchester scale in 30 patients with FTD. Patients were selected on the basis of a SPECT examination. The following differences between patients with FTD and were noted:
personal neglect,
hyperorality,
stereotyped behaviours and perseveration,
logopenia with preserved spatial orientation.
Kertesz et al. [12] developed a Frontal Behavioural Inventory (FBInv) in order to delineate specific behaviours, which would make possible a reliable diagnosis of frontal dementia. The inventory may be used both for the purpose of an introductory evaluation and to perform retrospective diagnosis. The items of the inventory were selected from the elementary diagnostic of the Lund-Manchester group and out of the most common syndromes of FTD observed in the patients examined by Kertesz et. al. [13]. The test was administered to 12 FTD patients, 16 DAT patients at the early stage of illness, and 11 patients with dementia caused by depression diagnosed by a psychiatrist and the Beck Depression Test during a pilot study. DAT patients were found to meet the criteria of NINCDS-ADRD. The depth of dementia was defined on the basis of scores gained on the Mattis Dementia Rating Scale (MDRS). FTD patients were selected taking into account clear cut behavioural symptoms, yet several developed logopenia at the later state of illness, and a typical Motor Neuron Disorder (MND) was stated in one of them. Frontal lobes atrophy was observed in 10 of those 12 patients with neuroimaging techniques. Pick disease was confirmed in 3 patients post-mortem, while in one cortico-basal symptoms were noted, while in two tau-negative ubiquitin inclusions as well as clinical symptoms of MND were stated. Results of the pilot study revealed that patients with frontal lobe lesions obtained much higher scores in the FBInventory than did the two control groups, something which was confirmed with ANOVA. No significant differences between the scores of patients with Alzheimer dementia and dementia following depression were noted. Patients with Alzheimer dementia were older, than patients with FTD and depression with one exception – all FTD patients were 65 years old at the onset of illness (which is a common time for falling ill in such cases). An analysis of the mean scores gained at FBInv revealed that the most common behavior disorders were: lack of insight, thinking rigidity, concreteness, personal neglect, inappropriate remarks due to disinhibition. A significant difference, however, was stated between the scores of FTD patients, and the control groups. The results made it possible to define a cutting point, and to operationalize the behavioural diagnosis of FTD. The point consists of 27 out of 72 points, which can be gained in FBInv, and includes all the FTD patients. Only one patient from amongst those with depressive dementia behaviour disorders was observed. Symptoms of depression were confirmed by vegetative symptoms as well as by scores recorded on the Beck Depression Inventory. All DAT patients, and most depressive patients scored below 24 points. Kertesz [14] notes, that the cutting points of FBInv may enable a grouping of the patients in order to perform a further examination. There are many inventories of behaviour evaluation that aim at screening deviant behaviours in geriatric, psychiatric or dementive patients [15–22]. Geriatric scales frequently combine cognition, behaviour, and everyday activities in order to measure global functional deterioration but they do not discriminate particular behavioural syndromes. Psychiatric behavioural scales also do not discriminate frontal and psychiatric symptoms. A direct evaluation of behaviour of FTD patients takes into account items measuring motor or cognitive perseverations, verbal inclusions, disinhibition, aspontaneity, and utilization behaviours. There were attempts to make the evaluation of symptoms of frontal lobe injuries more formal with the use of executive interviews [23]. Such direct tests often are a part of neurological examination and supplement FBInv. Logopenia appears at the later stages of FTD, and is sometimes connected with verbal and oral apraxia. Frequent hesitations in speech, prozodic disorders, the leaving out of initial consonants, and stuttering was also noted. Yet the scores of items evaluating those disorders were as a rule lower as they appear at a later stage of the illness in FTD patients. Those symptoms used to be called a Primary Progressive Aphasia (PPA) [24]. At the later phases of PPA personality disorders are quite common [26].
Some modification including new items were introduced to FBInv with time. The new version consists of a series of 24 items enabling an evaluation of social behaviour disorders as well as motor behaviours and the syndrome of alien hand. The last 5 items apply to behaviours appearing at the late stage of FTD. They create a lot of anxiety in family members who are reluctant to speak about them. The author believe that the inventory discussed may be of use in the case of PPA if administered along with specific language tests.
The aim of our research was to show the diagnostic value of FBInv [12–14, in distinguishing FTD from such syndromes as VAD or depression as well as in evaluating the efficacy of neurotherapy.
Material and Method
The examination included 112 patients, 68 men and 46 women, who were diagnosed and treated at the Reintegrative-Training Centre of the Foundation for Brain Damaged Persons in Kraków, and in Clinic for Developmental Psychiatry, Psychotic Disorders and the Old Age Psychiatry at the Medical University of Gdańsk, suffering from various types of dementia including depression. The demographic characteristics of the patients are presented in Table 1.
Table 1.
Demograpic data of examined patients.
| Variables | DAT | VAD | FTD | DD | PPA |
|---|---|---|---|---|---|
| Age (mean; SD) | 69.84±9.7 | 65.73±8.4 | 56.41±9.8 | 56.18±12.3 | 61.36±6.9 |
| Education* (mean; SD) | 2.4±3.2 | 2.4±3.2 | 2.1±2.3 | 2.9±3.8 | 2.6±3.2 |
| Onset of illness (mean; SD) | 4.3±5.8 | 4.4±5.8 | 3.4±6.7 | 6.9±5.3 | 3.9±5.8 |
| Duration of neurotherapy (mean; SD) | 6.4±3.5 | 8.4±3.5 | 4.2±1.2 | 7.8±1.2 | 8.4±3.5 |
Years of education after finishing primary school.
The group consisted of 41 patients with Alzheimer dementia including 25 men and 16 women; the mean age was 69.84 (SD 9.7), 14 patients with vascular dementia including 9 men and 5 women; the mean age was 65.73 (SD 8.4), 18 FTD patients including 11 men and 9 women; with a mean age of 56.41 (SD 9.8), 27 DD patients including 16 men and 11 women; mean age 56.18 (SD 12.3), 12 PPA patients including 7 men and 5 women; mean age 61.36 (SD 6.9).
The following tasks were applied:
clinical interview,
neuropsychological tests [Wechsler Inteligence Scale-Revised – WAIS-R [26]; Wechsler Memory Scale-Revisd – WMS-R [27]; Western Aphasia Battery – Revised – WAB-R [28]; Lines Evaluation Test [29], Face Recognition Test [30,31],
screening with MMSE to evaluate the depth and progression of dementia and with the Polish version of the Boston Naming Test (BNTvPL) [32] which aimed at discrimination of language and behavioural variants of Fronto-Temporal Dementia,
-
Frontal Behaviour Inventory (FBInv) adapted for Polish [9]. The inventory was filled out by the patients caregivers. The final version of the FBInv consists of questions that include both correct as well as incorrect, negative aspects of behaviour – out of which the carer selects those he thinks describe the patient best. The particular questions can be put in other words if the carer has problems with understanding them. The particular items are grouped into two types of behaviour. They are:
negative behaviors, such as apathy, aspontaneity, indifference, thinking rigidity, concreteness, personal neglect, distractibility, inattention, loss of insight, logopenia, verbal apraxia, and alien hand syndrome [the last 3 items were included to evaluate the specific motor and linguistic behaviours that might occur in FTD].
positive behaviours, connected with disinhibition, such as perseveration, irritability, excessive jocularity, unpredictability, irresponsibility, inappropriateness, impulsivity, restlessness, aggression, hyperorality, hypersexuality, utilization behaviour, and incontinence.
Procedure
The inventory was given to a patient caregiver, and the examiner provided explanations of the meaning of particular questions in the case of such a need.
Results
The scores gained by the examined patients with FTD did not differ in as far as sex is concerned, therefore, further analysis will be conducted for both men and women together in each group.
Evaluation of cognitive functions
The screening of cognitive functions taking into account intellectual abilities, memory, visual functions, language functions, and general features of dementia in individual groups is presented in Table 2.
Table 2.
Scores of examined patients in individual tests assessing cognitive processes.
| Index | Scale | Variables | ||||
|---|---|---|---|---|---|---|
| DAT | VAD | fvFTD | DD | PPA | ||
| WAIS – R[1] | ||||||
| I.Q. Full | 100 | 73.5 | 87.8 | 79.3 | 88.35 | 78.1 |
| I.Q. Verbal | 100 | 79.6 | 87.2 | 67.1 | 89.4 | 66.8 |
| I.Q. Nonverbal | 100 | 67.5 | 88.3 | 91.5 | 87.3 | 89.3 |
| WMS – R[2] | ||||||
| Instant logical memory | 24 | 13.4 | 15.7 | 19.1 | 21.6 | 16.7 |
| Delayed logical memory | 24 | 3.8 | 7.4 | 11.3 | 16.5 | 10.4 |
| Instant visual reproduction | 41 | 9.5 | 15.3 | 32.6 | 34.7 | 30.2 |
| Delayed visual reproduction | 41 | 2.6 | 6.7 | 11.9 | 26.8 | 21.7 |
| Visual-spacial orientation | ||||||
| Lines Evaluation Test | 30 | 13.1 | 19.2 | 26.7 | 28.7 | 26.7 |
| Face Recognition Test | 54 | 13.3 | 24.5 | 51.3 | 52.8 | 50.3 |
| Language Functions – WAB[3] | ||||||
| Aphasia Quotient – AQ | 100 | 87.3 | 92.7 | 85.1 | 97.3 | 64.3 |
| Cortex Quotient – CQ | 100 | 68.6 | 81.5 | 89.3 | 94.5 | 81.2 |
| General intellectual functions | ||||||
| MMSE | 30 | 19.0 | 20.8 | 19.3 | 23.9 | 21.9 |
WAIS – R Wechsler Adult Intelligence Scale;
WMS-R – Wechsler Memory Scale – Revisited;
WAB – Western Aphasia Battery.
Intellectual functions
An analysis of WAIS-R scores shows that the lowest global I.Q. was noted in patients with Alzheimer dementia, fvFTD patients, and PPA patients. Therefore, it does not discriminate the examined groups. Yet, it may be observed that the distribution of results in nonverbal I.Q. and verbal I.Q. is different in the groups. Thus, patients with Alzheimer dementia gained significantly lower scores in nonverbal I.Q. when compared with their verbal I.Q. On the other hand, the proportions are reversed in fvFTD and PPA patients as their nonverbal I.Q. is much higher than nonverbal I.Q.
The above is most probably connected with the different profile of disorders in Alzheimer dementia, in which visual-spatial deficits connected with a performance part of WAIS-R are more pronounced, while language disorders dominate in PPA and FTD patients (five patients with Semantic Dementia – SD were included in that group, and this might have influenced the results).
Memory
WMS-III test revealed disorders of logical memory in all examined patients, yet the depth of those disorders is different. Most severely impaired were DAT patients, less PPA and VAD patients, and the least DD patients. All of them presented difficulties in performing tasks with delay. It should be noted that DAT patients, as might be expected, exhibit more clear cut impairments in visual memory than other groups. In fact they practically do not accomplish any delay tasks whatsoever. On the other hand, FTD and PPA patients score much worse in auditory memory than in the visual memory. On reproduction with delay they score worse but they are able to remember considerable amounts of information.
Visual-spatial functions
The most pronounced disorders of visual-spatial functions occurred as was expected for DAT patients both in the Benton Test as well as in the Face Recognition Test, the disorders were less pronounced in VAD patients. FTD and PPA patients revealed non-significant disorders, while DD patients stayed within a norm. Those results can be explained due to the observation of patients performance. It can be noticed that FTD and PPA patients tend to abandon the tasks, which may be the cause of a lower scoring. Visual-spatial dysfunctions may be also connected with deficits in memory selectivity, as the patients spare much less time to individual tasks.
Language functions
Western Aphasia Battery (WAB) showed the occurrence of aphasic disorder in all examined patients with the exception of patients with depression. As expected most pronounced disorders were observed in PPA patients – with Aphasia Quotient =64.3, slightly lower in AD patients – with AQ=87.3, and in FTD patients – with AQ=85.1. The least pronounced disorders were stated in VAD patients – with AQ=92.7. Depressive patients were on the borderline of the norm.
Slightly surprising are the profiles of the cortical quotients as they show the reverse tendency. The worst scores were gained by DAT patients – with a Cortical Quotient =68.6. It was probably due to the fact that Picture recognition scores are included in the CQ, and as we know this is frequently impaired in Alzheimer dementia.
General traits of dementia
Comparison of the scores of the examined groups in the MMSE Test did not reveal statistically significant differences among the examined groups. The lowest mean, however, was gained by patients with DAT, and the lowest patients with depression and PPA, which was in agreement with expectations.
Behavioural changes
The FBInv scores of patients with various types of dementia are presented in Table 3. It may be observed that the highest scores (much above the cutting point) were obtained by FTD patients. The scores of PPA patients were higher than in the control groups, but not as high as in the case of patients with FTD. The scores of VAD patients were similar to the scores of FTD patients. It confirms the observations of other authors that the so called frontal type occurs within VAD – which must be connected with dissociation of the frontal subcortical structures taking place in VAD [33,34]. It was stated that disinhibition symptoms, which prevail over apathy and withdrawal, are helpful in distinguishing FTD patients from VAD or DD patients [35].
Table 3.
FBInv scores of patients with various types of dementia.
| Type of dementia | N | Sex | Age | Scores of FBInv | |||
|---|---|---|---|---|---|---|---|
| M | K | X | SD | X | SD | ||
| AD | 41 | 25 | 16 | 69.8 | 9.7 | 10.8 | 7.8 |
| VAD | 14 | 9 | 5 | 69.3 | 8.6 | 25.1 | 8.4 |
| FTD | 18 | 11 | 9 | 56.8 | 9.8 | 38.1 | 6.7 |
| DD | 27 | 16 | 11 | 56.1 | 12.3 | 9.1 | 8.3 |
| PPA | 12 | 7 | 5 | 67.5 | 6.9 | 15.3 | 12.5 |
| Total | 112 | 68 | 46 | 63.9 | 9.46 | 19.68 | 8.74 |
Frequency of the occurrence of individual frontal lobe symptoms in 18 patients with bvFTD, correlated with the data gathered from the structured interview is shown in Table 4. This will enable the reader to better see the spectrum of behaviour disorders that appear in bvFTD patients.
Table 4.
Frequency of occurrence of individual frontal lobe symptoms in 18 patients with bvFTD.
| Symptoms | % | Symptoms | % |
|---|---|---|---|
| Loss of insight | 86 | Hyperorality | 35 |
| Inappropriate remarks | 76 | Irritability | 34 |
| Perseveration | 75 | Restlessness | 34 |
| Logopenia, anomia | 75 | Puissance | 31 |
| Personal neglect | 69 | Impulsivity | 29 |
| Apathy | 65 | Careless driving* | 29 |
| Forgetfulness** | 61 | Incontinence | 26 |
| Inattention, distractibility | 59 | Aspontaneity | 19 |
| Indifference, emotional flatness | 57 | Excessive touching of objects (compulsiveness) | 19 |
| Disorganization, inability to plan | 52 | Hypersexuality | 18 |
| Social withdrawal*** | 51 | Excessive Jocularity (moria) | 18 |
| Financial errors | 47 | Kleptomania | 17 |
| Thinking rigidity, concreteness | 44 | Emotional lability | 12 |
| Loss of insight | 43 | Childish behaviour | 11 |
| Verbal mistakes | 41 | Paranoia | 9 |
| Aggression | 39 | Echolalia | 9 |
This is not specific as a result of the overlap in symptoms with the loss of impulsivity and the ability to evaluate;
As a result of the lack of specificity the following have been erased: forgetfulness and wandering off;
As a result of the overlapping of symptoms with apathy social withdrawal is not specific.
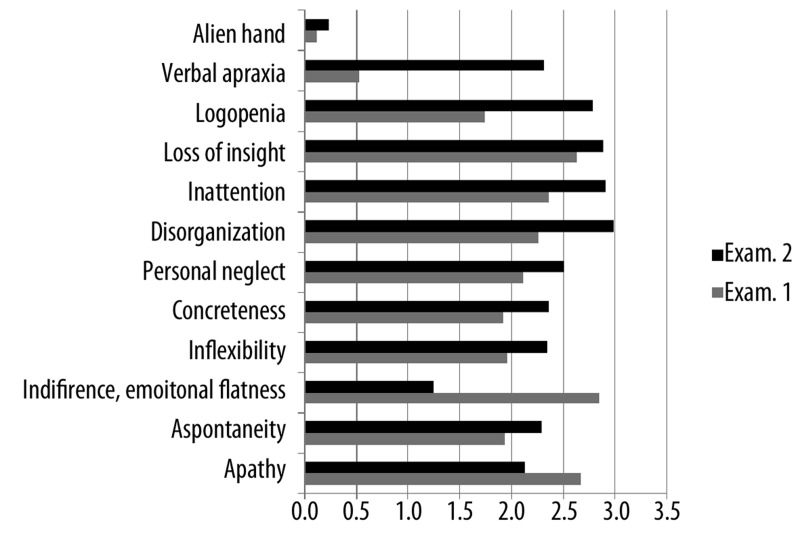
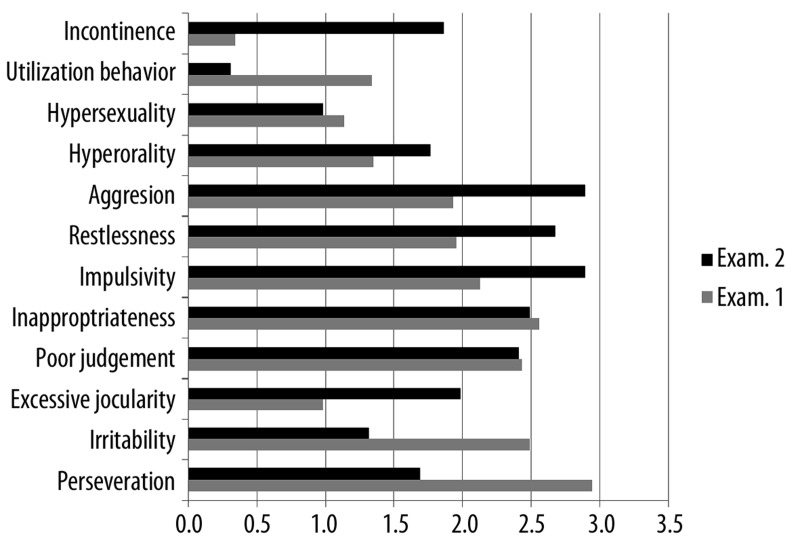
In our study bvFTD patients were given a half-a-year neuropsychotherapy aimed at a reduction of frontal symptoms in accordance with a program elaborated by Pąchalska [36]. The patients behaviour was then evaluated with FBInv. The results of the study are shown in Table 5, and in Figure 1A, B. It may be observed that rehabilitation resulted in an improvement of some deviant behaviours. Thus, a significant decrease of indifference and emotional flatness as well as of apathy was observed. Yet, some disorders, mainly the behaviours connected with disinhibition did increase along with the progression of the illness despite neurotherapy. Those behaviours such as impulsivity, restlessness, aggressiveness, and excessive jocularity. Behaviours resulting from difficulties in passing from an idea to action as well as apathy also increased. Those were apraxia of speech, concreteness, disorganization, and inattention. As might have been expected alien hand symptoms as well as incontinence also increased.
Table 5.
Scores of bvFTD patients in individual traits of FBInv in I and II examination.
| No. | TRAIT | Mean evaluation | ▲* | |
|---|---|---|---|---|
| Exam. 1 | Exam. 2 | |||
| 1 | Apathy | 2.67 | 2.13 | −20.22% |
| 2 | Aspontaneity | 1.93 | 2.29 | 18.81% |
| 3 | Indifference, Emotional Flatness | 2.85 | 1.25 | −56.14% |
| 4 | Inflexibility | 1.96 | 2.35 | 19.64% |
| 5 | Concreteness | 1.92 | 2.36 | 22.92% |
| 6 | Personal neglect | 2.11 | 2.51 | 19.15% |
| 7 | Disorganization | 2.26 | 2.98 | 31.81% |
| 8 | Inattention | 2.36 | 2.91 | 23.18% |
| 9 | Loss of insight | 2.63 | 2.89 | 9.89% |
| 10 | Logopenia | 1.74 | 2.78 | 59.77% |
| 11 | Verbal apraxia | 0.53 | 2.31 | 335.85% |
| 12 | Perseveration | 2.95 | 1.69 | −42.85% |
| 13 | Irritability | 2.49 | 1.32 | −46.99% |
| 14 | Excessive Jocularity | 0.98 | 1.98 | 102.04% |
| 15 | Utilization Behaviour | 2.43 | 2.41 | −1.03% |
| 16 | Inappropriateness | 2.56 | 2.49 | −2.81% |
| 17 | Impulsivity | 2.13 | 2.89 | 35.68% |
| 18 | Restlessness | 1.95 | 2.67 | 36.92% |
| 19 | Aggression | 1.93 | 2.89 | 49.74% |
| 20 | Hyperorality | 1.35 | 1.76 | 30.67% |
| 21 | Hypersexuality | 1.13 | 0.98 | −13.27% |
| 22 | Compulsiveness | 1.34 | 0.31 | −76.87% |
| 23 | Incontinence | 0.34 | 1.86 | 447.06% |
| 24 | Alien hand | 0.02 | 0.23 | 1050.00% |
The difference between the mean of the CHI group, and the mean of the FTD group devided by the mean FTD group, in percentage.
Figure 1A.
Mean scores of bvFTD patients (the first 12 traits of the Inventory in I and II examination.
Figure 1B.
Mean scores of patients with bvFTD (the other 12 traits of the Inventory in I and II examination.
Mean scores of patients with bvFTD in I i II examination in the first 12 traits of the inventory, i.e. negative behaviours resulting from difficulty in passing from an idea to action as well as apathy is shown in Figure 1A.
Mean scores of bvFTD patients in I and II examination in the other 12 trait of the inventory, which measure positive behaviours connected with disinhibition are shown in Figure 1B.
To sum up, it is worth pointing out that the above results are in agreement with the results obtained in the studies of other authors that, however, were performed much later than our study [34].
Discussion
In the course of diseases following degeneration of the brain a number of behaviour disorders and other psychopathological symptoms occur, beside the classical cognitive deficits. Such symptoms as psychotic disorders, restlessness, mood disorders, sleep disorders, journeying and many others, which accompany degenerative processes are called behavioural and psychological symptoms [37].
In Table 6, a differential analysis of FTD and DAT was presented on the basis of both our own research [9] and other studies [33,38–43].
Table 6.
Differential analysis of characteristics of Fronto-Tempotal Dementia [FTD] and Dementia of Alzheimer Type [DAT]* [Source: Pachalska [9]].
| Function | FTD | DAT |
|---|---|---|
| Clinical characteristics | ||
| Deficit pattern | Predominance of disorders of executive functions over memory deficits | Predominance of memory deficits over disorders of executive functions |
| Controling and monitoring behavior |
Behavioral variant (frontal): Hyperorality, disinhibition, inappropriateness and uncontrollable eating. Language abilities are well preserved Linguistic variant: lack of behavior disorders at the initial stage. |
Wandering, indifference |
| Initial symptoms | Disorders of language or personality as well as social behaviors disorders | Memory deficits connected with visual-spatial disorders |
| Age at the onset of illness | Typically before 65 years of age | 80 years on average |
| Sex of the patient | Men mainly | No sex preference (both men and women fall ill) |
| Course | Progression to decease 8.7±1.2 years. Years of outlive shorter for frontal variant (behavioural) bvFTD | Progression to decease 11.8±0.7 years |
| Progression of illness |
Early stage: loss of insight, disorders of personality/behaviour or language Late stage: memory loss, decrease of cognitive abilities, possible mutism |
Early stage: memory disorders, general decrease of cognitive abilities Late stage: disorders of personality/behaviour, disorders of functional communication |
| Brain damage | Massive atrophy of frontal lobes and anterior temporal lobes often including limbic structures, hypoperfusion and hypometabolism | Cortical atrophy, mainly of posterior cortex and hippocampus |
| Memory | Episodic memory and general orientation is preserved; deficit of semantic memory, inability to remember past events | Episodic memory and general orientation is disturbed; semantic memory is preserved at the early stage of illness, ability to remember past events is preserved at the early stage of illness |
| Language | Depending upon variant subtype: Behavioural variant (frontal): Well preserved linguistic abilities Linguistic variant: Progressive non-fluent aphasia: Errors in content and structure of utterances, possible logopenia Semantic dementia: Wrong meanings of utterances. Difficulties with differentiation of meaning of utterances (connected mainly with the loss of word-image relationships) |
Depending upon progression of illness: I (early deterioration) stage: Errors in content of utterances. Difficulties with distinguishing meanings (connected with auditory discrimination and difficulties with recognizing objects). II (mean deterioration) stage: Errors in content and structure of utterances. Difficulties with differentiation of meaning and structure of utterances III (deep deterioration)stage: Errors in content and structure of utterances including phonemic paraphasia |
| Naming | Symptoms depend upon the subtype of FTD | Paraphrasing. Changing semantics/phonetic indications. Loss of basic concepts. |
| Writing | Symptoms depend upon the subtype of FTD | Spelling mistakes, coping abilities worse than spontaneous writing |
Information summing up our own studies; Pąchalska [9] and studies of other authors: Kertesz and Munoz [33]; Hornberger et al. [34]; Neary et al. [35]; Harciarek and Jodzio [38]; O’Keeffe et al. [39]; Barcikowska [40]; Bidzan et al. [41]; Grochmal-Bach et al. [42] Kertesz [43]; Cummings & Bathgate [47]; Rasmusson et al. [51]; Geerlings et al. [57]; Diehl [73]; Cardarelli [79].
It can be noticed that executive functions disorders are more pronounced than memory disorders in Fronto-Temporal Dementia, while the reverse pattern of disorders occurs in Alzheimer Dementia.
It is worth pointing out, however, that the intensity of those symptoms as well as the clinical picture varies in accordance with the degree of degenerative changes [44,45], and with so called cognitive reserve [46]. The behavioural manifestation of the process depends both upon neurobiological factors, such as the localization of changes, as well as the dynamics of the process of dementia [9].
Clear cut differences in behavioural manifestations evaluated with FBInv were noted in our study. The most pronounced behavioural disruptions were observed in the bvFTD group. Both negative behaviours connected with passing from thought to action and apathy as well as positive behaviours following disinhibition were noted. The results confirm the usefulness of FBInv for description and the distinguishing of bvFTD from other types of dementia as well as an evaluation of neurotherapy results.
It should stressed here that classic descriptions of the frontal syndrome pointed to the appearance of a number of early behavioural symptoms before clinical disorders of memory [34].
Behaviours and symptoms most frequent in FTD as observed in our study (Table 3) are in agreement with the typical clinical picture of that disease [47]. The possibility of noting differences and similarities among the examined groups was increased by including groups of patients with a decrease in cognitive functions following various degenerative processes. Biochemical aspects were taken into account by the inclusion of one group with taupathy (FTD) and amyloidopathy (Alzheimer disease), forms conditioned by the primary vascular process, and functional disorders of cognitive functions following depression. The neuroanatomic discrimination is reflected in our results.
Since speech disorders are quite frequent in FTD Semantic Dementia as well as Primary Progressive Aphasia treated as a clinical variant of FTD, this is distinguished by some authors alongside with the temporal and frontal form [35]. Such symptoms as: stereotyped utterances, echolalia, perseveration, mutism, semantic aphasia, semantic deficit, and spontaneous speech reduction are considered to belong to the prodromal symptoms of FTD [48].
It seems questionable whether processes at the basis of progressive aphasia and FTD are identical or close to each other. Those nosographic doubts follow from the fact that the level of behavioural disorders registered in FBInv was significantly different in both groups. Thus, a high behavioural manifestation was noted in the case of vascular dementia. Vascular dementia may follow many vascular degenerations of varied localization. It should be also borne in mind that a clinical picture caused by vascular factors varies to a considerable degree, and thus does not fit a classic description of dementia [49].
It is true that diagnostic criteria contrast vascular dementia with primary degenerative diseases of the brain. Yet, the border line may be not clear, Since vascular factors are present also in primary degenerative processes such as Alzheimer Disease for example. Such vascular changes were observed in about 1/3 of Alzheimer patients [50].
Some of the vascular degenerations (amyloid angiopathy, leukoencephalopathy) are observed in almost all the examined cases of Alzheimer Disease [51]. Those changes increase the impairment of cognitive functions [52].
It is generally accepted that behavioural disorders are more frequent in vascular dementias than in Alzheimer Disease, at least at the early stages of the illness. This concerns mainly disorders of affect [53,54] something that was also confirmed in the present study.
A similar level of deviations was noted in the study of persons with DAT and with depression, which may suggest some connections between those diseases. It might seem surprising if we realize that two different disorders are being compared there: one following the slow degeneration of brain tissue, and the other that passes sometimes even without treatment. Yet, the connection between Alzheimer Disease, or dementia in its broader sense, and depression have much in common.
The more frequent occurrence of mood disorders (particularly of a depressive type) in DAT has already been mentioned. Yet, the present study makes it necessary to point out that depressive disorders are connected with a decrease of cognitive ability, which sometimes remind one of dementia due to their degree [55,56]. Quite often depression appears at the early phases of dementia, even in the preclinical stages. And it masks the development of dementia [57]. Hence, episodes of depression are treated as a significant factor pointing to the possibility of the occurrence of dementia, and of the Alzheimer type in particular [58].
Those complex relations may be, at least in part, explained on a neurobiological basis. The structure which unites those processes is hippocampus. Degenerative changes in Alzheimer Disease start in the hippocampus and olfactory cortex, and then expand to the frontal, temporal, and parietal associative cortex [59]. Moreover, the loss of subcortical neurons in the basal nucleus and bluish nucleus occurs quite often [34,60].
In the case of the relation between Alzheimer Dementia and depression the basic role seems to be played by the damage of the hippocampus, which leads to impairment of the mechanisms of control of the corticosteroids level. In DAT both an increase in the level of basic cortisol, and a lack of decrease of its level after the application of deksametason was observed [61–63].
The damage to the hippocampus causing an increase in the CRH level leads to an excessive activity of the hypothalamic-pituitary-adrenal pivot, which is often observed in depression too. Disruptions of the control of the corticosteroids level in depressions may also be connected with disorders of intellectual functions [64,65]. For our studies it is of importance that a hippocampus lesion occurs at the early or even preclinical stages of DAT [37,66].
Not only biological mechanisms indicating the connection of depression with Alzheimer Disease are taken under consideration. Some studies point to a significant loss of neurons in the bluish nucleus in Alzheimer patients, in whom depression was diagnosed at the same time [67,68]. Forstl et al. [69] noted also a more marked degeneration of neurons in the bluish nucleus in patients with Alzheimer Dementia, in whom depression was stated before the clinical development of dementia.
We should also take into account some other factors, while discussing the possible neurobiological mechanisms of creating psychological and behavioural symptoms of the diseases examined here. It is worth pointing out that the pathogenesis of psychological and behavioural symptoms is very complex. It is possible that morphological degenerations observed in dementias predestine some behaviours, which, however, are determined by premorbid personality traits as well as environmental factors [70].
Psychological and behavioural symptoms depend also upon the stage of dementia progression. Hence, aggressive behaviours appear more often in periods preceding faster progression [71,72]. At this present stage of the analysis of the results an evaluation of the process dynamics was not performed.
Better knowledge of the psychological picture, and of behavioural symptoms in particular is of great practical value. It should be borne in mind that dementive processes are proceeded by an signalling period, during which behaviour disorders may dominate over cognitive disorders, which at that time are difficult to distinguish during a clinical examination. Especially in the case of FTD, the symptoms of dementia may be neglected for a long time [34,73]. In consequence, such persons are often given the wrong diagnosis, which often results in a delay of proper treatment.
How can this be explained?
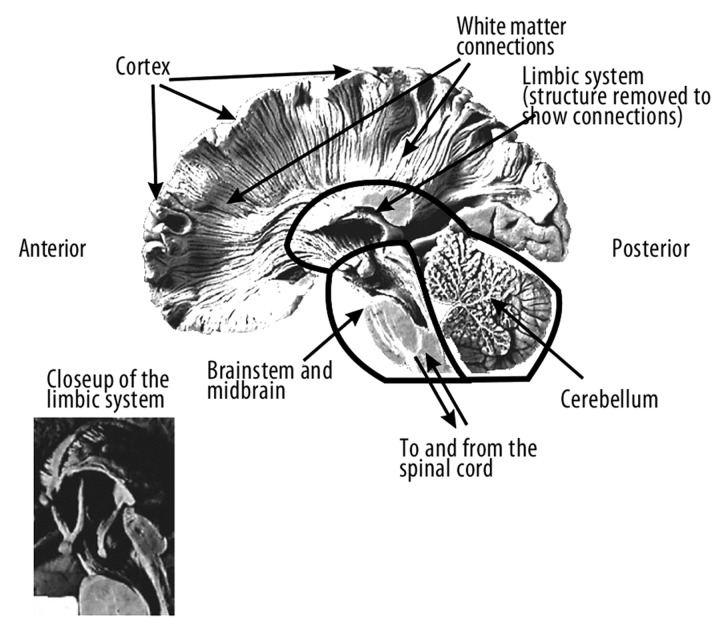
Naturally, the limbic structures, which play an essential role in basic drives and their affective correlates, may not be damaged at the early stage of FTD. The lesions may be noted after cutting of the ways leading from the frontal lobes to the limbic system or if that system is destroyed as a consequence of neurodegenerative changes.
Ablation of the frontal part of the temporal lobe connected with the bilateral ablation of the amygdalia body in animals is followed by dramatic changes in sexual and aggressive behaviours [30]. Klüver-Bucy syndrome, including hypersexuality, lack of fear reaction and visual agnosia, show how dramatic the results of lesions of the limbic structures for emotional-motivational functions can be. FTD patients often exhibit Klüver-Bucy Syndrome. They exhibit various deviant social behaviors, such as disinhibition, verbal or physical aggression, and executive functions disorders connected with apathy [36]. Lesions leading to the above mentioned pathological behaviours are difficult to grasp. Dysfunctions observed in those patients remind one of the behaviours of animals with lesions disrupting the activity of the limbic system. Microgenetic theory of a symptom [74] developed on the basis of an evolutionary, four-dimensional model of brain work [75] may be of help in understanding those disorders. Damage to the limbic system or of one of its connections with other brain structure including the frontal lobes (Figure 2) disrupts the normal activity of the brain, and due to that basic limbic-cortical dysfunction many patients with dementia exhibit difficulties with “adult” or “civilized” behaviours [36,75].
Figure 2.
Medial cross-section of the human brain and white matter connections (From: Pachalska and MacQueen [75]).
Behaviour disorders are often the first symptom of an illness, which may enable putting an early by a well trained evaluator [36]. The microgenetic model of brain work makes us aware that in Fronto-Temporal Dementia the whole of the brain systems become disrupted and not only specific cognitive functions as in the case of local brain injuries. The function of those systems are at first disorganized only in part, but then undergo global disruption, after the destabilization reaches a level, which causes a loss of integrity. It needs to be stressed that even in the case of the linguistic variant of FTD (lvFTD), neither the course nor the nature of the disorders observed, speech and language disorders, recalls aphasia [9,76,77]. There is a difference in reaction of the dynamic system that receives one strong and localized blow as is the case in a brain stroke; and of the system, which loses its energy and undergoes entropy as in the case of dementia. In such case the changes are vertical: the upper levels of complex brain processes lose energy, become disorganized, and finally disintegrate, in contradistinction to the “horizontal” effects of focal lesions, when one part of the system is damaged, and others remain untouched.
As cortical dementia traits grow, which occurs both in Alzheimer disease as well as in FTD, the process of neurodegeneration runs counter to microgenesis, that is: from the upper areas to the lower (deeper) areas of the brain. The deeper the neurodegenerative changes in the brain are, the less specific will be the symptoms. And after the illness assaults the lowest level (brain stem and midbrain), basic life functions will also undergo entropy: will lose energy and disintegrate, which results in decease. Therefore, at the final stage of any neurogenerative dementive disease differences in the clinical picture, which might be clear at the very beginning, become difficult to distinguish [78,79]. In other words, each dementia:
starts with an upset of one’s own identity, due to the arising disturbances,
runs in a way specific for the basic pathomechanism of a given type of dementia,
with time its specific traits are lost and each ends in a similar way.
Conclusions
To sum up, it should be stressed that the scores of the present study enable differentiation due to the specific behavioural profiles of the various dementive syndromes and are of significance for the course of therapy. At the same time they enable evaluation of the efficacy of neurotherapy, and hence they make possible a raising in the quality of life of persons with various types of dementia.
The results obtained in the present study confirmed the diagnostic value of FBInv in the differential diagnosis of various types of FTD and in the evaluation of neurotherapy efficacy.
Footnotes
Source of support: Departmental sources
References
- 1.Kaczmarek BLJ. Regulatory function of the frontal lobes. A neurolinguistic perspective. In: Perecman WE, editor. The frontal lobes revisited. Nowy Jork: IRBN Press; 1987. pp. 225–40. [Google Scholar]
- 2.Kaczmarek BLJ. Płaty czołowe a język i zachowanie człowieka. Lublin: Wydawnictwo Popularnonaukowe „ Linea”; 1993. [in Polish] [Google Scholar]
- 3.Gustafson L, Nielson L. Differential diagnosis of presenile dementia on clinical grounds. Acta Psychiatr Scand. 1982;65:194–207. doi: 10.1111/j.1600-0447.1982.tb00840.x. [DOI] [PubMed] [Google Scholar]
- 4.Brun A, Gustafson L . The Lund longitudinal dementia study. A 25-year perspective on neuropathology, differential diagnosis and treatment. In: Corain B, Nicolini M, Winblad B, et al., editors. Alzheimer’s disease advances in clinical and basic research. Chichester, New York, Bristone, Toronto, Singapore: Wiley; 1993. pp. 4–18. [Google Scholar]
- 5.Brun A, Englund E, Gustafson L, et al. Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J Neurol Neurosurg Psychiatry. 1994;57(4):416–18. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber R, Snowden JS, Craufurd D. Frontotemporal dementia and Alzheimer’s disease: Retrospective differentiation using information from informants. J Neurol Neurosurg Psychiatry. 1995;59:61–70. doi: 10.1136/jnnp.59.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez OL, Gonzalez MP, Becker JT, et al. Symptoms of depression and psychosis in Alzheimer’s disease and frontotemporal dementia. Neuropsychiatry Neuropsychol Behav Neurol. 1996;9:154–61. [Google Scholar]
- 8.Gregory CA, Hodges JR. Frontotemporal dementia: Use of consensus criteria and prevalence of psychiatric features. Neuropsychiatr Neuropsychol Behav Neurol. 1996;9:145–53. [Google Scholar]
- 9.Pąchalska M. Behawioralny wariant otępienia czołowo-skroniowego. In: Pąchalska M, Bidzan L, editors. Otępienie czołowo-skroniowe: ujęcie interdyscyplinarne. Kraków: Wydawnictwo Akademii Krakowskiej; 2011. [in Polish] [Google Scholar]
- 10.Lebert F. Behavioral changes, non-cognitive assessment and management in frontotemporal dementia. In: Pasquier F, Lebert F, Scheltens Ph, editors. Frontotemporal Dementia. The Netherlands: ICG Publications; 1996. pp. 71–82. [Google Scholar]
- 11.Miller BL, Ikonte C, Ponton M, et al. A study of the Lund-Manchester research criteria for frontotemporal dementia: Clinical and single-photon emission CT correlations. Neurology. 1997;48:937–42. doi: 10.1212/wnl.48.4.937. [DOI] [PubMed] [Google Scholar]
- 12.Kertesz A, Davidson W, Fox H. Frontal Behavioral Inventory: Diagnostic criteria for frontal lobe dementia. Can J Neurol Sci. 1997;24:29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- 13.Kertesz A, Nadkarni N, Davidson W, Thomas AW. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc. 2000;6:460–68. doi: 10.1017/s1355617700644041. [DOI] [PubMed] [Google Scholar]
- 14.Kertesz A. Pick complex and Pick’s disease. Eur J Neurol. 1996;3:280–82. doi: 10.1111/j.1468-1331.1996.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 15.Hersch EL, Kral VA, Palmer RB. Clinical value of the London Psychogeriatric Rating Scale. J Am Geriatr Soc. 1978;26:348–54. doi: 10.1111/j.1532-5415.1978.tb03682.x. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GE. Development and validation of the Geriatric Evaluation by Relatives Rating Instrument (GERRI) Psychol Rep. 1983;53:479–88. doi: 10.2466/pr0.1983.53.2.479. [DOI] [PubMed] [Google Scholar]
- 17.Reisberg B, Borenstein J, Salob SP, et al. Behavioral symptoms in Alzheimer’s disease: Phenomenology and treatment. J Clin Psychiatry. 1987;48(Suppl):9–15. [PubMed] [Google Scholar]
- 18.Niederehe G. Trims Behavioral Problem Checklist (BPC) Psychopharmacol Bull. 1988;24:771–73. [PubMed] [Google Scholar]
- 19.Mungas D, Weiler P, Franzi C, Henry R. Assessment of disruptive behavior associated with dementia: The Disruptive Behavior Rating Scales. J Geriatr Psychiatry Neurol. 1989;2:196–202. doi: 10.1177/089198878900200405. [DOI] [PubMed] [Google Scholar]
- 20.Baumgarten M, Becker R, Gauthier S. Validity and reliability of the Dementia Behavior Disturbance Scale. J Am Geriatr Soc. 1990;38:221–26. doi: 10.1111/j.1532-5415.1990.tb03495.x. [DOI] [PubMed] [Google Scholar]
- 21.Drachman DA, Swearer JM, O’Donnell BF, et al. The Caretaker Obstreperous-Behavior Rating Assessment (COBRA) Scale. J Am Geriatr Soe. 1992;40:463–80. doi: 10.1111/j.1532-5415.1992.tb02012.x. [DOI] [PubMed] [Google Scholar]
- 22.Cummings JL, Miller B, Hill MA, Neshkes R. Neuropsychiatric aspects of multi-infarct dementia and dementia of the alzheimer type. Arch Neurol. 1987;44:389–93. doi: 10.1001/archneur.1987.00520160031010. [DOI] [PubMed] [Google Scholar]
- 23.Royall DR, Mahurin RK, Cornell J. Bedside assessment of executive cognitive impairment: The Executive Interview (EXIT) J Am Geriatr Soc. 1992;40:1221–26. doi: 10.1111/j.1532-5415.1992.tb03646.x. [DOI] [PubMed] [Google Scholar]
- 24.Mesulam MM. Primary progressive aphasia-differentiation from Alzheimer’s disease. Ann Neurol. 1987;22:533–34. doi: 10.1002/ana.410220414. [DOI] [PubMed] [Google Scholar]
- 25.Kertesz A, Hudson L, Mackenzie IRA, Munoz DG. The pathology and nosology of primary progressive aphasia. Neurology. 1994;44:2065–72. doi: 10.1212/wnl.44.11.2065. [DOI] [PubMed] [Google Scholar]
- 26.Brzeziński J, Gaul M, Hornowska E, et al. Pracownia Testów Psychologicznych PTP. 1996. Skala Inteligencji D. Wechslera dla Dorosłych. Wersja Zrewidowana. WAIS-R (PL). Podręcznik. Warszawa. [in Polish] [Google Scholar]
- 27.Pachalska M, MacQueen BD. Autoryzowana Wersja Polska. Kraków: Fundacja na Rzecz Osób z Dysfunkcjami Mózgu; 2000. Skala Pamięci Wechslera (WMS-R) [in Polish] [Google Scholar]
- 28.Kertesz A. Western Aphasia Battery – Test Booklet. New York: Grune and Stratton; 1982. [Google Scholar]
- 29.Benton AL, Hamsher K, Varney NR, Spreen O. Contribution in neuropsychological assessment: A clinical manual. New York: Oxford University Press; 1983. [Google Scholar]
- 30.Dekosky ST, Heilman KM, Bowers D, Valenstein E. Recognition and discrimination of emotional facecs and pictures. Brain & Language. 1980;9:206–14. doi: 10.1016/0093-934x(80)90141-8. [DOI] [PubMed] [Google Scholar]
- 31.Keane J, Calder AJ, Hodges JR, Young AW. Face and emotion processing in frontal variant frontotemporal dementia. Neuropsychologia. 2002:655–65. doi: 10.1016/s0028-3932(01)00156-7. [DOI] [PubMed] [Google Scholar]
- 32.Pąchalska M, MacQueen BD. Bostoński Test Nazywania [Boston Naming Test, BNT] – Autoryzowana Wersja Polska. Kraków: Fundacja na Rzecz Osób z Dysfunkcjami Mózgu; 1988. [in Polish] [Google Scholar]
- 33.Kertesz A, Munoz D. Clinical and pathological overlap between frontal dementia, progressive aphasia and corticobasal degeneration-the Pick complex. Neurology. 1997;48:A293. doi: 10.1159/000051212. [DOI] [PubMed] [Google Scholar]
- 34.Hornberger M, Savage S, Hsieh S, et al. Orbitofrontal dysfunction discriminates behavioral variant frontotemporal dementia from Alzheimer’s disease. Dement Geriatr Cogn Disord. 2010;30(6):547–52. doi: 10.1159/000321670. [DOI] [PubMed] [Google Scholar]
- 35.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 36.Pąchalska M. Rehabilitacja Neuropsychologiczna. Lublin: Wydawnictwo UMCS; 2008. [in Polish] [Google Scholar]
- 37.Bookheimer SY, Strojwas MH, Cohen MS, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343:450–56. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harciarek M, Jodzio K. Neuropsychological differences between frontotemporal dementia and Alzheimer’s disease: A review. Neuropsychology Review. 2005;15(3):131–45. doi: 10.1007/s11065-005-7093-4. [DOI] [PubMed] [Google Scholar]
- 39.O’Keeffe FM, Murray B, Coen RF, et al. Loss of insight in frontotemporal dementia, corticobasal degeneration and progressive supranuclear palsy. Brain. 2007;130(Pt 3):753–64. doi: 10.1093/brain/awl367. [DOI] [PubMed] [Google Scholar]
- 40.Barcikowska M. Pacjent z zaburzeniami pamięci u lekarza podstawowej opieki zdrowotnej. Psychogeriatria Polska. 2007;4(4):223. [in Polish] [Google Scholar]
- 41.Bidzan L, Pąchalska M, Grochmal-Bach B, et al. Behavioral and psychological symptoms and the progression of dementia of the Alzheimer type in nursing home residents. Medical Science Monitor. 2008;14(11):CR559–67. [PubMed] [Google Scholar]
- 42.Grochmal-Bach B, Bidzan L, Pachalska M, et al. Aggressive and impulsive behaviors in Frontotemporal dementia and Alzheimer’s disease. Med Sci Monit. 2009;15(5):CR248–54. [PubMed] [Google Scholar]
- 43.Kertesz A. Clinical features and diagnosis of frontotemporal dementia. Front Neurol Neurosci. 2009;24:140–48. doi: 10.1159/000197893. [DOI] [PubMed] [Google Scholar]
- 44.Cohen-Mansfield J, Marx MS, Rosenthal AS. Dementia and agitation in nursing home residents: how are they related? Psychol Aging. 1990;5:3–8. doi: 10.1037//0882-7974.5.1.3. [DOI] [PubMed] [Google Scholar]
- 45.Beck C, Frank L, Chumbler NR, et al. Correlates of disruptive behavior in severely cognitively impaired nursing home residents. Gerontologist. 1998;38:189–98. doi: 10.1093/geront/38.2.189. [DOI] [PubMed] [Google Scholar]
- 46.Pąchalska M. Rehabilitacja Neuropsychologiczna. Lublin: Wydawnictwo UMCS; 2008. [in Polish] [Google Scholar]
- 47.Cummings JL, Bathgate D. Behaviour in frontotemporal dementia, Alzheimer’s disease and vascular dementia. Acta Neurol Scand. 2001;103(6):367–78. doi: 10.1034/j.1600-0404.2001.2000236.x. [DOI] [PubMed] [Google Scholar]
- 48.Ihori N. A case of frontotemporal lobar degeneration with progressive dysarthria. Behav Neurol. 2006;17(2):97–104. doi: 10.1155/2006/320638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet. 2003;2:89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 50.Rockwood K, Ebly E, Hackinski V, et al. Presence and treatment of vascular risk factors in patients with vascular cognitive impairment. Arch Neurol. 1997;54:33–39. doi: 10.1001/archneur.1997.00550130019010. [DOI] [PubMed] [Google Scholar]
- 51.Rasmusson DX, Brandt J, Steele C, et al. Accuracy of clinical diagnosis of Alzheimer disease and clinical features of patients with non-Alzheimer disease neuropathology. Alzheimer Dis Assoc Disord. 1996;10:180–88. doi: 10.1097/00002093-199601040-00002. [DOI] [PubMed] [Google Scholar]
- 52.Roman G. Perspectives in the treatment of vascular dementia. Drugs of Today. 2000;36:641–53. doi: 10.1358/dot.2000.36.9.593781. [DOI] [PubMed] [Google Scholar]
- 53.Cummings JL, Mega M, Gray K, et al. The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 54.Bidzan L. Zaburzenia niekognitywne w zespołach otępiennych typu Alzheimera i naczyniopochodnych. Rocznik Psychogeriatryczny. 1998;1:67–79. [in Polish] [Google Scholar]
- 55.Kendell RE. The stability of psychiatric diagnoses. Brit J Psychiat. 1974;124:352–56. doi: 10.1192/bjp.124.4.352. [DOI] [PubMed] [Google Scholar]
- 56.Reding M, Haycox J, Blass J. Depression in patients referred to a dementia clinic: a threee-year prospective study. Arch Neurol. 1985;42:894–96. doi: 10.1001/archneur.1985.04060080080019. [DOI] [PubMed] [Google Scholar]
- 57.Geerlings MI, Schoevers RA, Beekman AT, et al. Depression and risk of cognitive decline and Alzheimer’s disease. Results of two prospective community-based studies in The Netherlands. Br J Psychiatry. 2000;176:568–75. doi: 10.1192/bjp.176.6.568. [DOI] [PubMed] [Google Scholar]
- 58.Speck CE, Kukull WA, Brenner DE. History of depression as a risk factor for Alzheimer’s disease. Epidemiol. 1995;6:366–69. doi: 10.1097/00001648-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Neuropathologica. 1991;82:259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 60.Bondareff W, Mountjoy CQ, Roth M. Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology. 1982;32:164–68. doi: 10.1212/wnl.32.2.164. [DOI] [PubMed] [Google Scholar]
- 61.Bilikiewicz A, Bidzan L. Zależność między poziomem kortyzolu w osoczu a stopniem upośledzenia intelektualnego w zespołach otępiennych pierwotnie zwyrodnieniowych. Psychiatr Pol. 1990;24:2–7. [PubMed] [Google Scholar]
- 62.Greenwald BS, Mathe AA, Mohs RC, et al. Cortisol and alzheimer’s disease: dexamethasone suppression, dementia severity and affective symptoms. Am J Psychiatry. 1986;143(4):442–48. doi: 10.1176/ajp.143.4.442. [DOI] [PubMed] [Google Scholar]
- 63.Spar JE, Gerner R. Does the dexamethasone suppression test distinquish dementia from depression? Am. J Psychiatry. 1982;139(2):238–40. doi: 10.1176/ajp.139.2.238. [DOI] [PubMed] [Google Scholar]
- 64.Rubinow DR, Post RM, Savard R. Cortisol hypersecretion and cognitive impairment in depression. Arch. Gen Psychiatry. 1984;41:279–83. doi: 10.1001/archpsyc.1984.01790140069008. [DOI] [PubMed] [Google Scholar]
- 65.Kropotov JD. Quantitative EEG, event related potentials and neurotherapy. San Diego: Academic Press, Elsevier; 2009. [Google Scholar]
- 66.Dickerson BC, Salat DH, Greve DN, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;5:404–11. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zubenko GS, Moosy J. Major depression in primary dementia: clinical and neuropathologic correlates. Arch Neurol. 1988;45:1182–86. doi: 10.1001/archneur.1988.00520350020008. [DOI] [PubMed] [Google Scholar]
- 68.Zweig RM, Ross CA, Hedreen JC. The neuropathology of aminergic nuclei in Alzheimer’s disease. Ann Neurol. 1988;24:233–42. doi: 10.1002/ana.410240210. [DOI] [PubMed] [Google Scholar]
- 69.Forstl H, Burns A, Luthert P, et al. Clinical and neuropathological correlates of depression in Alzheimer’s disease. Psychol Med. 1992;22:877–84. doi: 10.1017/s0033291700038459. [DOI] [PubMed] [Google Scholar]
- 70.Kolanowski AM, Garr M. The relation of premorbid factors to aggressive physical behavior in dementia. J Neurosci Nurs. 1999;31:278–84. doi: 10.1097/01376517-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Walsh JS, Welch HG, Larson EB. Survival of outpatients with Alzheimer-type dementia. Ann Intern Med. 1990;113:429–34. doi: 10.7326/0003-4819-113-6-429. [DOI] [PubMed] [Google Scholar]
- 72.Moritz DJ, Fox PJ, Luscombe FA, Kraemer HC. Neurological and psychiatric predictors of mortality in patients with Alzheimer disease in California. Arch Neurol. 1997;54:878–85. doi: 10.1001/archneur.1997.00550190066016. [DOI] [PubMed] [Google Scholar]
- 73.Diehl J. Frontotemporal dementia: patient characteristics, cognition, and behaviour. Int J Geriatr Psychiatry. 2002;17(10):914–18. doi: 10.1002/gps.709. [DOI] [PubMed] [Google Scholar]
- 74.Brown JW, Pąchalska M. The nature of the symptom and its relevance for neuropsychology. Acta Neuropsychologica. 2003;1(1):1–11. [Google Scholar]
- 75.Pachalska M, MacQueen BD. The Microgenetic Revolution in Contemporary Neuropsychology and Neurolinguistics. In: Weber M, Weekes A, editors. Whiteheadian Approaches to Consciousness in Psychology, Neuropsychiatry and Philosophy of Mind. New York: SUNY Press; 2008. pp. 164–85. [Google Scholar]
- 76.Cohen L, Benoit N, Van Eeckhout P, et al. Pure progressive aphemia. J Neurol Neurosurg Psychiatry. 1993;56:923–24. doi: 10.1136/jnnp.56.8.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kertesz A, Munoz DG. Pick’s disease and Pick complex. Introductory nosology. In: Kertesz A, Munoz DG, editors. Pick’s disease and Pick complex. Nowy Jork: Willey-Liss, Inc; 1998. pp. 1–11. [Google Scholar]
- 78.Fukui T, Kertesz A. A quantitative study of brain atrophy in primary progressive aphasia and frontotemporal dementia. J Int Neuropsychol Soc. 1998;4:216. [Google Scholar]
- 79.Cardarelli R, Kertesz A, Knebl JA. Frontotemporal dementia: a review for primary care physicians. Am Fam Physician. 2010;82(11):1372–77. [PubMed] [Google Scholar]