Summary
Background
There are no absolute criteria for identifying those girls with idiopathic central precocious puberty (CPP) who will benefit from gonadotropin-releasing hormone analog (GnRHa) treatment. Our objective was to predict at initial evaluation the differences between adult height (AH) and target height (TH) and (for untreated girls) the time between puberty onset and first menstruation.
Material/Methods
The 122 girls with CPP who reached their AH included 70 who were given GnRHa because their predicted AH was <155 cm (n=24), their luteinising hormone (LH)/follicle-stimulating hormone peaks (FSH) ratio was >0.66 (n=41) and/or their estradiol was >15 pg/ml (n=40). The other 52 were untreated because their predicted AH was >155 cm. Multiple linear regressions were performed on several subsets of variables.
Results
Treated: the difference between AH and TH (−0.6±5.4 cm) was predicted by (using SDS) =3.68 (height at initial evaluation – TH) − 1.94 (height at initial evaluation-predicted AH) − 4.23; R2=0.73. Untreated: the difference between AH and TH (1.7±4.3 cm) was predicted by =2.76 (height at initial evaluation – TH) − 3.68 LH/FSH peaks ratio − 3.49; R2=0.77. Time between puberty onset and first menstruation (years) was predicted by =12.2 – 1.06 age CPP − 0.4 (height at initial evaluation – TH); R2=0.75.
Conclusions
A greater difference between height at initial evaluation and TH (SDS) is associated with a greater AH in treated and untreated girls, as are smaller differences between height at initial evaluation and predicted AH in treated and lower LH/FSH peaks ratios in untreated girls.
Keywords: final height, gonadotropin releasing hormone analogues, idiopathic precocious puberty, mathematical model, puberty, precocious puberty, precocious puberty treatment
Background
Central precocious puberty (CPP) in girls is defined as the development of sexual characteristics before the age of 8 years due to the premature activation of the hypothalamo-pituitary-ovarian axis. CPP is idiopathic in the majority of young female patients [1]. It is sometimes difficult to distinguish between CPP and premature thelarche, which is defined by non-pathological isolated early breast development, generally during the first 2 years of life.
The premature secretion of estradiol increases the growth rate and accelerates bone maturation. This can shorten the growing period, resulting in short adult height (AH). Treatment with gonadotropin-releasing hormone (GnRH) analog (GnRHa) blocks the pituitary-ovarian axis and thus the secretion of estradiol, slowing bone age progression and preserving growth potential [2,3]. However, the effect of this treatment on the AH varies, mainly because the progression of idiopathic CPP varies. There are unsustained forms [4,5], also called slowly progressing forms [6,7], in addition to the rapidly progressing form, but it is difficult to distinguish between the rapidly and the slowly progressing forms. Partsch et al. [8] suggested that CPP is more a continuum of clinical presentation and progression rate, ranging from normal variants like premature thelarche, to unsustained or slowly progressive forms, and rapidly progressing forms. These may represent different positions along a continuum of hypothalamic GnRH neuron activation [9]. This variability makes randomized prospective trials comparing treated and untreated girls inappropriate.
It is therefore not easy to decide to treat a given girl with idiopathic CPP with GnRHa. The Consensus Conference Group has recommended that progressive pubertal development be documented for 3–6 months before starting GnRHa treatment, and that the responses to the GnRH test and estradiol assays should be used [2]. The factors associated with better results of GnRHa treatment on height are younger chronological age at puberty or treatment [10,11,12–15], a shorter interval between the onset of CPP and treatment [12,16], a greater height at initial evaluation [10,11,16–18], target height (TH) [10,16,18], predicted AH before treatment [11,18], and the duration of the treatment [12,15,18].
This study was done to help physicians decide which of these girls should be given GnRHa treatment. We analyzed retrospective data for 122 girls with idiopathic CPP who had reached their AH. Some were treated with GnRHa, and the others were followed without treatment. Multiple linear regressions were performed on several subsets of the variables [19,20]. The objective was to predict at initial evaluation the AH and (for untreated girls) the time between the onset of puberty and first menstruation. We evaluated these criteria, as it is important for discussing treatment with the patient’s parents.
Material and Methods
Ethics statement
Written informed consent for the evaluations was obtained from the children’s parents and was included in their hospital medical record. The Ethical Review Committee (Comité de Protection des Personnes, Ile de France III) stated that “This research was found to conform to generally accepted scientific principles and research ethical standards and to be in conformity with the laws and regulations of the country in which the research experiment was performed.”
Patients
This retrospective study was carried out on 122 girls, first seen for idiopathic CPP between 1982 and 2002 in a university pediatric hospital by one of us (R Brauner). They had all reached their AH (growth during the preceding year of less than 1 cm in a menstruated girl). CPP was diagnosed on the appearance of breast development before the age of 8 years accompanied by the presence of pubic or axillary hair (n=46), a growth rate greater than 2 SD scores (SDS, n=59) and/or a bone age more than 2 years greater than chronological age (n=39). Organic intracranial lesions were excluded by neuroradiological evaluation in all cases, as were ovarian [21] and adrenal disorders. Plasma 17-hydroxyprogesterone, delta4-androstenedione and testosterone concentrations were measured in those girls whose first sign was pubic or axillary hair development to exclude abnormal androgen secretion and congenital adrenal hyperplasia [22]. Plasma thyroxin and thyroid stimulating hormone concentrations were measured to exclude hypothyroidism and 24-h urinary cortisol was measured to exclude hypercortisolism in those who were overweight.
The 122 girls were selected from a cohort of 350 girls with idiopathic CPP [23], 242 of whom were over 15 years old. The 19 adopted girls were excluded because their TH was not available. When the AH was not available in the hospital record, a letter was sent to the parents asking for the age and latest height in her health records and her growth in the past year, data that were collected by their pediatrician. This letter was returned to us because of a change of address in 101 cases, whose characteristics at initial evaluation were similar to those of the 122 girls included in the study. Thus, we compared the characteristics analysed in Table 1 in the 101 excluded and 122 included girls and found that the differences were not significant for each of them.
Table 1.
Characteristics at initial evaluation and adult heights of girls with idiopathic CPP.
| Treated (n=70) |
Untreated (n=52) |
P | Treated n (%) |
Untreated n (%) |
||
|---|---|---|---|---|---|---|
| Age at onset of puberty, yr | 6.5±1.5 | 7.1±0.9 | <0.02 | <6 yr | 15 (21.4) | 3 (5.8) |
| Age at evaluation, yr | 7.4±1.5 | 8.0±1.0 | NS | |||
| Interval from onset, yr | 0.9±0.6 | 0.9±0.6 | NS | |||
| Bone age, yr | 9.1±1.8 | 9.1±1.6 | NS | |||
| Bone age advance, yr | 1.7±1.4 | 1.1±1.1 | <0.01 | >2 yr | 25 (35.7) | 14 (26.9) |
| Height, cm | 128.9±9.6 | 134.2±7.6 | 0.0005 | |||
| Height, SDS | 1.9±1.4 | 2.2±1.0 | NS | >2 SDS | 30 (42.9) | 31 (59.6) |
| Growth rate, SDS | (n=69) 2.3±2.1 |
(n=50) 2.3±2.2 |
NS | >2 SDS | 35 (50.7) | 24 (48.0) |
| BMI, SDS | 1.3±1.1 | 1.3±1.5 | NS | >2 SDS | 15 (21.4) | 16 (30.8) |
| Estradiol, pg/mL | (n=67) 26.7±18.7 |
(n=51) 16.7±12.0 |
0.0003 | >15 pg/mL | 40 (57.1) | 15 (29.4) |
| LH peak, IU/L | 14.4±16.5 | 6.4±13.3 | <0.0001 | |||
| FSH peak, IU/L | 15.0±8.4 | 12.7±7.0 | NS | |||
| LH/FSH peaks ratio | 0.9±0.7 | 0.5±0.5 | <0.0001 | >0.66 | 41 (58.6) | 13 (25.0) |
| Insulin, U/mL | (n=22) 12.5±5.7 |
(n=16) 16.2±11.3 |
NS | |||
| Target height, cm | 161.0±5.0 | 161.4±4.1 | NS | |||
| Predicted adult height*, cm | (n=64) 159.5±7.2 |
(n=50) 164.5±7.2 |
<0.0001 | <155 cm | 14 (21.9) | 3 (6.0) |
| Adult height, cm | 160.4±5.3 | 163.0±5.2 | <0.007 | <−2 S DS | 7 (10.0) | 2 (4.0) |
| Adult-target heights, cm | −0.6±5.4 | 1.7±4.3 | <0.03 | <−5 cm | 10 (14.3) | 1 (1.9) |
| Adult- predicted adult heights, cm | (n=64) 1.0±6.2 |
(n=50) −1.4±5.6 |
<0.04 | <−5 cm | 11 (17.2) | 10 (20.0) |
Predicted adult height at onset of treatment: 158.8±7.5 cm.
Protocol
The initial evaluation included determinations, in a fasting state, of height, weight, pubertal stage, bone age, and evaluation of the hypothalamo-pituitary-ovarian axis by measuring basal and GnRH (100 μg/m2)-stimulated luteinizing hormone (LH) and follicle-stimulating hormone (FSH) peaks and the plasma concentrations of estradiol. Aliquots (n=38) of plasma were kept at −22°C to measure insulin concentrations.
The patients were assigned to 1 of 2 groups: Group 1 patients (n=70) were given GnRHa and Group 2 patients (n=52) were followed without treatment.
The criteria for treatment were (Table 1): 1) a predicted AH <155 cm at the initial evaluation (n=14), or at the evaluations performed every 4 months (n=10 after 1.0±0.9 (0.1–2.9) years), 2) an LH/FSH peaks ratio >0.66 (n=41) and/or 3) a plasma estradiol concentration >15 pg/mL (n=40). Treatment (D-Trp-GnRH, 3.75 mg, i.m., every 24–26 days; half doses in patients weighing <20 kg) was continued for at least 2 years. This interval between injections was chosen because estrogen activity reappears, according to the vaginal maturation index, when the interval exceeds 27–29 days (our unpublished observations). Oral cyproterone acetate (12.5 mg twice a day) was given for 15 days before and for 1 month after the first GnRHa injection to prevent the formation of ovarian cysts in response to the gonadotropin surge. Therapy was stopped when the bone age was approximately 12–12.5 years, except for 15 girls whose bone ages were 10.5–11.8 years. In these, the decision to stop the therapy was taken case-by-case with the parents because of chronological age or height judged to be sufficient for the increase of estradiol secretion, or, more frequently, slow growth rate (<3 to 4 cm) during the previous year. The patients were seen every 6 months after the start of therapy for clinical evaluation and measurements of height and bone age before deciding to stop therapy. Plasma estradiol was measured when there was a doubt about the persistence of breast development because of adipose tissue deposition – it was undetectable. The treatment produced no side-effects.Group 2 patients (n=52) were followed without treatment because their predicted AH was >155 cm in all but 3 cases (147, 153, 153.6 cm) (Table 1). They were seen every 4 months until they reached an acceptable predicted AH for clinical and bone age evaluation, if indicated, and then each year until the AH.
Methods
Height, growth rate and body mass index (BMI, weight in kg/height in m2) are expressed as SDS for chronological age [24,25]. The pubertal stage was rated [26]. Bone age was assessed by one of us [27]. TH was calculated from reported parental heights [28]. Predicted AH was calculated [29], except in 8 cases (6 treated and 2 untreated) whose predicted AH could not be calculated because they had a bone age of <7 years. We used the column “advanced” when the bone age advance was greater than 1 year [29].
Plasma LH, FSH and estradiol concentrations were measured using different immunoassays during the study period. When the assay method for a given hormone was changed, it was cross-correlated with the previous method. Thus, the results for a given parameter are comparable throughout the whole period. An LH/FSH peaks ratio after GnRH test of >0.66 was considered to be pubertal [30].
Data are expressed as means ±SD. Paired values were compared with a Wilcoxon test. Groups were compared with a Mann-Whitney U test. Percentages were compared with the Chi-squared test. Correlations were analyzed using Spearman’s test.
Least squares fitting multiple linear regressions [19,20] were performed on many subsets of the variables shown in Table 1, and they were evaluated through a cross-validation procedure. The models selected were those that gave the best trade-off between quality and simplicity. We looked for formulae that could be used at initial evaluation to predict the difference (cm) between AH and TH. The differences predicted for each patient were compared to the actual observed differences. A difference, particularly a loss, of 3 cm was considered significant, as this value corresponds to approximately 0.5 SD. We also tried to predict the time between the onset of puberty and first menstruation for the untreated girls.
Results
1. Comparison of the groups at initial evaluation
Group 1 patients were younger at the onset of puberty, but the intervals between the onset of puberty and evaluation were similar in the 2 groups (Table 1). Breast development was at Tanner’s stage 2 in 33% of the group 1 patients, and stages 3 and 4 in 67% of them, whereas these percentages were 65% and 35% for the group 2 patients (P=0.001). Group 1 girls had significantly greater bone age advance, plasma estradiol concentrations, LH peaks, LH/FSH peaks ratio, and lower predicted AH than the Group 2 girls.
2. Treated group
The time between the onset of puberty and treatment was 1.5±0.9 (0.2–4.7) years. The time between the initial evaluation and the onset of treatment was 0.5±0.8 years. Treatment was initiated at 7.9±1.5 years and stopped at 10.8±0.9 years. During the treatment (2.8±1.3 years), the bone age increased from 9.9±1.6 to 12.1±0.7 years and the height from 132.5±8.5 to 148.2±4.7 cm. The predicted AH at the end of treatment (160.8±5.0 cm) was similar to the actual AH. The height gain between the end of treatment and AH was 12±4 (0 to 23.5) cm. It was negatively correlated with the chronological (r=−0.46, P=0.0001) and bone (r=−0.37, P<0.007) ages at the end of treatment. The time between the end of treatment and the first menstruation was 1.3±0.8 (0.1 to 3.3) years.
The AH was greater than the predicted AH before treatment (P<0.005), but similar to the TH (Table 1). It was positively correlated with the heights at initial evaluation and at treatment initiation, TH, predicted AH, growth rate during the first year of treatment; it was negatively correlated with the ages at the end of treatment and at first menstruation (Table 2).
Table 2.
Factors influencing the adult height, adult height-predicted adult height, and adult height-target adult height of girls with idiopathic CPP.
| Treated (n=70) | Untreated (n=52) | |||
|---|---|---|---|---|
| r | P | r | P | |
| Adult height (cm) | ||||
| Height at initial evaluation, cm | 0.39 | 0.0013 | 0.33 | 0.02 |
| Height at treatment, cm | 0.41 | 0.0007 | – | – |
| Target height, cm | 0.46 | 0.0001 | 0.61 | <0.0001 |
| Predicted adult height, cm | 0.53 | <0.0001 | 0.70 | <0.0001 |
| Growth rate during the first year of treatment, SDS | 0.28 | 0.02 | – | – |
| Age at the end of treatment, yr | −0.47 | <0.0001 | – | – |
| Age at first menstruation, yr | −0.41 | 0.0007 | – | – |
| LH peak, IU/L | – | – | −0.27 | 0.05 |
| Adult height – predicted adult height (cm) | ||||
| Height at initial evaluation, cm | 0.30 | 0.017 | – | – |
| Breast development (Tanner’s stage) | 0.30 | 0.016 | – | – |
| Bone age, yr | 0.59 | <0.0001 | 0.42 | 0.003 |
| Bone age advance, yr | 0.64 | <0.0001 | 0.41 | 0.004 |
| Estradiol, pg/mL | 0.31 | 0.016 | – | – |
| Age at first mother’s menstruation, yr | – | – | 0.29 | 0.04 |
| Adult height – target height (cm) | ||||
| Height at initial evaluation, SDS | – | – | 0.54 | 0.0001 |
| Height at treatment, cm | 0.28 | 0.019 | – | – |
| Predicted adult height, cm | 0.51 | <0.0001 | 0.40 | 0.005 |
| Age at onset of puberty, yr | – | – | −0.32 | 0.02 |
| Age at evaluation, yr | – | – | −0.42 | 0.003 |
| LH peak, IU/L | – | – | −0.32 | 0.02 |
The difference between the AH and predicted AH just before treatment was 2.2±6.4 (−12.8 to 20) cm. It was positively correlated with the height at initial evaluation, the stage of breast development, bone age and bone age advance before treatment, and with the plasma estradiol concentration (Table 2). These differences were similar in the patients younger than and older than 6 years at the onset of puberty.
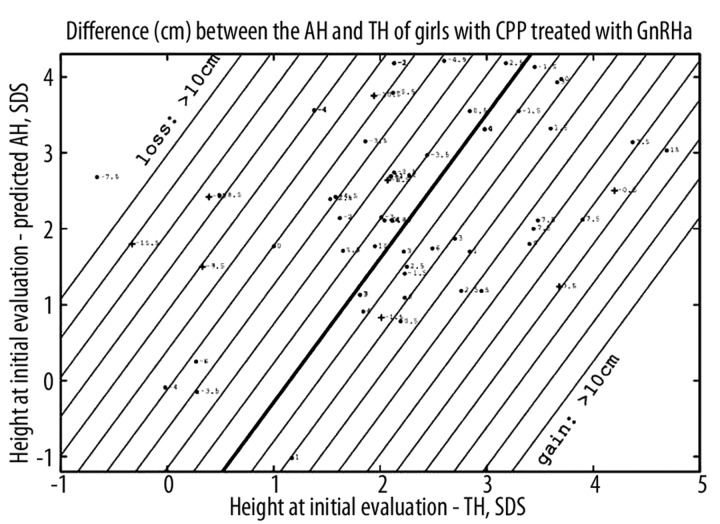
The difference between the AH and TH was −0.6±5.4 (−15.5 to 15) cm, and was positively correlated with the height at treatment initiation and the predicted AH. This difference (cm) can be predicted at the initial evaluation by the formula (using SDS) =3.68 (height at initial evaluation – TH) − 1.94 (height at initial evaluation – predicted AH) − 4.23 with R2=0.73 (Figure 1).
Figure 1.
Predictions of the difference (cm) between the adult height (AH) and target height (TH) of girls with CPP treated with GnRHa. Straight lines are contour plots, every cm. Each point corresponds to the predicted difference, with the actual difference indicated. The point is changed to a cross when the actual value is more than 3 cm lower than the calculated one. Three patients located in the upper part were deleted.
The actual AH of 9 girls (14%) was >3 cm lower than the calculated AH. Ten patients had an AH that was >5 cm lower than their TH.
3. Untreated group
Three girls were aged 3.5, 4.5 and 5.3 years, 12 were between 6 and 6.9 years, and 37 were between 7 and 8 years. The 3 youngest had plasma estradiol concentrations of 9, 15 and 88 pg/mL and a prepubertal LH/FSH peaks ratio; they had menstruated at 10.5, 11 and 12 years; and their AH was greater than their TH.
The AH was similar to the predicted AH, but greater than the TH (P<0.01, Table 1). It was positively correlated with the height at initial evaluation, TH and the predicted AH (Table 2).
The difference between the AH and predicted AH was −1.4±5.6 (−13.4 to 17) cm. It was positively correlated with the bone age and bone age advance, and with the age of the mother at her first menstruation.
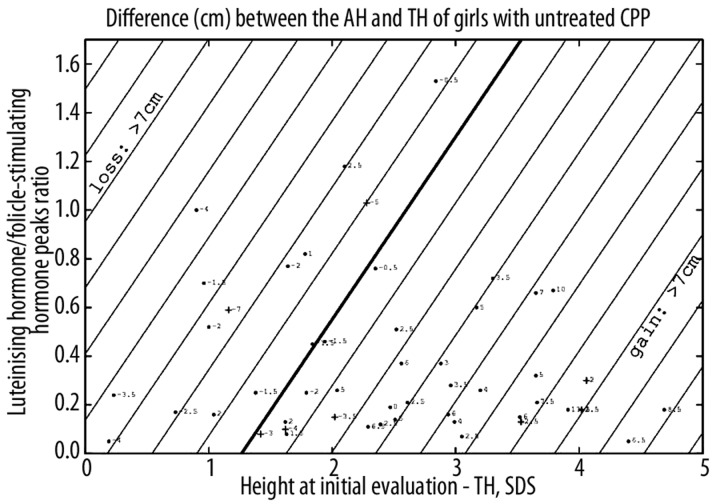
The difference between the AH and TH was 1.7±4.3 (−7 to 13.5) cm. It correlated positively with the height at initial evaluation, predicted AH, and negatively with the age at onset of puberty, the age at evaluation and the LH peak. This difference (cm) can be predicted at the initial evaluation by the formula (using SDS) =2.76 (height at initial evaluation – TH) − 3.68 LH/FSH peaks ratio −3.49 with R2=0.77 (Figure 2).
Figure 2.
Predictions of the difference (cm) between adult height (AH) and target height (TH) of girls with untreated CPP. Straight lines are contour plots, every cm. Each point corresponds to the predicted difference, with the actual difference indicated. The point is changed to a cross when the actual value is more than 3 cm lower than the calculated one. Two patients located in the upper part were deleted.
The actual AH of 9 girls (17%) was >3 cm lower than the calculated AH, and 4 of the 9 (44%) had a BMI >2 SDS, while 6 (66%) had a bone age advance greater than 2 years at initial evaluation; these percentages were 30.8 and 26.9% in the whole untreated group (Table 1). Only 1 patients had an AH that was >5 cm lower than her TH (7 cm).
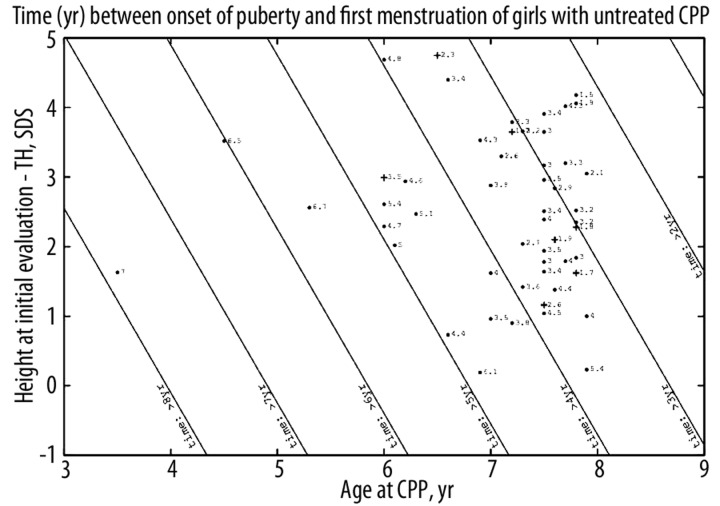
The 52 girls included 39 who began menstruating before they were 11 years old (19/51 in their mothers). The time between the onset of puberty and first menstruation (years) can be calculated with the formula 12.2 – 1.06 age CPP − 0.4 (height at initial evaluation – TH, SDS) with R2=0.75 (Figure 3).
Figure 3.
Predictions of the time (yr) between the onset of puberty and the first menstruation of girls with untreated CPP. TH: target height. Straight lines are contour plots, every year. Each point corresponds to the predicted time, with the actual time indicated. The point is changed to a cross when the actual time is more than one year less than the calculated one.
Discussion
We have identified a model that can be used at the initial evaluation to predict the difference between AH and TH. This difference is probably the best expression of the effect of CPP and its treatment on the growth potential – height SDS at the initial evaluation is influenced by the previous growth acceleration due to CPP; this difference is more informative than the adult height SDS.
1. Deciding on GnRHa treatment
The time between the onset of puberty and the initiation of treatment was greater than 2 months, to avoid treating transient forms. We based our decision to treat on a low or deteriorating predicted AH, a pubertal LH/FSH response to the GnRH test, and a plasma estradiol concentration suggesting hypothalamic GnRH neuron activation and/or high estrogen activity, but the treated and untreated girls had similar THs, heights SDS, growth rates, BMIs and insulin concentrations. This agrees well with our data [31] showing that the growth rates of CPP patients are independent of the plasma estradiol concentrations, which is correlated with the LH/FSH peaks ratio. This suggests that the degrees of hypothalamo-pituitary-ovarian activation determines the LH/FSH peaks ratio, and, hence, the plasma estradiol concentration, the bone age advance, and the predicted AH, but not the growth rate. The LH/FSH peaks ratio is significantly correlated with the anterior pituitary height [32]. These degrees of activation may be determined by genetic factors and could explain the variability of the evolution in CPP. These data explain that greater height at initial evaluation is associated with a greater AH in treated and untreated girls. Similarly, a greater AH is associated with a smaller difference between the height at initial evaluation and the predicted AH (all expressed as SDS) in treated girls (Figure 1) and a lower LH/FSH peaks ratio in untreated girls (Figure 2). We retrospectively evaluated the plasma insulin concentrations, as obesity seems to contribute to the earlier onset of puberty [33]; we found no difference between the 2 groups. Obesity seems to be a factor in loss of height between the actual and the calculated AH in the untreated group (see below). The effect of caloric reduction on the evolution of the height prediction has yet to be evaluated.
2. Untreated group
There is a question as to whether these patients actually have CPP, despite the prepubertal response to the GnRH test in 75% and estradiol concentration in 70%. Only 3 were younger than 6 and 12 were aged 6 to 7 years. Among this group, 21% had menstruated before the age of 10 and 75% before 11 years, suggesting an increased estrogen secretion. The other 25% may not have suffered from progressing CPP.
The decision to not treat these girls was mainly based on their having a predicted AH of >155 cm. A greater height at initial evaluation, expressed as cm or SDS, and predicted AH are associated with a greater AH and a larger difference between AH and TH. We have identified a model that can be used to predict this difference at the initial evaluation.
We analysed the characteristics of the 9 untreated girls whose actual AH was >3 cm lower than the calculated AH according to the model. Compared to the whole untreated group (Table 1), they represent 4/18 with breast development at Tanner’s stage 3 or 4, 6/14 with a bone age advance greater than 2 years, 4/16 with a BMI greater than 2 SDS, 5/15 with a plasma estradiol concentration greater than 15 pg/mL, and 3/13 with an LH/FSH peaks ratio greater than 0.66.
The time between the onset of CPP and the first menstruation was longer in the girls who were youngest at the onset of CPP and in those having a smaller difference between height at initial evaluation and TH.
3. Clinical use of the formulae
We have set up a web page and spreadsheets to simplify the use of the above formulae in day-to-day practice. The formulae can be tried by simply inserting the raw data, such as the values for the TH, the height at initial evaluation and the predicted AH in the girl to be treated, and the LH/FSH peaks ratio or the age at onset of puberty for the girl left untreated. These resources are available at: http://www.kamick.org/lemaire/med/girls-cpp.html.
4. Study limitations
The girls excluded because of the change in their address may introduce bias. We postulate that their similarity to the girls who were included limits this bias. The decision to stop treatment was based on a bone age of approximately 12–12.5 years, which is associated with better growth until the FH [3]. However, the bone ages of 15 girls were lower when the decision to stop the GnRHa treatment was taken, because of their chronological age or their height was judged to be sufficient for the increase of estradiol secretion, or more frequently their growth rate had been slow (<3 to 4 cm) during the previous year.
The final heights of a few girls were collected from their health records held by their pediatricians. The reported parental height is less accurate than the measured heights, as males seem to overestimate their height [34]. These authors recommend accurately measuring the heights of both parents, given the considerable variations between the reported and measured heights in both sexes.
Conclusions
We have identified models for predicting the difference between the AH and TH of girls with idiopathic CPP – a greater difference between the height at initial evaluation and the TH (SDS) is associated with a greater AH in both treated and untreated girls. The second factor of the model is a smaller difference between the height at initial evaluation and the predicted AH in treated girls and a lower LH/FSH peaks ratio in untreated girls. In these, a bone age advance greater than 2 years should be an additional indicator for treatment. Psychosocial and ethical factors should also be considered.
The interval between the onset of CPP and the first menstruation can also be predicted for untreated girls.
We have thus provided a way of identifying those girls who can be left untreated without any great risk to their AH, provided they are closely monitored. These models will undoubtedly be optimized as a result of their use in other centers and in prospective studies.
Acknowledgements
We thank Monique Pouillot for technical help and Dr Owen Parkes for editing the manuscript.
We thank Nadia Brauner, G-SCOP, INPGrenoble, UJF, CNRS, Grenoble, 38000, France for helpful discussions and the CNRS for financial support through its Groupe de Recherche en Recherche Opérationnelle program.
Abbreviations
- AH
adult height
- BMI
body mass index
- CPP
central precocious puberty
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- GnRHa
gonadotropin-releasing hormone analog
- LH
luteinizing hormone
- SDS
SD scores
- TH
target height
Footnotes
Competing interests
The authors declare no competing interests.
Web page for using these formulae: http://www.kamick.org/lemaire/med/girls-cpp.html
Source of support: Departmental sources
References
- 1.Bridges NA, Christopher JA, Hindmarsh PC, Brook CGD. Sexual precocity: sex incidence and aetiology. Arch Dis Child. 1994;70:116–18. doi: 10.1136/adc.70.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carel J-C, Eugster EA, Rogol A, et al. on behalf of the members of the ESPE-LWPES GnRH Analog Consensus Conference Group. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. 2009;123(4):e752–62. doi: 10.1542/peds.2008-1783. [DOI] [PubMed] [Google Scholar]
- 3.Brauner R, Adan L, Malandry F, Zantleifer D. Adult height in girls with idiopathic true precocious puberty. J Clin Endocrinol Metab. 1994;79:415–20. doi: 10.1210/jcem.79.2.8045957. [DOI] [PubMed] [Google Scholar]
- 4.Zipf WB, Kelch RP, Hopwood NJ, et al. Suppressed responsiveness to gonadotropin-releasing hormone in girls with unsustained isosexual precocity. J Pediatr. 1979;95:38–43. doi: 10.1016/s0022-3476(79)80079-7. [DOI] [PubMed] [Google Scholar]
- 5.Palmert MR, Malin HV, Boepple PA. Unsustained or slowly progressive puberty in young girls: initial presentation and long-term follow-up of 20 untreated patients. J Clin Endocrinol Metab. 1999;84:415–23. doi: 10.1210/jcem.84.2.5430. [DOI] [PubMed] [Google Scholar]
- 6.Fontoura M, Brauner R, Prevot C, Rappaport R. Precocious puberty in girls: early diagnosis of a slowly progressing variant. Arch Dis Child. 1989;64:1170–76. doi: 10.1136/adc.64.8.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreiter M, Burstein S, Rosenfield RL, et al. Preserving adult height potential in girls with idiopathic true precocious puberty. J Pediatr. 1990;117:364–70. doi: 10.1016/s0022-3476(05)81074-1. [DOI] [PubMed] [Google Scholar]
- 8.Partsch C-J, Heger S, Sippell WG. Management and outcome of central precocious puberty. Clin Endocrinol. 2002;56:129–48. doi: 10.1046/j.0300-0664.2001.01490.x. [DOI] [PubMed] [Google Scholar]
- 9.Pescovitz OH, Hench KD, Barnes KM, et al. Premature thelarche and central precocious puberty: the relationship between clinical presentation and the gonadotropin response to luteinizing hormone-releasing hormone. J Clin Endocrinol Metab. 1988;67:474–79. doi: 10.1210/jcem-67-3-474. [DOI] [PubMed] [Google Scholar]
- 10.Lazar L, Padoa A, Philip M. Growth pattern and final height after cessation of gonadotropin-suppressive therapy in girls with central sexual precocity. J Clin Endocrinol Metab. 2007;92:3483–89. doi: 10.1210/jc.2007-0321. [DOI] [PubMed] [Google Scholar]
- 11.Pasquino A-M, Pucarelli I, Accardo F, et al. Long-term observation of 87 girls with idiopathic central precocious puberty treated with gonadotropin-releasing hormone analogs: impact on adult height, body mass index, bone mineral content, and reproductive function. J Clin Endocrinol Metab. 2008;93:190–95. doi: 10.1210/jc.2007-1216. [DOI] [PubMed] [Google Scholar]
- 12.Oerter Klein K, Barnes KM, Jones JV, et al. Increased final height in precocious puberty after long term treatment with LHRH agonists: the National Institutes of Health experience. J Clin Endocrinol Metab. 2001;86:4711–16. doi: 10.1210/jcem.86.10.7915. [DOI] [PubMed] [Google Scholar]
- 13.Heger S, Partsch C-J, Sippel WG. Long-term outcome after depot gonadotropin-releasing hormone agonist treatment of central precocious puberty: final height, body proportions, body composition, bone mineral density, and reproductive function. J Clin Endocrinol Metab. 1999;84:4583–90. doi: 10.1210/jcem.84.12.6203. [DOI] [PubMed] [Google Scholar]
- 14.Paul D, Conte FA, Grumbach MM, Kaplan SL. Long term effect of gonadotropin-releasing hormone agonist therapy on final and near-final height in 26 children with true precocious puberty treated at a median age of less than 5 years. J Clin Endocrinol Metab. 1995;80:546–51. doi: 10.1210/jcem.80.2.7852518. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, Niimi H, Matsuo N, et al. Results of long-term follow-up after treatment of central precocious puberty with leuprorelin acetate: evaluation of effectiveness of treatment and recovery of gonadal function. The TAP-144-SR Japanese Study Group on central precocious puberty. J Clin Endocrinol Metab. 2005;90:1371–76. doi: 10.1210/jc.2004-1863. [DOI] [PubMed] [Google Scholar]
- 16.Brito VN, Latronico AC, Cukier P, et al. Factors determining normal adult height in girls with gonadotropin-dependent precocious puberty treated with depot gonadotropin-releasing hormone analogs. J Clin Endocrinol Metab. 2008;93:2662–69. doi: 10.1210/jc.2007-2183. [DOI] [PubMed] [Google Scholar]
- 17.Oostdijk W, Rikken B, Schreuder S, et al. Final height in central precocious puberty after long term treatment with a slow release GnRH agonist. Arch Dis Child. 1996;75:292–97. doi: 10.1136/adc.75.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arrigo T, Cisternino M, Galluzzi F, et al. Analysis of the factors affecting auxological response to GnRH agonist treatment and final height outcome in girls with idiopathic central precocious puberty. Eur J Endocrinol. 1999;141:141–44. doi: 10.1530/eje.0.1410140. [DOI] [PubMed] [Google Scholar]
- 19.Weisstein EW. Linear Regression. From MathWorld-A Wolfram Web Resource; http://mathworld.wolfram.com/LinearRegression.html. [Google Scholar]
- 20.Witten IH, Frank E. Data Mining Practical machine learning tools and techniques. 2nd Edition. Morgan Kaufmann Eds; San Francisco: 2005. [Google Scholar]
- 21.Brauner R, Bashamboo A, Rouget S, et al. Clinical, biological and genetic analysis of prepubertal isolated ovarian cyst in 11 girls. PLoS One. 2010;5(6):e11282. doi: 10.1371/journal.pone.0011282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armengaud J-B, Charkaluk M-L, Trivin C, et al. Precocious pubarche: distinguishing late-onset congenital adrenal hyperplasia from premature adrenarche. J Clin Endocrinol Metab. 2009;94:2835–40. doi: 10.1210/jc.2009-0314. [DOI] [PubMed] [Google Scholar]
- 23.Prété G, Couto-Silva A-C, Trivin C, Brauner R. Idiopathic central precocious puberty in girls: presentation factors. BMC Pediatr. 2008;8:27. doi: 10.1186/1471-2431-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sempé M, Pédron G, Roy-Pernot MP. In Auxologie, méthode et séquences. Paris, France: Théraplix; 1979. [Google Scholar]
- 25.Rolland-Cachera MF, Cole TJ, Sempé M, et al. Body mass index variations: centiles from birth to 87 years. Eur J Clin Nutr. 1991;45:13–21. [PubMed] [Google Scholar]
- 26.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford: Stanford University Press; 1959. [Google Scholar]
- 28.Tanner JM, Goldstein H, Whitehouse RH. Standards for children’s height at ages 2–9 years allowing for height of parents. Arch Dis Child. 1970;47:755–62. doi: 10.1136/adc.45.244.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with Greulich Pyle hand standards. J Pediatr. 1952;50:432–41. doi: 10.1016/s0022-3476(52)80205-7. [DOI] [PubMed] [Google Scholar]
- 30.Oerter KE, Uriarte MM, Rose SR, et al. Gonadotropin secretory dynamics during puberty in normal girls and boys. J Clin Endocrinol Metab. 1990;71:1251–58. doi: 10.1210/jcem-71-5-1251. [DOI] [PubMed] [Google Scholar]
- 31.Brauner R, Malandry F, Fontoura M, et al. Idiopathic central precocious puberty in girls as a model of the effect of plasma estradiol level on growth, skeletal maturation and plasma insulin-like growth factor I. Horm Res. 1991;36:116–20. doi: 10.1159/000182143. [DOI] [PubMed] [Google Scholar]
- 32.Pérignon F, Brauner R, Argyropoulou M, Brunelle F. Precocious puberty in girls: pituitary height as an index of hypothalamo-pituitary activation. J Clin Endocrinol Metab. 1992;75:1170–72. doi: 10.1210/jcem.75.4.1400889. [DOI] [PubMed] [Google Scholar]
- 33.Kaplowitz PB, Slora EJ, Wasserman RC, et al. Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics. 2001;108:347–53. doi: 10.1542/peds.108.2.347. [DOI] [PubMed] [Google Scholar]
- 34.Cizmecioglu F, Doherty A, Paterson WF, et al. Measured versus reported parental height. Arch Dis Child. 2005;90:941–42. doi: 10.1136/adc.2005.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]