Summary
Background
To examine the effect of probiotics as adjunctive therapy for the treatment of rheumatoid arthritis (RA). A sample size of 30 subjects was calculated to determine a moderate effect.
Material/Methods
A three month double-blind, placebo-controlled study was performed using probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 capsules administered orally. Inclusion criteria required at least 4 swollen and 4 tender joints and stable medications with no steroids for at least 1 month prior to and during the study. Twenty-nine patients with RA were randomized to treatment. ACR20 responses, serum cytokine levels and safety parameters were assessed.
Results
Fifteen patients were randomized to the probiotic group, and 14 to placebo. Three subjects in the probiotic (20%) and one in the placebo group (7%) achieved an ACR20 response (p= 0.33). There was no statistically significant difference between individual components of the ACR20 criteria. Changes in cytokines favored placebo over probiotic. There was a significant improvement in the Health Assessment Questionnaire (HAQ) score in the probiotic group from visit 1 to visit 3 (p=0.02) but no between-group differences.
Conclusions
Due to inclusion criteria, patients selected for the study had stable RA with chronic synovitis, and thus it may have been difficult for an adjunctive therapy to demonstrate improvement within 3 months. Although probiotics did not clinically improve RA as measured by the ACR20, it is interesting that there was functional improvement seen within the probiotic group compared to placebo.
Keywords: rheumatoid arthritis (RA), probiotics, randomized controlled trial, lactobacillus
Background
Rheumatoid arthritis (RA) is the most common inflammatory arthritis affecting approximately 1% of Canadian adults. It has been shown that patients with inflammatory arthritis have increased permeability of the gut due to inflammation of the gastrointestinal (GI) tract [1]. This allows food antigens and potentially harmful micro-organisms to enter the bloodstream. Patients with inflammatory arthritis have been shown to have elevated antibodies to these antigens [2,3], in some cases leading to immune complexes in capillaries supplying the joint capsule [4]. A meta-analysis found a statistically and long-term clinically significant benefit of fasting and a vegetarian diet in RA patients [5], while another study demonstrated that this approach altered the intestinal flora [6].
Probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” [7]. It is not fully clear how probiotics work, but there is good evidence that they reduce gut permeability [8] and modulate immunity. This modulation may come via increasing local secretory IgA immune responses to pathogens [9], reducing overgrowth of pathogenic bacteria, or down-regulating inflammatory immune factors such as TNF-α [10].
The use of probiotics to prevent or treat arthritis is quite unexplored but some studies have indicated the potential benefit. Within the scope of animal models, oral treatment with probiotics has been shown to decrease the clinical severity in arthritic changes [11]. Combination treatment of Methotrexate and probiotics (Enterococcus faecium) has also shown a potentiating effect, reducing clinical parameters compared to animals who receive only the methotrexate treatment [12,13]. Amongst patients with ankylosing spondylitis, the imbalanced GI micoflora [14] implies that a displacement of pathogens (in this case sulphate reducing bacteria) and addition of commensals such as lactobacilli or bifidobacteria, might provide some physiological and symptomatic relief. A study using an RA model in Lewis rats has shown that Lactobacillus rhamnosus GG ingestion can provide some improvement in inflammation at joint sites both clinically and histologically [15]. A clinical study that was notably underpowered (n=21 patients) and only looked at mild RA, did not find a statistically significant difference in the activity of RA with the use of L. rhamnosus GG, although more subjects in the probiotic group reported improvement in subjective wellbeing [16]. The more recently anti-inflammatory effects mediated by L. rhamnosus GR-1 [10] and L. reuteri[17] suggested that these strains would be worth assessing in RA.
The purpose of the study was to examine the utility of probiotics as an adjunctive therapy (in addition to patients’ pharmacotherapy) for the treatment of RA. It was hypothesized that the probiotics L. rhamnosus GR-1 and L. reuteri RC-14, would confer anti-inflammatory effects and alleviate symptoms, and help increase daily activities of patients with RA.
Material and Methods
Design and sample size
This study was conducted according to clinical practice guidelines for a prospective, randomized, double-blind, placebo-controlled study, with 2 parallel arms. The trial was registered on the clinicaltrials.gov site under the Lawson Health Research Institute. It was approved by the University of Western Ontario Ethics Review Board. Randomization was done by random number generation (labeled ordered 1 to 30). Capsule blinding was completed by the research pharmacist at the St. Joseph’s Health Care Center (SJHC).
As this was a pilot study, given past data we felt a sample size of 30 to 40 (15 to 20 per arm) would allow us to see 30 to 40% more patients within an ACR20 in the probiotic group depending on the placebo response that was estimated. Other literature supported this sample size [16].
Recruitment
Subjects were recruited from SJHC Rheumatology clinic. To participate in the study, patients had to be between 18 to 80 years of age, diagnosed with RA (according to ACR criteria), and with at least 4 swollen and tender joints, be on stable DMARDs, steroids and/or NSAIDs for at least one month prior to randomization, and not have received intra-articular steroids within one month before enrolment or during the study. Patients were excluded if they had inflammatory bowel disease or leaky gut, were currently consuming probiotics with refusal to have a 2 week washout period, had known allergies to the study product or placebo content, planned to have surgery during the time of the study, had any illness that could impair their ability to comply with the study, or were enrolled in another clinical trial.
Probiotic
The active agent in this study was a probiotic capsule containing L. rhamnosus GR-1 and L. reuteri RC-14, kindly provided by Chr Hansen, Denmark. Each capsule contained 2 billion colony- forming unit (CFU) viable bacterial cells in addition to the inactive ingredients; dextrose, potato starch, microcrystalline cellulose and magnesium stearate. The placebo capsules contained the same ingredients described above without the bacteria. Study medications were received by the Research Pharmacist who ensured the medications were properly labeled and stored. They were then distributed to patients by the research coordinator.
Protocol
Participants read the letter of information for the study and provided signed consent. They were randomized to receive one capsule taken orally, twice daily, containing placebo or probiotic (Figure 1). The subjects continued to take the placebo or probiotic, in addition to their stable RA medications, for three months. The study medication was prepared by the pharmacist (who was the sole un-blinded person during the study), and dispensed by study personnel. The pharmacist was not involved in study design, recruitment, or analysis, and did not have any contact with patients during the study. Pill counts were done by the research coordinator at the second and third visit to evaluate compliance.
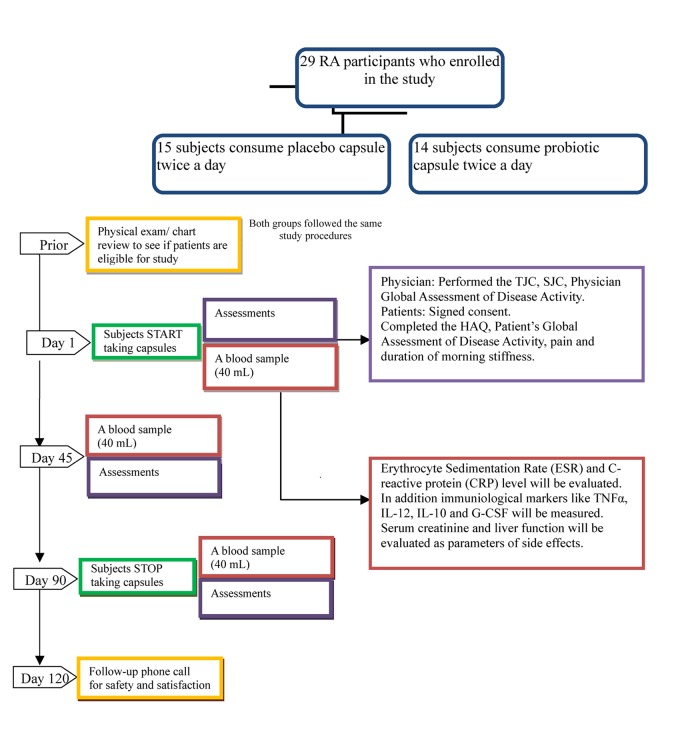
Figure 1.
Methodology Flow Chart.
Subjects came in for three visits during the study. On the first visit at day 0, subjects were randomized and started on capsules. The second visit was at day 45 and the third visit was at day 90. At each visit, blood samples were collected and used to evaluate Erythrocyte Sedimentation Rate (ESR), C-reactive protein (CRP), and levels of 15 inflammatory cytokines. The following cytokines were measured simultaneously in serum using a multiplexed immunoassay (Millipore, MA): IL-1α, IL-1β, IL-6, IL-8, TNF-α, IL-12p70, IL-15, IL-10, GM-CSF, G-CSF, IL-17, sCD40 ligand, MIP-1α, MIP-1β, MCP-1. Serum creatinine and liver function tests were evaluated as parameters of side-effects of the probiotic treatment.
At each visit, the swollen and tender joint counts (both 28 and 64/66) and Physician Global Assessment of Disease activity (10 cm VAS) were assessed. Patients were asked to complete the following self-administered, questionnaires: Health Assessment Questionnaire (HAQ, a 0–3 scale), Patient’s Global Assessment of Disease Activity (10 cm VAS), Patient Assessment of Pain (10 cm VAS) and duration of morning stiffness in minutes.
A follow-up phone call took place 30 days after the completion of the study medication. If a subject withdrew from the study prematurely, he/she was asked to receive a follow-up telephone call 30 days from the date they last took the study product. During the phone call, the participant was asked about any medications taken in the past month and about any possible side effects (unwanted effects or health problems) that they may have experienced.
The primary outcome for this study was the difference in the number of patients that achieved an ACR20 response in the probiotic versus the placebo group. Changes in the individual components of the ACR20 criteria and in cytokine levels were considered secondary outcomes.
Statistical methods
The primary outcome was measured by comparing the number of patients who achieved ACR20 response in each treatment group using a Chi square analysis. Secondary outcomes were compared at baseline and at visit three using independent sample t-tests to compare patients on placebo versus probiotic. Paired t-tests were used to detect significant changes in each patient from visit one to visit three (as the study was small between groups differences could adjust for potential baseline imbalances) (Table 1). For between group analyses, cytokine levels were standardized to the patients’ baseline inflammatory state using C-reactive protein levels specific to each time point. Fold difference was calculated for each group as median cytokine level of visit 3 divided by visit 1, then Wilcoxon tests done to determine statistical differences. Randomization was done by the pharmacy.
Table 1.
Mean changes in patients from baseline to final visit (ITT analysis).
| Probiotic group 15/15 completed | Placebo group 11/14 completed | p-value for between groups differences (final – baseline) except for ACR20 | |
|---|---|---|---|
| DAS | −2.1 (SD±1.1) | −2.9 (SD±0.6) | 0.77 |
| SJC (64) | −0.4 (SD±3.8) | −1.36 (SD±4.1) | 0.98 |
| SJC (28) | −0.4 (SD±3.3) | −1.0 (SD±3.6) | 0.47 |
| TJC (66) | −0.73 (SD±9.5) | 0.27 (SD±8.5) | 0.87 |
| TJC (28) | 0.2 (SD±5.5) | −0.55 (SD±7.1) | 0.43 |
| MD Global | 0.6 (SD±1.8) | 0.0 (SD±0.8) | 0.04* |
| Patient Global | −0.06 (SD±1.7) | 0.31 (SD±1.2) | 0.39 |
| Morning stiffness (min) | 8.25 (SD±26.4) | 7.3 (SD±57.5) | 0.28 |
| HAQ | −0.16 (SD±0.2) | −0.05 (SD±0.2) | 0.11 |
| Pain | −0.25 (SD±1.9) | 0.02 (SD±2.3) | 0.41 |
| Fatigue | −0.42 (SD±1.6) | 1.3 (SD±2.2) | 0.28 |
| ESR | −4.0 (SD±9.8) | 0.27 (SD±6.8) | 0.76 |
| CRP | 1.8 (SD±8.4) | 1.2 (SD±4.8) | 0.75 |
| Patient overall status (0–5) | −0.43 (SD±0.7) | 0.1 (SD±0.6) | 0.16 |
| # Achieving ACR20 (%) | 20% | 7% | 0.33 |
| IL-1 alpha | −22.9 (SD±93.4) | −52.5 (SD±257.2) | 0.15 |
| IL-1 beta | 3.0 (SD±12.4) | −16.1 (SD±54.7) | 0.06* |
| IL-6 | −5.0 (SD±15.1) | −16.4 (SD±50.5) | 0.004* |
| IL-8 | 5.8 (SD±13.8) | 0.5 (SD±9.8) | 0.48 |
| TNF-alpha | −0.2 (SD±3.7) | −5.2 (SD±19.8) | 0.03* |
| IL-12 p70 | −0.96 (SD±7.7) | −75.6 (SD±168.3) | 0.001* |
| IL-15 | 1.1 (SD±4.8) | −12.4 (SD±51.3) | 0.04* |
| IL-10 | −3.2 (SD±14.3) | −4.2 (SD±23) | 0.12 |
| GM-CSF | 70.1 (SD±234) | −463.3 (SD±1654) | 0.05* |
| G-CSF | 1.6 (SD±6.2) | 0.79 (SD±15.7) | 0.25 |
| IL-17 | 0.46 (SD±1.6) | −5.6 (SD±14.2) | 0.02* |
| sCD40 ligand | 4049.0 (SD±8000) | −3.8 (SD±20714) | 0.16 |
| M1P-1 alpha | 7.3 (SD±18.6) | −15.4 (SD±31.6) | 0.04* |
| M1P-1 beta | 7.9 (SD±31.8) | −19.8 (SD±95) | 0.12 |
| MCP-1 | 22.8 (SD±84.9) | 83.1 (SD±227.5) | 0.01# |
All values with means and SD;
favours placebo;
favours probiotics.
Results
Over two hundred patients were screened over a one year period, most of whom did not have sufficient swollen joints or had enough active disease where DMARD treatment was changed. A total of twenty-nine subjects participated in the study; fifteen were randomized to the probiotic group and 14 to the placebo group. Baseline characteristics of the study groups are shown in Table 2. Through box plots we determined that the cytokine values for patient 28 were outliers. Hence, we excluded this patient’s cytokine results as they skewed the data for the probiotic group. However, the clinical results for this patient were included in the stats. There were three cytokines significantly different between the study groups at baseline: IL-8, IL12p70 and MIP-1β were lower in the probiotic group compared to placebo (Table 3). There were no other significant differences between groups at baseline. In the placebo group, two participants were lost to follow-up and one was withdrawn due to increased disease activity.
Table 2.
Baseline characteristics of the study groups.
| Probiotic group n=15 | Placebo group n=14 | p-value | |
|---|---|---|---|
| Sex F: M | 14:1 | 13:1 | |
| Mean age (years) (SD) | 63.8 (SD±7.5) | 59.1 (SD±9.1) | 0.66 |
| Mean disease duration (years)(SD) | 19 (SD±12.4) | 13.7 (SD±8.4) | 0.08 |
| Current DMARDs | |||
| % using Methotrexate | 73% | 78% | |
| % receiving other DMARDs | Leflunomide (20%) Hydroxychloroquine (40%) Sulfasalazine (33%) Myochrysine (0%) |
Leflunomide (21%) Hydroxychloroquine (50%) Sulfasalazine (28%) Myochrysine (14%) |
|
| % on combination DMARDs | 66% | 64% | |
| % not using any DMARDs | 6% | 7% | |
| % using oral steroids | 26% | 21% | |
| DAS | 4.18 (SD±1.05) | 4.83 (SD±0.91) | 0.48 |
| SJC (64) | 9.5 (SD±5.3) | 8.5 (SD±3.6) | 0.58 |
| SJC (28) | 7.7 (SD±5.2) | 8.8 (SD±4.5) | 0.81 |
| TJC (66) | 13.6 (SD±13.6) | 8.7 (SD±4.1) | 0.22 |
| TJC (28) | 7.5 (SD±5.8) | 7.8 (SD±4.1) | 0.19 |
| MD Global Assessment (0–10) | 2.28 (SD±1.2) | 2.13 (SD±0.9) | 0.62 |
| Patient Global (0–10) | 2.77 (SD±1.6) | 4.38 (SD±1.9) | 0.87 |
| Morning Stiffness (minutes) | 33.5 (SD±40.2) | 45.7 (SD±67.5) | 0.56 |
| HAQ (0–3) | 0.97 (SD±0.5) | 1.22 (SD±0.6) | 0.98 |
| Pain (0–10) | 3.7 (SD±1.8) | 5.2 (SD±2.5) | 0.09 |
| Fatigue (0–10) | 3.3 (SD±1.8) | 4.4 (SD±2.2) | 0.19 |
| ESR (SD) | 15.4 (SD±13.2) | 20.2 (SD±14.6) | 0.36 |
| CRP (SD) | 3.8 (SD±3.2) | 7.4 (SD±8.8) | 0.15 |
| Patient overall status (0–5) | 3.07 (SD±0.6) | 2.77 (SD±0.5) | 0.63 |
All values with means are given with SD also.
Table 3.
Mean cytokine values at baseline and at 90 days.
| Cytokine | Baseline | D=90 | ||||
|---|---|---|---|---|---|---|
| Probiotic n=15 |
Placebo n=14 |
p-value | Probiotic n=15 |
Placebo n=11 |
p-value | |
| IL-1 alpha | 80.3 (SD±170.7) | 194.5 (SD±640.1) | 0.18 | 57.39 (SD±122.2) | 194.88 (SD±473.3) | 0.31 |
| IL-1 beta | 11.8 (SD±25.0) | 43.1 (SD±86.5) | 0.09 | 14.8 (SD±27.7) | 33.8 (SD±45.4) | 0.21 |
| IL-6 | 20.4 (SD±53.5) | 66.2 (SD±97.7) | 0.07 | 15.4 (SD±39.6) | 61.3 (SD± 72.9) | 0.056 |
| IL-8 | 9.8 (SD±8.0) | 12.7 (SD±14.9) | 0.04* | 15.6 (SD±19.7) | 15.8 (SD±14.3) | 0.97 |
| TNF-alpha | 4.42 (SD±4.03) | 13.2 (SD±26.6) | 0.057 | 11.1 (SD±12) | 4.2 (SD±3.2) | 0.049 |
| IL-12 p70 | 123.2 (SD±10.4) | 123.25 (SD±231.6) | 0.01* | 9.4 (SD±23.6) | 73.1 (SD±104.3) | 0.03 |
| IL-15 | 5.05 (SD±7.73) | 23.3 (SD±66.0) | 0.08 | 6.1 (SD±9.1) | 16.5 (SD±25.9) | 0.17 |
| IL-10 | 16.1 (SD±43.0) | 23.7 (SD±35.6) | 0.67 | 12.9 (SD±29.5) | 24.4 (SD±22.9) | 0.29 |
| GM-CSF | 176.7 (SD±314.2) | 987.9 (SD±2574.6) | 0.054 | 246.8 (SD±456.2) | 742.6 (SD± 1300) | 0.19 |
| G-CSF | 10.3 (SD±5.6) | 13.6 (SD±16.3) | 0.64 | 11.9 (SD±8.9) | 17.4 (SD±14.2) | 0.25 |
| IL-17 | 3.7 (SD±4.8) | 11.58 (SD±31.9) | 0.07 | 4.2 (SD±5.7) | 8.9 (SD± 21.6) | 0.43 |
| sCD40 ligand | 2.6 (SD±2.4) | 2.8 (SD±3.1) | 0.48 | 3.0 (SD± 2.5) | 3.0 (SD±2.7) | 0.95 |
| M1P-1 alpha | 26.5 (SD±20.4) | 60.3 (SD±101.4) | 0.03* | 33.8 (SD±32.9) | 59.2 (SD± 86.4) | 0.32 |
| M1P-1 beta | 58.1 (SD±69.2) | 103.8 (SD±172.8) | 0.23 | 66 (SD±67.3) | 99.2 (SD±106.8) | 0.35 |
| MCP-1 | 311.9 (SD±234.6) | 351.7 (SD±352.1) | 0.27 | 334.7 (SD±224) | 494.7 (SD±430.1) | 0.24 |
All values with means are given with SD also;
values found to be significantly different.
At the first visit, the mean swollen joint count (64 SJC) and tender joint count (66 TJC) for the placebo group was 8.5 and 8.9 respectively, which was not statistically different from the probiotic treated group (9.5 and 13.6 respectively). At the last visit (day 90), the mean SJC /TJC were 7.3/6.7 for the placebo group and 9.1/13.7 for the probiotic group (p=NS, Table 1). The within-group changes were not statistically significant for SJC or TJC from visit 1 to visit 3.
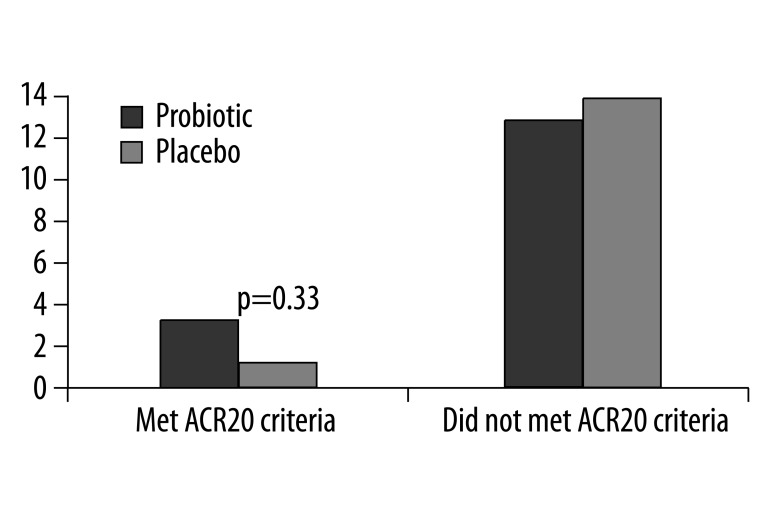
At the end of the study, three participants in the probiotic group and one in the placebo group achieved an ACR20 response (Figure 2). This difference was not statistically significant (p=0.33), which may have been to the low number of responders. Aside from HAQ, there were no within-group differences found when comparing the individual components of the ACR20 criteria at the beginning of the study to the end. In addition, there were no statistically significant differences in changes in cytokine levels within each group, there were no significant within-group changes or between-group changes in DAS.
Figure 2.
Number of patients that met and did not meet ACR20 criteria at 3 months in the placebo and probiotic groups.
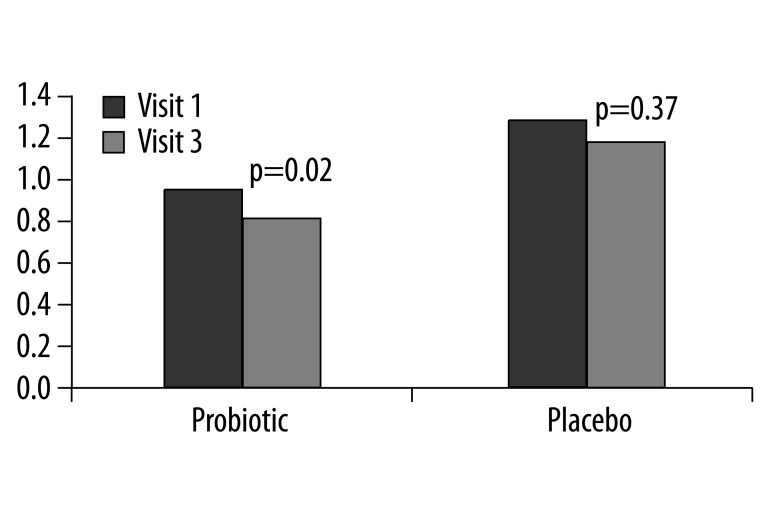
At the initial visit the HAQ scores for each group were not significantly different (Placebo 1.22, Probiotic 0.97 p=0.98). However when comparing the within-group changes at completion of the trial, the probiotic group showed an improvement in the HAQ score, from 0.97 at the initial visit to 0.80 at the final visit (p=0.02) (Figure 3). There was no significant change seen within the placebo group for the HAQ score.
Figure 3.
Mean values of HAQ at visit 1 compared to visit 3 for the placebo and probiotic groups. There were no between group differences.
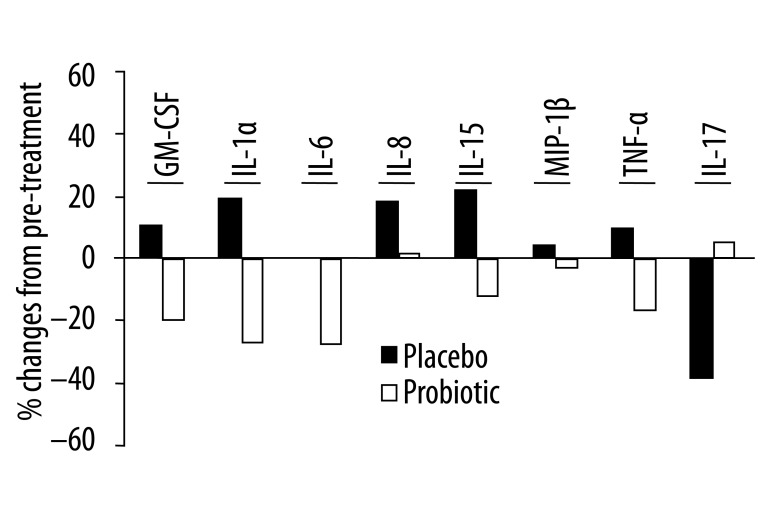
A fold change analysis was performed on the data from the cytokine analysis (Figure 4), reflecting changes within the individual compared to changes within the group. Although not significant, there was a trend for reduced secretion of pro-inflammatory cytokines, especially GM-CSF, IL-1α, IL-6, IL-15 and TNF-α, following probiotic treatment compared to placebo.
Figure 4.
Percent change in serum levels of inflammatory cytokines before and after 3 months of treatment with probiotics or placebo in patients with rheumatoid arthritis. Percent change within each group was calculated as (median fold change for post/pretreatment − 1) ×100. None of these changes reached statistical significance.
There were no adverse effects from the probiotics reported by the subjects. In addition, there were no significant changes in creatinine levels and liver transaminases. The results did not change when studying completers only, as the drop out rate was low.
Discussion
There is evidence that probiotics have anti-inflammatory effects even at sites distant from where they are administered. According to Hatakka and colleagues (2003), probiotics could indeed induce improved patient wellbeing in RA patients over-and-above continuation of their standard medications. Participants in the current study reported a significant improvement in the HAQ in the probiotic group. The HAQ has been well validated in the literature, and may better reflect the functional status of patients compared to laboratory values or physical exams [18]. The significance of this finding, however, is uncertain as there were no between-group differences in the HAQ scores.
Using the primary outcome of the number of patients who achieved an ACR20 response, there was no evidence that probiotics clinically improved RA over and above standard medication. In addition, there were no significant differences observed when comparing individual components of the ACR20 criteria or cytokine markers within each group. These findings are similar to the L rhamnosus GG pilot study of Hatakka and colleagues in 2003, where more subjects in the probiotic group reported improvement in subjective wellbeing; however there were no significant laboratory findings.
Specifically addressing cytokine results, there was a decrease in serum levels of IL-1α, IL-6, IL-10, IL-12p70 and TNF-α following probiotics treatment in rheumatoid patients. Nevertheless, placebo caused a significantly greater decline in the production of IL-6, IL-12p70 and TNF-α, as well as IL-15, IL-17, GM-CSF and MIP-1α. Each of these cytokines play an important role in the pathogenesis of RA and, excluding MIP-1α, are presently undergoing study in clinical trials as potential targeted treatments to reduce joint inflammation and damage in RA [19,20]. TNF-α was reduced substantially in the present study, in line with expectations from in vitro experiments [10]. TNF-blocking agents are currently approved for clinical use and show the most promise, yet this therapy is most effective when combined with another therapy such as methotrexate [21]. It would be interesting to examine the clinical effectiveness of a combined probiotics and TNF antagonist treatment. Although there has been animal data to suggest that combination therapy of methotrexate and probiotics is more effective compared to methotrexate alone [12], a subset analysis was not possible in the current study due to low numbers of subjects.
More favorably, MCP-1 levels increased significantly less with probiotics treatment. MCP-1 has contrasting roles whereby it can enhance synovitis by facilitating the recruitment of leukocytes into the joint [22,23], but it can also promote a protective Th2 response [24]. Likely for these reasons, plus the uncertainty of its role in RA, there are no human trials that target MCP-1 in RA at present [25,26]. Further research is required to decipher the influence of MCP-1 on the inflammatory process in human RA.
When considering the results of the current study, one set-back was the enrolment criteria. In an attempt to control for variables – especially those that would exert an immunomodulatory effect, patients who were to have changes to their medications were excluded from participating. The most frequent reason for medication changes was a flare in disease activity. Another foundational criteria was that patients had to have four swollen and four tender joints on exam. This potentially biased recruitment of patients with stable RA and chronic synovitis. Thus, it may have been asking too much for an adjunctive therapy to demonstrate improvement within three months. McDougall and colleagues [27] found a similar trend of less change in patients with long standing RA when attempting to change gut antigens using a strict vegetarian diet.
Ethically, patients with active disease could not be given an experimental agent when a proven DMARD was available, nor could patients be taken off DMARDs and put on placebo or probiotics as monotherapy. It is possible but unlikely that with increased recruitment numbers, and therefore increased power, a significant difference may have been seen. Recruitment, however, was ongoing for 12 months, and the project had limitations including no funding source and two medical students (MP, ST) working on the project.
Previous studies have indicated that this probiotic capsule could augment the efficacy of antimicrobial agents given for a short duration [28–30]. This was not the case here, with no augmentation of disease modifying anti-rheumatic drugs noted.
Conclusions
In conclusion, while probiotics were well tolerated and suppressed production of several inflammatory cytokines systemically, their activity was not better than placebo and no overall clinical improvement can be claimed. It remains possible that probiotics had a favorable anti-inflammatory effect at the cellular level in rheumatoid joints.
Acknowledgements
The authors wish to acknowledge the help of Shannon Mifflin in analyzing the serum samples. Chr. Hansen, Denmark provided the probiotic and placebo capsules. Dr. Summers’ lab analyzed the cytokines.
Footnotes
Source of support: Maria Pineda received funding from the Schulich Summer Research Training Program (SRTP), at the University of Western Ontario
References
- 1.Mielants H, De Vos M, Cuvelier C, Veys EM. The role of gut inflammation in the pathogenesis of spondyloarthropathies. Acta Clin Belg. 1996;51:340–49. doi: 10.1080/22953337.1996.11718528. [DOI] [PubMed] [Google Scholar]
- 2.Tiwana H, Walmsley RS, Wilson C, et al. Characterization of the humoral immune response to Klebsiella species in inflammatory bowel disease and ankylosing spondylitis. Br J Rheumatol. 1998;37:525–31. doi: 10.1093/rheumatology/37.5.525. [DOI] [PubMed] [Google Scholar]
- 3.Hvatum M, Kanerud L, Hallgren R, Brandtzaeg P. The gut-joint axis: cross reactive food antibodies in rheumatoid arthritis. Gut. 2006;55:1240–47. doi: 10.1136/gut.2005.076901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danning CL, Boumpas DT. Commonly used disease-modifying antirheumatic drugs in the treatment of inflammatory arthritis: an update on mechanisms of action. Clin Exp Rheumatol. 1998;16:595–604. [PubMed] [Google Scholar]
- 5.Müller H, de Toledo FW, Resch KL. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: a systematic review. Scand J Rheumatol. 2001;30:1–10. doi: 10.1080/030097401750065256. [DOI] [PubMed] [Google Scholar]
- 6.Peltonen R, Nenonen M, Helve T, et al. Faecal microbial flora and disease activity in rheumatoid arthritis during a vegan diet. Br J Rheumatol. 1997;36:64–68. doi: 10.1093/rheumatology/36.1.64. [DOI] [PubMed] [Google Scholar]
- 7.Food and Agriculture Organization of the UN and WHO. Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. Food and Agriculture Organization of the United Nations and World Health Organization Expert Consultation Report; 2001, Oct 1 –4; Cordova, Argentina. Available from: ftp://ftp.fao.org/docrep/fao/009/a0512e/A0512E00.pdf. [Google Scholar]
- 8.Strowski MZ, Wiedenmann B. Probiotic carbohydrates reduce intestinal permeability and inflammation in metabolic diseases. Gut. 2009;58:1044–45. doi: 10.1136/gut.2009.179325. [DOI] [PubMed] [Google Scholar]
- 9.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–84. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Kim SO, Sheikh HI, Ha SD, et al. G-CSF-mediated inhibition of JNK is a key mechanism for Lactobacillus rhamnosus-induced suppression of TNF production in macrophages. Cell Microbiol. 2006;8:1958–71. doi: 10.1111/j.1462-5822.2006.00763.x. [DOI] [PubMed] [Google Scholar]
- 11.Kano H, Kaneko T, Kaminogawa S. Oral intake of Lactobacillus delbrueckii subsp. Bulgaricus OLL1073R-1 prevents collagen-induced arthritis in mice. J Foo Prot. 2002;65(1):153–60. doi: 10.4315/0362-028x-65.1.153. [DOI] [PubMed] [Google Scholar]
- 12.Rovensky J, Svik K, Matha V, et al. Combination treatment of rat adjuvant-induced arthritis with methotrexate, probiotic bacteria Enterococcus faecium, and selenium. Ann NY Acad Sci. 2005;1051:570–81. doi: 10.1196/annals.1361.101. [DOI] [PubMed] [Google Scholar]
- 13.Rovensky J, Stancikova M, Svik K, et al. Treatment of adjuvant-induced arthritis with the combination of methotrexate and probiotic bacteria Escherichio coli O83 (Colinfant®) Folia Microbiol. 2009;54:359–63. doi: 10.1007/s12223-009-0045-2. [DOI] [PubMed] [Google Scholar]
- 14.Stebbings S, Munro K, Simon MA, et al. Comparison of the faecal microflora of patients with ankylosing spondylitis and controls using molecular methods of analysis. Rheumatology (Oxford) 2002;41:1395–401. doi: 10.1093/rheumatology/41.12.1395. [DOI] [PubMed] [Google Scholar]
- 15.Baharav E, Mor F, Halpern M, Weinberger A. Lactobacillus GG bacteria ameliorate arthritis in Lewis rats. J Nutr. 2004;134:1964–69. doi: 10.1093/jn/134.8.1964. [DOI] [PubMed] [Google Scholar]
- 16.Hatakka K, Martio J, Korpela M, et al. Effects of probiotic therapy on the activity and activation of mild rheumatoid arthritis – a pilot study. Scand J Rheumatol. 2003;32:211–15. doi: 10.1080/03009740310003695. [DOI] [PubMed] [Google Scholar]
- 17.Christensen HR, Frøkiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168:171–78. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe F, Cathey MA. The assessment and prediction of functional disability in rheumatoid arthritis. J Rheumatol. 1994;18:1298–306. [PubMed] [Google Scholar]
- 19.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429–42. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 20.Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373(9664):659–72. doi: 10.1016/S0140-6736(09)60008-8. [DOI] [PubMed] [Google Scholar]
- 21.De Stefano R, Frati E, Nargi F, et al. Comparison of combination therapies in the treatment of rheumatoid arthritis: leflunomide-anti-TNF-alpha versus methotrexate-anti-TNF-alpha. Clin Rheumatol. 2010;29(5):517–24. doi: 10.1007/s10067-009-1349-y. [DOI] [PubMed] [Google Scholar]
- 22.Koch AE. Chemokines and their receptors in rheumatoid arthritis: future targets? Arthritis Rheum. 2005;52(3):710–21. doi: 10.1002/art.20932. [DOI] [PubMed] [Google Scholar]
- 23.Iwamoto T, Okamoto H, Toyama Y, Momohara S. Molecular aspects of rheumatoid arthritis: chemokines in the joints of patients. FEBS J. 2008;275(18):4448–55. doi: 10.1111/j.1742-4658.2008.06580.x. [DOI] [PubMed] [Google Scholar]
- 24.Gu L, Tseng S, Horner RM, et al. Control of TH2polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404(6776):407–11. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 25.Daly C, Rollins BJ. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: therapeutic opportunities and controversies. Microcirculation. 2003;10(3–4):247–57. doi: 10.1038/sj.mn.7800190. [DOI] [PubMed] [Google Scholar]
- 26.Dawson J, Miltz W, Mir AK, Wiessner C. Targeting monocyte chemoattractant protein-1 signalling in disease. Expert Opin Ther Targets. 2003;7(1):35–48. doi: 10.1517/14728222.7.1.35. [DOI] [PubMed] [Google Scholar]
- 27.McDougall J, Bruce B, Spiller G, et al. Effects of a very low-fat, vegan diet in subjects with rheumatoid arthritis. J Altern Complement Med. 2002;8(1):71–75. doi: 10.1089/107555302753507195. [DOI] [PubMed] [Google Scholar]
- 28.Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8:1450–54. doi: 10.1016/j.micinf.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Martinez RC, Franceschini SA, Patta MC, et al. Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14. Lett Appl Microbiol. 2009;48:269–74. doi: 10.1111/j.1472-765X.2008.02477.x. [DOI] [PubMed] [Google Scholar]
- 30.Martinez RC, Franceschini SA, Patta MC, et al. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: a randomized, double-blind, placebo-controlled trial. Can J Microbiol. 2009;55:133–38. doi: 10.1139/w08-102. [DOI] [PubMed] [Google Scholar]