Summary
Background
Heart rhythm turbulence (HRT) is a novel tool for evaluation of cardiovascular mortality. Liver cirrhosis is associated with hemodynamic and myocardial disturbances termed cirrhotic cardiomyopathy. In the stable stage of liver cirrhosis, systolic and myocardial dysfunction is correlated with brain natriuretic peptide (BNP).
The aim was to evaluate HRT and its correlation with NT-proBNP, echocardiographic and biochemical parameters in patients with decompensation of liver cirrhosis.
Material/Methods
The study included 18 patients with decompensated liver cirrhosis and 18 healthy volunteers. Participants underwent echocardiography and 24-hour ECG monitoring. Serum NT-proBNP and other biochemical parameters were measured. Turbulence onset (TO) and turbulence slope (TS) were used to indicate HRT.
Results
Mean HR (87/min vs. 75/min), TO (−0.385% vs. −0.92%), NT-proBNP (304.85 pg/ml vs. 83.2 pg/ml), LAd (42.5 mm vs. 34.5 mm), RVdd (29.5 mm vs. 25 mm), SPAP (36.5 mmHg vs. 22.5 mmHg) were significantly (p<0.05) higher in patients with liver cirrhosis. Patients with normal TO and TS had better stage in Child-Pugh classification (P=0.04) than patients with abnormal values. Significant negative correlation was found between creatinine and TO, and between mean HR and TS, and significant positive correlation was found between LAd and TS. LV diastolic dysfunction was noted in a majority of cirrhotic patients (n=16).
Conclusions
Patients with decompensated cirrhosis had elevated levels of NT-proBNP and LV diastolic dysfunction. TO values in cirrhotic patients differed significantly from the control group. These findings can indicate risk of symptomatic heart failure development and may be a marker of cirrhotic cardiomyopathy. HRT parameters seem not to be appropriate death predicators.
Keywords: liver cirrhosis, heart rhythm turbulence, NT-proBNP
Background
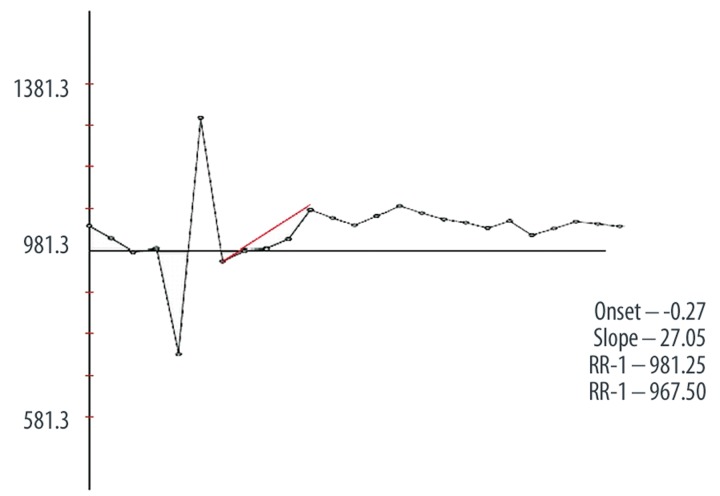
Heart rhythm turbulence (HRT) is a novel tool for evaluation of cardiovascular mortality. HRT is especially used to evaluate risk of death in a patient with ischemic heart disease and congestive heart failure [1]. HRT describes a short-term heart rhythm (HR) fluctuation in sinus rhythm that follows the ventricular premature beat. Early acceleration followed by late deceleration of sinus rhythm is observed. This phenomenon usually includes 15–20 consecutive beats. It is associated with autonomic nervous system activity and baroreceptor reflex [2]. Two phases of HRT are estimated by the following parameters: turbulence onset (TO) and turbulence slope (TS) (Figure 1).
Figure 1.
Scheme of estimation turbulence onset (To) and turbulence slope (Ts).
Normal values of these parameters are calculated as TO <0% and TS >2.5 ms/RR [3]. Recent findings suggest that TO and TS values are comparable or even better predictors of sudden cardiac death in myocardial infarction than are accelerated basic HR, ventricular arrhythmia, lowered left ventricular ejection fraction (LVEF<30%), and age over 65 years. The highest risk of death was demonstrated for patients after MI with impaired HRT [1,4,5].
Cirrhosis is associated with various hemodynamic and myocardial disturbances termed cirrhotic cardiomyopathy [6,7]. Impaired cardiac contractility, systolic and diastolic dysfunction, electromechanical abnormalities, chronotropic incompetence and various arrhythmias are observed in cirrhotic patients [8–12]. Increased or normal cardiac output, decreased blood pressure and total peripheral resistance are also noted. Increased cardiac load results in contractility disturbances and decreased cardiac output. Diastolic function is also affected, which is associated with the increase in left ventricular filling pressure. These pathological processes are associated with LV hypertrophy and myocardial fibrosis [13,14], and is also observed during surgery, pharmacotherapy, stress, transjugular intrahepatic portosystemic shunt placement (TIPS) or liver transplantation [15–17].
Recent findings suggest that systolic myocardial dysfunction is positively correlated with brain natriuretic peptide (BNP) in the stable stage of cirrhosis, so this parameter seems to be a marker of cirrhotic cardiomyopathy [18–21]. A recently published study by Pimeta et al. [22] proved that elevated BNP level is also associated with cardiac contractility impairment in decompensated cirrhosis. This biomarker may by an independent mortality predictor in medium-term survival in end-stage cirrhosis.
Until now, HRT and its correlation with NT-proBNP in patients with cirrhosis have not been evaluated. The aim of our study was to evaluate the HRT and its correlation with NT-proBNP, creatinine, ammonium, sodium, total bilirubin, albumin, INR and echocardiographic parameters in patients with decompensation of cirrhosis. We estimated a role of HRT and NT-proBNP evaluation in patients with cirrhosis decompensation.
Material and Methods
The study included 18 patients admitted to the Department of Infectious and Liver Diseases (Medical University of Lodz) between 01.01.2009 and 01.06.2009 due to cirrhosis and liver deteriorated function (presence of ascites, jaundice and/or hepatic encephalopathy). Exclusion criteria were: previous history of ischemic heart disease, arterial hypertension, atrial fibrillation and other arrhythmias that disturbed ECG record, chronic kidney disease (stage 3 and higher), diabetes mellitus, autoimmunological diseases, neoplasmatic diseases (including primary hepatic cancer), nicotine addiction, alcohol dependence, gastrointestinal hemorrhage (during the most recent 2 months). Diagnosis of cirrhosis was confirmed by physical examination, laboratory results, ultrasonography and, if needed, liver biopsy. The etiology of cirrhosis was alcoholic (in 10 patients), hepatitis B and C (both in 3 patients) and idiopathic cirrhosis (in 2 patients). The control group consisted of 18 volunteers matched for age and sex, not treated for liver diseases or for any diseases that were excluding patients from the study. Twelve months after discharge from hospital, cirrhotic patients’ status (survive or not survive for any reason) was checked. Information was obtained from patients during visits in hospital ambulatory or, in case of patient’s death, from telephone contact with family. On admission medical history was taken, patients were examined, and blood samples for laboratory tests were taken. Patients who met inclusion criteria underwent echocardiography, 24-hour ECG monitoring and NT-proBNP was estimated, and then typical treatment was administered including spironolactone, loop diuretics, and beta-adrenolytics.
Twenty-four-hour ECG was monitored using an Aspect 702 recorder (Aspel Zabierzów, Poland). Data were analyzed by automatic computer system – HolCARD 24W (Aspel Zabierzów, Poland). Automatic detection of QRS complexes and HRT calculation were performed. TO and TS were used to indicate rhythm turbulence. ECG records with at least 5 premature ventricular beats with prematurity >20% were accepted as adequate for interpretation [23,24]. Analysis and calculation of TO and TS were performed automatically by HRT application that was a part of a commercial system for HRT analysis. Results of these analyses were verified by a cardiologist experienced in 24-hour ECG records. Results of unsatisfactory quality or quantity of data were excluded from final analysis. Normal values were TO <0% and TS >2.5 ms/RR. Finally, satisfactory 24-hour ECG records were obtained from 14 patients.
NT-proBNP serum level was estimated using an electrochemiluminescent immunoassay kit – (analisator MODULAR ANALITICS E 170, Roche Diagnostics GmbH, D-68296 Mannheim, Germany). Measurement range was of 5–35000 pg/ml. Elevated bilirubin (<35 mg/dl) and triglycerides levels (TG <4000 mg/dl) do not affect method of NT-proBNP measurement. Normal values were <125 pg/ml. Other biochemical (serum), morphological (whole blood) and coagulology (plasma) analyses were performed using standard methods. Peripheral venous blood samples were used for all estimations.
The echocardiogram was carried out using HP Sonos 2500 (Hewlett Packard, Santa Clara, CA, USA) echocardiograph with a 3.5 MHz probe. The examination was performed with 2D guidance, color Doppler and spectral or continues Doppler. The following parameters were measured: RV internal diastolic diameter (RVdd), systolic pulmonary artery pressure (SPAP), left atrium diameter (LAd), left ventricle end-diastolic and end-systolic diameter (LVEds and LVEsd), intraventricular septum diastolic diameter (IVSdd). Systolic function of the left ventricle was assessed by the LV ejection fraction (LVEF) according to modified Simpson formula, and systolic function of the right ventricle was assessed by estimation of tricuspid annular plane systolic excursion (TAPSE). Diastolic function of the left ventricle was assessed by estimation of E/A wave of mitral inflow ratio, deceleration time of E wave mitral inflow (DT), and propagation velocity of E wave mitral inflow (Vprop.).
According to study protocol, patients with cirrhosis were divided into 3 groups according to TO and TS values. Group I (n=7) comprised subjects with normal TO and TS values. Group II (n=7) comprised subjects with abnormal TO (3 patients) or TS (4 patients). None of the study participants met criteria of group III – abnormal TO and TS. All healthy volunteers had normal TO and TS values.
The study was performed in agreement with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by local Bioethics Committee.
Our study was relatively small (n=36), and was a pilot study. However, results of the study encouraged us to continue our study for 3–5 years so as to include about 100 patients. Thus we will be able to compare present results and conclusions with data obtained from a larger study group.
Statistical analysis
Statistical analysis was performed using STATISTICA 8 PL (StatSoft Inc.). Results are presented as median, average, minimum and maximum values. Statistical significance was indicated for P<0.05. Variable distribution was assessed by Shapiro-Wilk W test. As continuous variables were non-normally distributed, we used nonparametric tests: Mann-Whitney and Wilcoxon test. Correlations between variables were tested by using the Spearman R test. We attempted to determine if RV or/and LV parameters and biochemical parameters of liver function have an impact on HRT and NT-proBNP.
Results
For the first 6 months of the study, 27 patients with presumptive diagnosis of decompensated liver cirrhosis were admitted to our department. Among them 4 patients had previously diagnosed ischemic heart disease, 2 patients had heart failure caused by alcoholic cardiomyopathy, 1 patient had hemorrhage from esophageal varices in the last 2 months, 1 patient had lung tumor (carcinoma plano epitheliale), and 1 patient did not give consent to participate in the study. Finally, 18 patients aged 33–73 years (13 men and 5 women) were enrolled to the study.
Clinical, echocardiographic and laboratory parameters are presented in Table 1.
Table 1.
Characteristic of patients with the liver cirrhosis and control group.
| Characteristic | Patients n=18 |
Control n=18 |
P | ||
|---|---|---|---|---|---|
| Female gender (n.%) | 5 | (27.8) | 5 | (31.25) | 0.673 |
| Age (years) | 52 | (33–73) | 52 | (33–75) | 0.876 |
| BMI (kg/m2) | 25.85 | (21.2–37.7) | 25.6 | (21.9–31.5) | 0.743 |
| HR max (beats/min) | 127 | (90–159) | 118.5 | (96–132) | 0.096 |
| HR min (beats/min) | 60 | (35–78) | 47.5 | (42–55) | <0.001 |
| HR mean (beats/min) | 87 | (66–116) | 75 | (67–87) | 0.009 |
| Child-Pugh Class | |||||
| A (n.%) | 2 | (11.1) | |||
| B (n.%) | 9 | (50.0) | |||
| C (n.%) | 7 | (38.9) | |||
| Blood analysis | |||||
| Creatinine (mg/dl) | 0.875 | (0.6–1.98) | 0.83 | (0.14–1.21) | 0.187 |
| Ammonium (mg/dl) | 99 | (77–228) | 36 | (20–78) | <0.001 |
| NT-proBNP (pg/ml) | 304.85 | (27.35–930.1) | 83.2 | (45.8–123.6) | 0.003 |
| Haemoglobin (g/dl) | 11.5 | (7.3–14.4) | 13.55 | (12.7–15.5) | <0.001 |
| Platelet count (×109/l) | 151.5 | (46–293) | 268.8 | (152.89–389.09) | <0.001 |
| Sodium (mmol/l) | 133 | (124–140) | 140 | (136–146) | <0.001 |
| Total bilirubin (mg/dl) | 2.665 | (0.19–9.66) | 0.585 | (0.26–1.02) | <0.001 |
| Albumin (g/dl) | 3.24 | (2.08–4.33) | 4.285 | (3.89–4.74) | 0.003 |
| INR | 1.465 | (0.98–2.32) | 1.035 | (0.79–1.23) | <0.001 |
| Echocardiography finding | |||||
| LA diameter (mm) | 42.5 | (34–55) | 34.5 | (27–39) | <0.001 |
| RV diastolic diameter (mm) | 29.5 | (22–38) | 25 | (20–30) | 0.004 |
| SPAP (mmHg) | 36.5 | (20–72) | 22.5 | (18–26) | <0.001 |
| TAPSE (mm) | 30 | (16–40) | 27 | (22–30) | 0.111 |
| EF (%) | 64 | (49–78) | 68 | (58–76) | 0.128 |
| LV end systolic diameter (mm) | 27.5 | (21–43) | 28.5 | (24–36) | 0.917 |
| LV end diastolic diameter (mm) | 45 | (35–53) | 48 | (40–56) | 0.183 |
| IVS diastolic diameter (mm) | 10.5 | (8–13) | 10 | (8–12) | 0.998 |
| E/A ratio | 0.8 | (0.4–2.8) | 1.15 | (0.8–1.4) | 0.008 |
| DT (ms) | 220 | (70–240) | 167.5 | (147–200) | <0.001 |
| Vprop. (cm/s) | 34 | (22–80) | 64 | (56–72) | <0.001 |
| HRT parameters | |||||
| TO (%) | −0.385 | (−2.75–2.53) | −0.92 | (−4.38–0.46) | 0.004 |
| TS (ms/RR) | 3.205 | (0.3–12.5) | 7.29 | (2.66–18.6) | 0.055 |
NT-proBNP – N-terminal fragment of Brain natriuretic peptide (BNP); INR – international normalized ratio; LA – left atrium; RV – right ventricle; LV – left ventricle; IVS – intraventricular septum SPAP – Systolic Pulmonary Artery Pressure; TAPSE – Tricuspid Annular Plane Systolic Excursion; E/A wave of mitral flow; DT – deceleration time of E wave mitral inflow; Vprop. – propagation velocity of E wave mitral inflow; HRT – Heart Rate Turbulence; TO – Turbulence onset; TS – Turbulence slope.
No significant differences were observed in age, BMI, women percentage, maximal HR, TAPSE, LVEF, LVEDd, LVESd, IVSDd, creatinine level between the study group and the control group. Mean HR, minimal HR, ammonium, NT-proBNP, total bilirubin, INR, LAd, RVdd, SPAP, DT, and TO were significantly higher in patients with cirrhosis compared to the control group. Hemoglobin level, PLT, sodium and albumin level, E/A ratio, and Vprop were significantly lower in patients with cirrhosis compared to the control group. No significant changes were noted in other studied parameters between group I and group II. Patients from group I had significantly better stage in Child-Pugh classification (p=0.04) than patients with abnormal TO or TS. Correlations of selected biochemical and echocardiographic parameters with TO, TS and NT-proBNP were calculated (Table 2).
Table 2.
Correlations of selected parameters with TO.TS and NT-proBNP.
| Parameter | TO | TS | NT-proBNP | |||
|---|---|---|---|---|---|---|
| R (Spearman) | P | R (Spearman) | P | R (Spearman) | p | |
| Creatinine (mg/dl) | −0.678322 | 0.015317 | 0.181818 | 0.571701 | −0.028571 | 0.922761 |
| Ammonium (mg/dl) | −0.428571 | 0.337368 | −0.464286 | 0.293934 | 0.142857 | 0.735765 |
| NT-proBNP (pg/ml) | −0.212121 | 0.556306 | 0.127273 | 0.726057 | – | – |
| LA diameter (mm) | 0.105844 | 0.718755 | 0.586552 | 0.027470 | 0.063947 | 0.828066 |
| RV diastolic diameter (mm) | 0.398239 | 0.158452 | 0.278767 | 0.334484 | −0.508861 | 0.063129 |
| SPAP (mmHg) | 0.184249 | 0.528330 | 0.421775 | 0.133063 | −0.033187 | 0.910328 |
| TAPSE (mm) | 0.364646 | 0.199888 | 0.183428 | 0.530201 | −0.209610 | 0.472008 |
| EF (%) | −0.121684 | 0.678584 | 0.172570 | 0.555223 | −0.059603 | 0.839603 |
| Vprop (cm/s) | −0.355855 | 0.068495 | 0.280605 | 0.156265 | −0.517265 | 0.003421 |
| LVEDd | 0.048553 | 0.809949 | 0.278715 | 0.159192 | −0.332070 | 0.073004 |
| HR mean (beats/min) | 0.171997 | 0.556558 | −0.646090 | 0.012550 | 0.049656 | 0.872020 |
NT-proBNP – N-terminal fragment of Brain natriuretic peptide (BNP); INR – international normalized ratio; LA – left atrium; RV – right ventricle; SPAP – Systolic Pulmonary Artery Pressure; TAPSE – Tricuspid Annular Plane Systolic Excursion; E/A wave of mitral flow; DT – deceleration time of E wave mitral inflow; Vprop. – propagation velocity of E wave mitral inflow; HRT – Heart Rate Turbulence; TO – Turbulence onset; TS – Turbulence slope.
Significant negative correlation was found between creatinine and TO. For other studied parameters, significant correlations were not found with TO value. Significant negative correlation was found between mean HR and TS, and between Vprop and NT-proBNP level. Significant positive correlation was found between LAd and TS. During the study period 7 patients died. We compared selected parameters between patients that died and patients that survived (Table 3.).
Table 3.
Comparison of selected parameters between patients that died and patients that survived.
| Selected parameters | Patients that survived (n=11) | Patients that died (n=7) | p | ||
|---|---|---|---|---|---|
| Age (years) | 50 | (37–73) | 54 | (33–62) | 0.497 |
| BMI (kg/m2) | 24 | (21.2–37.7) | 27.4 | (21.4–28.7) | 0.821 |
| NT-proBNP (pg/ml) | 252 | (27.35–813.4) | 397 | (122.6–930.1) | 0.286 |
| LA diameter (mm) | 44 | (37–50) | 41 | (34–55) | 0.174 |
| RV diastolic diameter (mm) | 31 | (22–38) | 29 | (25–33) | 0.717 |
| SPAP (mmHg) | 38 | (20–72) | 32 | (20–42) | 0.390 |
| TAPSE (mm) | 30 | (22–40) | 30 | (16–32) | 0.526 |
| EF (%) | 62 | (49–78) | 68 | (58–74) | 0.258 |
| E/A ratio | 0.9 | (0.4–2.8) | 0.6 | (0.5–0.8) | 0.024 |
| Vprop (cm/s) | 42 | (30–80) | 32 | (22–38) | 0.037 |
| DT (ms) | 210 | (70–220) | 220 | (220–240) | 0.004 |
| LVEDd (mm) | 47 | (35–63) | 42 | (38–50) | 0.298 |
| LVESd (mm) | 29 | (21–43) | 26 | (24–32) | 0.239 |
| IVSDd (mm) | 10 | (8–13) | 11 | (10–12) | 0.189 |
| HR mean (beats/min) | 88 | (66–105) | 87 | (73–116) | 0.841 |
| TO (%) | −0.12 | (−0.62–2.53) | −0.91 | (−2.75– −0.05) | 0.083 |
| TS (ms/RR) | 3.4 | (0.3–11.24) | 3.01 | (0.73–12.5) | 0.790 |
NT-proBNP – N-terminal fragment of Brain natriuretic peptide (BNP); INR – international normalized ratio; LA – left atrium; RV – right ventricle; SPAP – Systolic Pulmonary Artery Pressure; TAPSE – Tricuspid Annular Plane Systolic Excursion; E/A wave of mitral flow; DT – deceleration time of E wave mitral inflow; Vprop. – propagation velocity of E wave mitral inflow; HRT – Heart Rate Turbulence; TO – Turbulence onset; TS – Turbulence slope.
Except for parameters of diastolic function (E/A ratio, DT and Vprop), any significant differences were noted in studied parameters between patients that died and patients that survived during the observational period. Diastolic function was significantly disturbed in patients that died during the observation period. There were no significant differences in all parameters regarding etiology of liver cirrhosis.
Discussion
Cirrhotic patients rarely present symptoms of apparent heart failure; however, some patients develop cardiomyopathy related to cirrhosis. At the beginning of this state we observed hyperkinetic circulation and increased or normal cardiac output; simultaneously, lowered peripheral resistance results in decreased blood pressure. Progressive fibrosis and hypertrophy of the myocardium results in disturbances of LV diastolic function [9,10,12,13,15]. In this condition, increased load may result in acute circulatory failure. Such a state may occur in patients after liver transplantation.
Histopathological findings and hemodynamic disturbances on early stages of cirrhotic cardiomyopathy are related to those observed in hypertrophic cardiomyopathy (HCM) [25]. Our study revealed significantly higher mean HR in patients with cirrhosis and significantly higher minimal HR compared to the control group. LV EF and TAPSE in cirrhotic patients did not differ significantly from the control group. This confirms that our patients developed hyperkinetic circulation. Disturbances of diastolic LV function were demonstrated for all parameters in the study group. Similar results were presented by Bernal et al. [26] in patients undergoing liver transplantation. Moreover, fluid overload and LV hypertrophy were noted in these patients. Our patients also presented other laboratory abnormalities typical for cirrhosis: decreased levels of hemoglobin, PLT, sodium, albumins and elevated concentrations of bilirubin and ammonium. Our results are consistent with previous data presented by Pimenta et al. [22], who also observed significantly higher BNP levels in cirrhotic patients, mainly in patients with cardiac systolic dysfunction, defined by low cardiac output and associated with an increased occurrence of death during 6 months observation. In our studies we also noted significantly elevated NT-proBNP concentration in patients with cirrhosis compared to the control group, which may be associated with excessive circulatory system load in decompensation of cirrhosis. Increased serum level of natriuretic peptides seems to be rather an effect of damage and structural lesion of myocardium and is not associated with hyperkinetic circulation. In our study we did not observe significant correlations between NT-proBNP and biochemical parameters, mean HR, LAd, RVdd, TAPSE and SPAP, as also noted by Pimenta et al. In contrast to the findings of Pimenta et al, we did not observe significant correlation between NT-proBNP and LVEF. Our results revealed that systolic LV function was not affected in cirrhotic patients. It is possible that classical echocardiography examination is not sufficiently sensitive to find mild LV systolic function disturbances that cause increased NT-proBNP in cirrhotic patients. Moreover, patients included for our studies had no past history of cardiovascular diseases. Because study participants had no previous cardiovascular diseases, it seems that increase in NT-proBNP is a result of myocardial damage caused by cirrhosis. In a stable stage of cirrhosis, elevated levels of NT-proBNP were also observed, and this biomarker seems to be an indicator of cirrhotic cardiomyopathy [18,19]. Readle-Hurst et al. [27] demonstrated that elevated NT-proBNP level may be a useful marker of LV diastolic function disturbances in liver cirrhosis. These authors suggest that NT-proBNP levels exceeding 290 pg/ml should undergo further cardiac evaluation. We also demonstrated significantly higher NT-proBNP level and LV diastolic dysfunction in cirrhotic patients, which confirms previous Readle-Hurst et al observations. Significant correlation was found between Vprop and NT-proBNP level, indicating that elevation of NT-proBNP levels follows diastolic dysfunction progress. In our studies, NT-proBNP levels and LVEF did not differ significantly for both values between patients that died during the study period and patients that survived. It is possible that NT-proBNP levels and LVEF, contrary to chronic heart failure, is not a predictor for survival in end-stage cirrhosis.
In cirrhotic patients, 1 HRT parameter differed significantly compared to the control group; TO value in cirrhotic patients amounted to −0.385% v/s −0.920% in the control group. TS value did not differ significantly between cirrhotic patients and the control group. In spite of these differences, TO and TS values were within normal limits in a majority of study participants. Only 3 patients had abnormal TO values, and 4 patients had abnormal TS values. Other studied parameters, including NT-proBNP, did not differ significantly between patients with normal TO and TS and patients with abnormal TO or TS. Correlations between NT-proBNP and TO and TS values were not found in any of the studied groups. Interestingly, significant negative correlation between creatinine level and TO occurred, which may indicate that development of renal failure correlates with improvement in TO value. There are no data that may explain this phenomenon. Significant negative correlation was found between mean HR and TS. There are not at present any available data on heart rate turbulence in cirrhosis in the literature. In studies performed on patients with congestive heart failure by Szymanowska et al. [28], contrary to our results, no correlations were found between mean HR and HRT parameters (TO, TS). Authors of the cited study observed significant negative correlation between TS and BNP, but they did not observe correlations between TO and biochemical parameters. They also demonstrated a correlation between progression of heart failure and worsening of HRT parameters. Kawasaki et al. [29] studied patients with HCM; revealing that TO and TS stayed within normal limits (TO −2.1±3.4 vs. −1.1±2.9 vs. −1.4±5.1%; p=0.22; TS 18.0±13.9 vs. 10.6±8.6 vs. 16.6±9.7 ms/beat; p=0.00084, respectively). As mentioned above, myocardial lesion in HCM is similar to damage observed in cirrhotic cardiomyopathy. These results are similar to our results obtained from patients with cirrhosis. Kawasaki et al did not find significant differences in HRT parameters between patients with HCM and the control group. A significant difference was observed in TS for patients after MI vs. the control group. In cited work, HRT parameters stayed within normal limits during a 27-month observation of patients with HCM and did not affect survival. These observations suggest that HCM is not associated with HRT disturbances. HRT disturbances are present when myocardium is damaged due to ischemic heart disease.
Our studies indicate that worsening HRT parameters are associated with deterioration of cirrhosis assessed by Child-Pugh classification. This observation agrees with a previous study by Szymanowska et al, which demonstrated that worsening HRT parameters were correlated with deterioration of congestive heart failure estimated by NYHA scale. Thus, abnormalities in HRT (mainly TO) parameters may be associated with worse prognosis in cirrhotic patients.
Conclusions
To conclude, our study demonstrates that patients with decompensation of cirrhosis have elevated levels of NT-proBNP and left ventricle diastolic dysfunction. In a majority of study participants, TO and TS, or at least 1 of these parameters, stayed within normal limits. However, TO values in cirrhotic patients differed significantly from the control group.
Increased NT-proBNP level is a result of myocardium lesion typical for cirrhotic cardiomyopathy (mainly diastolic dysfunction) and not a result of biochemical changes and hyperkinetic circulation. Elevated NT-proBNP level can indicate risk of symptomatic heart failure development. NT-proBNP may be marker of cirrhotic cardiomyopathy. No correlations between NT-proBNP and TO and TS were found. Patients with normal TO and TS had significantly lower Child-Pugh class than patients with abnormal TO or TS. HRT parameters do not appear to be appropriate death predicators in cirrhosis decompensation. These parameters can be estimated in complex evaluation of autonomic disturbances that occurred in cirrhosis. This problem requires further studies.
Abbreviations
- HR
heart rate
- NT-proBNP
N-terminal fragment of brain natriuretic peptide (BNP)
- INR
international normalized ratio
- LA
left atrium
- LAd
left atrium diameter
- RV
right ventricle
- RVdd
right ventricle diastolic diameter
- LV
left ventricle
- LVEds
left ventricle end-diastolic diameter
- LVEsd
left ventricle end-systolic diameter
- IVS
intraventricular septum
- IVSdd
intraventricular septum diastolic diameter
- SPAP
Systolic Pulmonary Artery Pressure
- TAPSE
Tricuspid Annular Plane Systolic Excursion; E/A wave of mitral flow
- DT
deceleration time of E wave mitral inflow
- Vprop
propagation velocity of E wave mitral inflow
- HRT
Heart Rate Turbulence
- TO
Turbulence onset
- TS
Turbulence slope
- EF
Ejection fraction
Footnotes
Source of support: Departmental sources
References
- 1.Schmidt G, Malik M, Barthel P, et al. Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet. 1999;353:1390–1396. doi: 10.1016/S0140-6736(98)08428-1. [DOI] [PubMed] [Google Scholar]
- 2.Davies LC, Francis DP, Ponikowski P, et al. Relation of heart rate an blood pressure turbulence following premature ventricular complexes to baroreflex sensitivity in chronic congestive heart failure. Am J Cardiol. 2001;87:737–42. doi: 10.1016/s0002-9149(00)01493-4. [DOI] [PubMed] [Google Scholar]
- 3.Bauer A, Malik A, Schmidt G, et al. Heart Rate Turbulance: Standards of Measurement, Physiological Interpretation, and Clinical Use. J Am Coll Cardiol. 2008;52:1353–65. doi: 10.1016/j.jacc.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 4.Barthel P, Schneider R, Bauer A, et al. Risk stratification after acute myocardial infarction by heart rate turbulence. Circulation. 2003;108:1221–26. doi: 10.1161/01.CIR.0000088783.34082.89. [DOI] [PubMed] [Google Scholar]
- 5.Hallstrom JO, Stein PK, Schneider R, et al. Characteristics of heart beat intervals and prediction of death. Int J Cardiol. 2005;100:37–45. doi: 10.1016/j.ijcard.2004.05.047. [DOI] [PubMed] [Google Scholar]
- 6.Baik SK, Lee SS. Cirrhotic cardiomyopathy. Orphanet encyclopedia. Jan, 2005. http://www.orpha.net/data/patho/GB/uk-CirrhoticCardiomyopathy.pdf.
- 7.Hamoudi WA, Lee SS. Cirrhotic cardiomyopathy. Annals Hepatol. 2006;5:132–39. [PubMed] [Google Scholar]
- 8.Möller S, Henriksen JH. Cardiovascular complications of cirrhosis. Gut. 2008;28:59–69. doi: 10.1136/gut.2006.112177. [DOI] [PubMed] [Google Scholar]
- 9.Wong F. Cirrhotic cardiomyopathy. Hepatol Int. 2009;3:294–304. doi: 10.1007/s12072-008-9109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee F, Glenn TK, Lee S. Cardiac dysfunction in cirrhosis. Best Practice & Research Clinical Gastroenterology. 2007;21:125–40. doi: 10.1016/j.bpg.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Henriksen JH, Fuglsang S, Bendtsen F, et al. Dyssynchronous electrical and mechanical systole in patients with cirrhosis. J Hepatol. 2002;36:513–20. doi: 10.1016/s0168-8278(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 12.Toris GT, Bikis CN, Tsourouflis GS, et al. Hepatic encephalopathy: An updated approach from pathogenesis to treatment. Med Sci Monit. 2011;17:53–63. doi: 10.12659/MSM.881387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll JD, Hess O. Assessment of normal and abnormal cardiac function. In: Zipes DP, Libby P, Bonow RO, Braunwald E, editors. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 7th edn. Vol. 2. Philadelphia, PA: Elsevier Saunders; 2005. p. 498. [Google Scholar]
- 14.Aurigemma GP, Gassch WH. Clinical practice. Diastolic heart failure. N Engl J Med. 2004;251:1097–105. doi: 10.1056/NEJMcp022709. [DOI] [PubMed] [Google Scholar]
- 15.Kelbaek H, Eriksen J, Brynjolf I, et al. Cardiac performance in patients with asymptomatic alcoholic cirrhosis of the liver. Am J Cardiol. 1984;54:852–55. doi: 10.1016/s0002-9149(84)80220-9. [DOI] [PubMed] [Google Scholar]
- 16.Lee SS. Cardiac abnormalities In liver cirrhosis. West J Med. 1989;151:530–35. [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Song D, Lee SS. Cirrhotic cardiomyopathy. Gastroenterol Clin Biol. 2002;26:842–47. [PubMed] [Google Scholar]
- 18.Wong F, Siu S, Liu P, et al. Brain natriuretic peptide: is it a predictor of cardiomyopathy in cirrhosis? Clin Sci. 2001;101:621–28. [PubMed] [Google Scholar]
- 19.Henriksen JH, Gotze JP, Fuglsang S, et al. Increased circulatring pro-brain peptide (BNP) in patients with cirrhosis: relation to cardiovascular dysfunction and severity of disease. Gut. 2003;52:1511–17. doi: 10.1136/gut.52.10.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Readle-Hurst TM, Welsch C, Forestrier N, et al. Validity of N-terminal propeptide of the brain natriuretic peptide in predicting left ventricular diastolic dysfunction diagnosed by tissue Doppler imaging in patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2008;20:865–73. doi: 10.1097/MEG.0b013e3282fb7cd0. [DOI] [PubMed] [Google Scholar]
- 21.Yildiz R, Yildirim B, Karincaloglu M, et al. Brain natriuretic peptide and severity of disease in non-alcoholic cirrhotic patients. J Gastroenterol Hepatol. 2005;20:1115–20. doi: 10.1111/j.1440-1746.2005.03906.x. [DOI] [PubMed] [Google Scholar]
- 22.Pimenta J, Paulo C, Gomes A, et al. B-type natriuretic peptide is related to cardiac function and prognosis in hospitalized patients with decompensated cirrhosis. Liver International. 2010;30:1059–66. doi: 10.1111/j.1478-3231.2010.02266.x. [DOI] [PubMed] [Google Scholar]
- 23.Schneider R, Barthel P, Watanabe M. Heart Rate Turbulence on Holter. In: Malik M, Camm JA, editors. Dynemic Electrocardiography. Vol. 20. Blackwell Futura Publishing; London: 2004. pp. 190–93. [Google Scholar]
- 24.Watanabe MA, Schmidt G. Heart rate turbulence: A 5-year revive. Heart Rhythm. 2004;1:732–38. doi: 10.1016/j.hrthm.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Mandell MS, Lindenfeld J, Tsou M-Y, Zimmerman M. Cardiac evaluation of liver transplant candidates. World Journal of Gastroenterology. 2008;14:3445–51. doi: 10.3748/wjg.14.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernal V, Pascual I, Esquivias P, et al. Cardiac hemodynamic profiles and pro-B-type natriuretic Peptide in cirrhotic patients undergoing liver transplantation. Transplant Proc. 2009;41:985–86. doi: 10.1016/j.transproceed.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Raedle-Hurst TM, Welsch C, Forestier N, et al. Validity of N-terminal propeptide of the brain natriuretic peptide in predicting left ventricular diastolic dysfunction diagnosed by tissue Doppler imaging in patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2008;20:865–73. doi: 10.1097/MEG.0b013e3282fb7cd0. [DOI] [PubMed] [Google Scholar]
- 28.Szymanowska K, Piątkowska A, Nowicka A, et al. Clinical significance of heart rate turbulence assessment in patients with chronic heart failure. Kardiol Pol. 2008;66:1289–95. [PubMed] [Google Scholar]
- 29.Kawasaki T, Azuma A, Asada S, et al. Heart Rate Turbulence and Clinical Prognosis in Hypertrophic Cardiomyopathy and Myocardial Infarction. Circ J. 2003;67:601–4. doi: 10.1253/circj.67.601. [DOI] [PubMed] [Google Scholar]