Summary
Stress cardiomyopathy is characterised by reversible left ventricular dysfunction. It simulates an acute coronary syndrome (ACS), presenting with precordial pain or dyspnoea, changes of the ST segment, T wave, or QTc interval on electrocardiogram, and raised cardiac enzymes. Typical findings are disturbances of segmental contractility (apical hypokinesia or akinesia), with normal epicardial coronary arteries. The true prevalence is unknown, as the syndrome may be under-diagnosed; it is more common in postmenopausal women. There is usually a trigger in the form of physical or psychological stress. The electrocardiographic, echocardiographic, and ventriculographic changes resolve spontaneously over a variable period of time (from days to months). There are a number of pathophysiological theories, none of which has been shown to be definitive, suggesting that all of them may be involved to some extent. The prognosis is generally favourable, and recurrence is very rare.
Keywords: Takotsubo, left ventricular ballooning syndrome, stress cardiomyopathy, myocardial disfunction
Definition, History, and Epidemiology
Stress cardiomyopathy or Takotsubo syndrome (TS) is an acute, reversible disorder of the heart characterised by left ventricular dysfunction [1]. Its diagnosis continues to be controversial [1–8] (Table 1). The most widely accepted diagnostic criteria are those of the Mayo Clinic [5], which requires normality of the epicardial coronary arteries (Table 2). Other proposed diagnostic criteria admit non-significant coronary artery lesions [6] (Table 3) and, in the absence of coronary angiography, accept the results of echocardiography, cardiac magnetic resonance imaging, or isotope ventriculography [7] (Table 4). Kawai et al. [8] define this syndrome as a nosological entity in which there is acute ballooning of the apex of the left ventricle (LV) of unknown aetiology (Table 5).
Table 1.
Abe and Kondo criteria.
Major criteria:
|
Minor criteria:
|
Exclusion criteria:
|
Table 2.
Diagnostic criteria of the Mayo Clinic.
|
|
|
|
Table 3.
Prasad criteria.
|
|
|
|
Table 4.
Segovia Cubero criteria.
Previous conditions (both obligatory):
|
Diagnostic criteria:
|
| Confirmed TADS: major criterion or 2 or more minor criteria, including an angiographic criterion. Probable TADS: 2 or more minor criteria, with no angiographic criterion. TADS: transient apical dysfunction syndrome. |
Table 5.
Kawai criteria.
Exclusion criteria:
|
Diagnostic references:
|
The LV adopts the shape of a “takotsubo” (in Japanese, “tako” means octopus and “tsubo” means pot; takotsubo is the pot that Japanese fishermen use as an octopus trap). The syndrome receives this name because of the morphological similarity between this object (narrow neck and broad base with a globular form) and the LV during ventricular systole (on echocardiography and ventriculography) [9]. It is also known as ampulla cardiomyopathy, a name referring to the wide-bodied, narrow-necked container used in ancient Greece and Imperial Rome [8,10]. The morphology of the LV has led to a number of different names being used [8–20]. In general, to establish the diagnosis, it is accepted that other diseases of known aetiology (cerebrovascular pathology, phaeochromocytoma, viral or idiopathic myocarditis, and epicardial ischemic disease) must be excluded [8].
In the 1990s, the Japanese authors Dote and Sato [21] were the first to describe this syndrome, calling it “takotsubo syndrome”. In 2001, Tsuchihashi et al. [15] published the first Japanese series, and the syndrome appeared as a new nosological entity. Since that time, case reports and series have been published [3,22–26] outside Asia [3,11,15,24,27], in African-American and white populations in the United States [25,28–32], in Europe [33–36], and in Oceania [37], excluding interracial, cultural, and geographic interactions. Due to the types of studies available, its true incidence is unknown, varying between 0.7% and 4.87% [25–38], although it may be under-diagnosed due to a lack of clinical suspicion [39–46].
It is more common in women (70–100% vs. 5–30% in men) [3,11–13,15,20–26,35,41–54], particularly in postmenopausal women (78–85.7%) [35,38]. Although it has been reported in younger patients, only 3% are under 50 years of age, and most of these are white individuals [30,34,35–55] and the mean age at onset is over 60 years [3,5,11,13,15,22–26,28,31,33, 38–41,51,54–62]. A trigger in the form of a stressful event can usually be detected in 27–100% [15–17,33,48,51,64,65], and this could worsen the prognosis [60]; this event may be physical [3,17,33,42,46,64,65] or psychological [3,11,15,22,24,26,33,49,64–67]. The trigger is not related to sex, but whites appear to be more susceptible to emotional triggers than Asians [66]. In contrast, a stressful trigger is only found in about 3% of cases of ischemic cardiopathy [41]. There is no clear a risk factor associated with TS, though the most frequent association appears to be systemic hypertension (13–80%), followed by hyperlipidemia (0–60%), diabetes mellitus (0–33%), smoking (0–50%), and a family history of cardiovascular disease (0–50%) [3,11,15,22,24,26,33,49,64–66,68].
Clinical Presentation and Complications
Although the syndrome may initially be asymptomatic (2–20%) [27,48], the clinical presentation is usually similar to an acute coronary syndrome. The most common symptom is precordial pain [6,7,34,69], although this may be atypical; it is reported in 50–100% of cases at onset [3,5,15,22–26,33–36,48,50,51,64–66,68,69] and is more common in whites [66]. African-Americans more commonly present with respiratory insufficiency [31]. Other presentations include abdominal symptoms (8–10%) [32,34,41,65], myalgia (0.5–3.33%) [24,27,66], dyspnea (7–60%) [6,15,31,32–36,48,64–68], palpitations (5–9%) [66], presyncope or syncope [6–22%], usually associated with an arrhythmia [5,13,21,32–36,42,46,49,50,70], or even cardiorespiratory arrest (0.5–8%) or sudden death (3%) [70,71]. Heart failure is a common form of onset of the syndrome (11–28%), presenting as acute pulmonary edema (3–50%) [5,6,15,35,48,65,66,68,69] or cardiogenic shock (2–46%) [6,15,16,21–26,31,32,35,36,46,53,72,73]. It was erroneously initially attributed to respiratory insufficiency (7–69%) due to a respiratory exacerbation of chronic obstructive pulmonary disease, pneumonia, or even respiratory distress [6,12,22,31,32,66,74–77].
All types of arrhythmias have been reported, with a very variable incidence (estimated frequency, 5.7%, which could be lower with beta-blockers); the most common arrhythmia is probably extrasystoles [78]. These arrhythmias, and the conduction blocks in particular, are usually transitory, and the prognosis appears to depend more on the degree of ventricular dysfunction than on the arrhythmia [79]. The causes of these arrhythmias are unknown, and a number of theories have been proposed. Furushima et al. [80], through electrophysiological study of an ST with a long QTc interval and torsade de pointes, which recurred after normalisation of the echocardiographic and electrocardiographic disturbances, observed changes in the myocardial-epicardial repolarisation gradients of the LV. Fisher et al. [81], using magnetic mapping by cardiac magnetic resonance, observed lesions similar to myocarditis or ischemia, and disturbances of repolarisation that persisted for longer than the structural changes (more than 6–12 months). When the arrhythmias develop during the resolution phase, they suggest that normalisation of ventricular function depends on a slow electric remodelling; this would explain recurrence of the arrhythmias and the high risk of sudden death despite complete recovery of the electrocardiographic and echocardiographic changes [82–88].
Other, rarer complications have been reported, such as pneumothorax (0.5–8.3%) [46,66], pericarditis in the subacute [29] or recovery [89], phases of the syndrome, and mechanical complications such as rupture of the free wall of the LV (0.5%) [5,66,90–93], perforation of the interventricular septum (0.5%) [66], or rupture of the papillary muscles [94].
The area of dyskinesia may give rise to intracavity thrombi, with an incidence of 2.5–9%; the thrombi are usually found at the apex, and thromboembolic phenomena have been reported in 0.8–14% of cases of TS [5,25,34,93,95].
The Electrocardiogram
Although the initial electrocardiogram (ECG) may be non-specific or normal, the majority (11–100%) present elevation of the ST segment, particularly affecting the anterior wall (36–100%) and, more rarely, the inferior or lateral walls (4–50% and 5–70%, respectively) or in aVR [3,6,7,13,15,21–26,33–36,50,54,64–66,70,96–99]; ST segment depression has been reported in 6–23% [13,64]. These changes may be very dynamic [98,99]. An absence of R-wave progression in the anterior wall may also be seen (7–32%) [17,22]. T wave inversion is very common (17–100%) [7,13,15,21,33–36,64,66,96–98], and may be associated with a poorer prognosis [62,98]; however, peaked T waves sometimes predominate (86%) [64,98]. A long QTc interval is seen in 50–100% [3,7,12,21–26,64]. Pathological Q waves are observed in 20–63% [6,7,15,21–26,33–44,64] and new bundle branch blocks develop in approximately 6–8% [5,21–25]. Racial differences exist in the electrocardiographic changes, with a higher frequency of inverted T waves in whites, and a higher incidence of ST elevation in Asians [64]; in blacks the initial ECG is more commonly normal, with a rapid progression to T wave inversion and a prolonged QTc interval [31]. The course of the changes [17,25,36,62,64,96–98] usually shows ST elevation or depression for 1 to 2 days, and there may even be new intraventricular conduction disturbances [6,17,25,36]. In the subacute phase, the ST changes normalise and disturbances of the QTc interval develop but disappear rapidly; T wave inversion develops in parallel with lengthening of the QTc interval but is of longer duration (1–4 months) [15,36]. Pathological Q waves (myocardial stunning) may be observed in the chronic phase, but disappear more rapidly than in cardiac ischemia, although they may persist indefinitely in 10% of cases [24].
T wave inversion and the long QTc interval persist after normalisation of the echocardiographic images, whereas R wave progression normalises in parallel with the improvement in motility [17]. All these electrocardiographic changes usually resolve within 3 weeks to 1 year [3,13,17,22,25,33,36,51,62,64,96–98].
Unsuccessful attempts have been made to differentiate TS from ACS based on ECG findings [56,91,100–104]. Ogura et al. [101] and Segovia Cubero et al. [7] observed that there are fewer pathological Q waves, a longer QTc segment, less ST depression and a higher V4-V6 to V1-V3 ratio of ST elevation in TS; they considered that an ST segment with a V4-V6/V1-V3 ratio greater than 1 had a high sensitivity and specificity for TS. Other authors observed that ST elevation is greater in patients with myocardial infarction [102–104], whereas ST elevation of less than 1.75 mm in V2 or less than 2.5 mm in V3 is more specific to TS [103].
Cardiac Biomarkers
Although no elevation of the biomarkers has been reported in about 5% of cases of this syndrome [14,25,64], there is typically a slight increase in the creatine kinase MB and in troponins I and T, although at lower levels than occur with acute myocardial infarction [5,15,21,33,36,37,41,64,66,102–104]. The echocardiographic, ventriculographic, and even electrocardiographic changes usually indicate severe cardiac disturbances, but these are not reflected in the enzyme levels, which are closer to values found in conditions such as myopericarditis or the non-ischemic cardiomyopathies [97,105]. At the present time, the cut-off point for the enzyme levels to differentiate between TS and ACS remains to be defined [106]; however, the majority of authors have not found increases of the cTnI of over 4.5 ng/ml or of CPK-MB of over 10.5 U/L, and higher values are usually suggestive of ischemia [106].
Measurement has also been performed of the plasma levels of brain natriuretic peptide (BNP) [106–109] and NT-proBNP [110], which have been correlated with the degree of cardiac failure and the fall in left ventricular ejection fraction (LVEF), although their correlation with survival is not known.
There is no evidence of a rise in catecholamines in 30% of patients in the acute phase [33,111]. When there is a rise above normal, it is very marked [46] for several weeks and falls progressively. Elevation of C-reactive protein (CRP) is detected in 50% of patients and is a sign of poor prognosis and predictor of mortality [36,64,112] in TS.
Echocardiography
The typical finding is of apical ballooning of the left ventricle. This is due to akinesia, hypokinesia, or dyskinesia of the apical and middle segments of the LV and hyperkinesia of the basal segments [8,24,28] (Figure 1). Some disturbances of contractility are similar to those that occur in systemic hypertension due to an increase in afterload [113]. The LVEF is low or very low from the initial phase, with values below 30% in some cases [16,23,26,32,105] and up to 75% [64]. In the subacute phase, global and segmental systolic function and the electrocardiographic changes improve over a period of days, weeks, or a few months, until they stabilise with an LVEF above 50% [5,11,15,16,21–24, 56–69,114–119]. In general, echocardiography is normal at 1 year [7,14,21–24]; the rate of recovery is more rapid in Asians than in whites [120].
Figure 1.
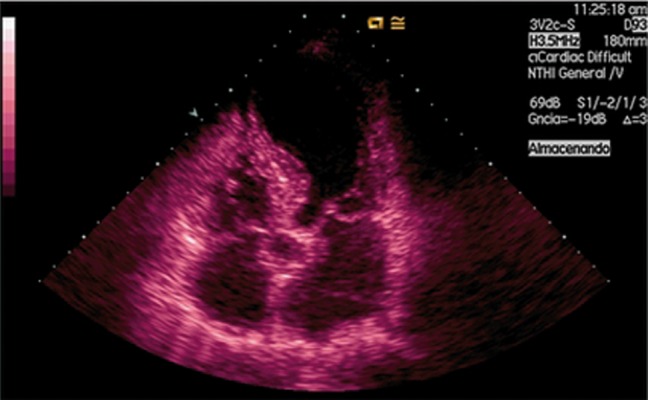
Echocardiographic image in apical ballooning which is also observed mitral prolapse (P2).
Despite this, there is a noticeable lack of use of quantitative echocardiographic techniques such as the strain, strain rate, or velocity vector imaging for the diagnosis and follow-up of these patients [121,122[ (Figure 2). The descriptions of diastolic function mainly use transmitral flow [123], and ignore information from the pulmonary veins or from tissue Doppler studies, and the diastolic dysfunction detected could be spurious. Furthermore, little use has been made of intravenous echocardiographic contrast, which has enabled intraventricular thrombi [34] and abnormalities of the coronary microcirculation to be detected, or dobutamine echocardiography, which can reveal underlying dysfunction [123].
Figure 2.
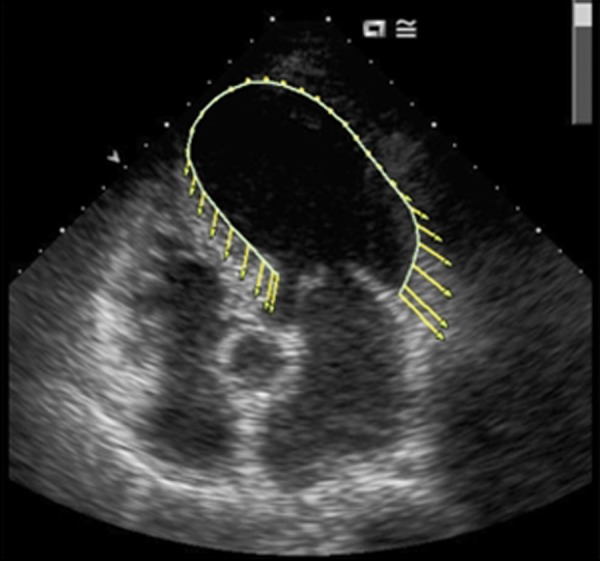
This is hybrid speckle tracking imagen of takotsubo patient. There is a pathological displacement curve in the apical segments and increased in the basal segments.
Changes in the LVEF are initially similar to those seen in ischemic heart disease, although recovery is better in patients with TS than in those with ACS [41]. Functional valve disturbances can also develop, including: 1) mild-moderate tricuspid regurgitation (50%) [54] associated with pulmonary hypertension (mean pulmonary arterial pressure 46.6±10.5 mmHg) [54] in 33%; 2) mild-moderate aortic insufficiency (12.5%) [54]; and 3) mitral insufficiency of grade 2 or higher, present in 9.7–62.5% [31,41]. Systolic anterior movement may be observed (6.67%) [54] and a gradient in the LV outflow tract (43–100 mmHg) (20–35.71%) [54,68]. These findings are usually associated with a Killip III–IV infarct (50%) and higher levels of cardiac enzymes [68]. Mitral insufficiency leads to a lower LVEF and a slow ventricular recovery (79%) [68], with complete recovery of the syndrome occurring at around 6 months, although 8.1% continue to present mitral insufficiency of grade 2/4 or higher [124]. Severe mitral insufficiency is a cause of death in 14.29% [68]. Tricuspid insufficiency reverts and the pulmonary hypertension disappears in 13.33% [54].
Catheterisation
Normality of the epicardial coronary arteries is normally a requirement for the diagnosis of TS [4,7,13,74]. Overall, it is considered that the number of vessels affected in TS is zero compared to 1.6±0.7 in ACS; however, other authors have found stenoses not greater than 50% in 10–21% [21], or even greater than 50% [5,34,125]. Some coronary artery lesions have a chronic morphology with no angiographic evidence of acute complication by virtue of its prevalence in the population at risk, whereas others are acute lesions giving rise to ischemia associated with the TS [95,125]. Angiography has revealed spontaneous coronary artery spasm in 1.4–10% [25,94,126], induced spasm in 4.5–71% [3,11,13] and vasoconstriction in 21–100% [3,21]. Asian individuals are more likely to develop vasospasm, but this likelihood may be due to the widespread use of provocation tests in Japan [127]. Ventriculography shows the same changes as echocardiography (Figure 3).
Figure 3.

Ventriculography image of apical ballooning which shows apical akinesia and basal hyperkinesis.
The TIMI frame count is higher in TS [2,3,21,22,34,128], which may be caused by diffuse coronary microvascular dysfunction, although it usually normalises after functional recovery [2].
Angiographic blush is present in two-thirds of patients at onset of the syndrome, and the severity is determined by the magnitude of the rise in the troponin levels and by the ECG changes [48,97]. This leads us to question whether TS is a distinct entity or is a stage of ischemia [129,130].
Imaging Studies
Multislice computed tomography (mCT) and cardiac magnetic resonance imaging (cMRI) are also useful in the diagnosis of apical ballooning. mCT is a non-invasive test that is suitable for the differential diagnosis between infarction and TS, showing not only the coronary artery circulation and its lesions, but also disturbances of cardiac contractility. According to cMRI, the myocardium initially affected is viable [131]. Delayed enhancement with gadolinium is usually present in myocarditis and ACS [2,25,132] and does not occur in 95% of patients with TS. However, cases of TS with delayed gadolinium uptake have been reported [133], giving rise to doubts of ischemia. Myocardial SPECT reveals a fall in technetium-99m tetrofosmin uptake in the acute phase, indicative of a decrease in myocardial perfusion [100,135,136]. Reversible alterations of a number of radioactive tracers, such as 123I-BMIPP and 123I-MIBG, could indicate a reduction in fatty acid and glucose metabolism, alterations of coronary microcirculation, or a possible imbalance in the sympathetic innervation between the apical and basal regions [137].
Biopsy
There is no specific histopathological pattern in TS. Focal lesions of myocytolysis are usually observed, with a reduction in the number of myocytes and a polymorphonuclear infiltrate with fibrosis [3,11,24,27]. However, there is controversy over the findings in the acute phase, with an inflammatory infiltrate [43] or myocyte necrosis [33] being found. Wittstein et al. [26] described bands of necrotic contraction without myocyte necrosis, similar to that observed in patients who die in tragic circumstances and in patients with catecholamine-induced cardiomyopathy [2,26], although these changes have also been observed in patients without inotropes and with no increase in the catecholamines [104]. Fatty infiltration of the myocardium or cardiac steatosis suggests focal damage [37,78,112,121] due to metabolic changes that can progress to a cardiomyopathy that usually precedes ventricular systolic dysfunction, as occurs in patients with type 2 diabetes mellitus. Rather than its myocardial distribution, the characteristics of the fat are similar to the infiltration in arrhythmogenic dysplasia [22], making it necessary to include this disorder in the differential diagnosis. Few biopsies of the LV are performed and, although the results are not specific, it does appear that there is an increased accumulation of periodic acid-Schiff-positive material, suggestive of myocardial hibernation [121].
Aetiology and Pathogenesis
The aetiology and pathogenesis of this disorder are unknown. One theory is the possible existence of myocardial stunning secondary to a primary metabolic disturbance characterized by dysfunctional metabolism of cardiomyocytes [138], affecting either glucose [139] or fatty acid metabolism, or due to mitochondrial disturbances [140–142]. Another possibility is stress-induced catecholamine release, producing cardiac stunning [143,144] through a direct lesion of the myocytes and vasoconstriction [24] secondary to increased calcium [142], which activates cAMP, causing damage to the cardiac cells and favouring free radical release. The apical region is the most vulnerable area due to the large number of adrenergic receptors [144]. Structurally, the third layer of the myocardial architecture is absent at the apex, and this region presents a rapid loss of elasticity after excessive dilatation. It is also more susceptible to developing ischemia due to a precarious coronary blood flow, as it is in a borderline region of the coronary circulation [18]. Segmental alterations of contractility may be due to the local release of catecholamines in this frontier region [145]. There are fewer sympathetic terminals in the base of the heart than at the apex; this makes the apex more vulnerable to a rapid rise in catecholamine levels and means that the apical myocardium has an increased response to sympathetic stimulation. In situations of stress, functional and/or structural differences in regional coronary blood flow may develop due to an alteration in the apex-base perfusion gradient, with a deficit developing at the apex [146].
Due to the oestrogen deficit, women in the perimenopausal and postmenopausal period have a higher risk of developing angina due to microvascular dysfunction associated with normal coronary arteries; this is caused by the influence of these hormones on the sympathetic system [147]. In a situation of stress, men release a larger quantity of catecholamines and are more sensitive to vasoconstriction; however, women are more vulnerable to sympathetic mediators, present more episodes of angina, and develop more marked disturbances of cardiac contractility and of LVEF [148,149]. However, it appears that a lack of oestrogens could be a primary cause of TS due to an indirect action on the nervous system or a direct action on the heart [150].
Peripheral endothelial function and microvascular function have been studied in the acute phase of TS, and no dysfunctions have been observed [151]. Another hypothesis that has been proposed is spasm of the epicardial arteries. It appears unlikely that the left ventricular dysfunction that occurs in this syndrome could be due only to coronary artery spasm. The disorder of motility affects the territories of the 3 coronary arteries. One possibility could be spasm of all the arteries at the same time [3,24,34], or vasoconstriction due to an increase in sympathetic tone caused by the stress, and that this provokes microvascular dysfunction [21,142].
Acute myocarditis forms part of the differential diagnosis [3,11,13,24,27], a theory based on the fact that in some cases positive results for viruses have been found in biopsies and serological tests [34,152]; however, there is no microbiological agent related with TS.
The pathogenesis of the dynamic gradient that may be detected in TS could be due to a geometric susceptibility of the heart – a sigmoid or bulging interventricular septum, a small LV [35,153–155], or abnormal mitro-aortic and septo-aortic angles [156]. Elderly women have a greater tendency to develop hypertrophy of the basal anterior septum. The angle of the septum is responsible for the increase in the speed in the outflow tract and simulates a hypertrophic cardiomyopathy [157]. It is also associated with an abnormal orientation of the mitral valve due to flaccidity, deformity of the mitral valve, false chordae, disturbances of the papillary muscles, or systolic anterior movement [158–160]; more rarely the obstruction is secondary to hyperdynamic contraction of the basal segments of the LV. The onset of symptoms usually occurs in the context of hypovolemia or major adrenergic stimuli. In some circumstances, the dynamic obstruction precedes apical ballooning, and has a good prognosis [35,155,160].
Ibañez et al. [161] used intravascular ultrasound (IVUS) to study the left anterior descending artery in patients with TS and found that all had unstable atheromatous plaques with the possibility of early spontaneous reperfusion. The duration of ischemia was short and the myocardial damage gave rise only to a small elevation of the enzymes; left ventricular dysfunction was due to stunning. This spontaneous lysis of the thrombus left the coronary arteries with a normal angiographic appearance. Another hypothesis by Ibañez et al. [162] is of a susceptible coronary vascular tree, as in the case of recurrent epicardial arteries. These abnormalities have also been described by other authors, with an incidence of 72–92% in normal hearts [3,5,162].
Another theory is the genetic basis [63,163]. Phenotypically, Mediterranean and Asian women, due to their constitutions and the presence of hypoplastic apical epicardial arteries, have a greater susceptibility to this dysfunction. However, genetic analyses have not identified a mutation or polymorphism [164].
Atypical Forms
At the present time, one-third of patients are reported to present a variant form of the disorder [130]. One of the most important variants is inverted takotsubo [17,40,50,52,58,60,76,165,166,170], which Bonnemeier called artichoke heart [171]; the apex is hypercontractile and the basal segments are akinetic. Aubert et al. [172] described transient midventricular ballooning [30] or hawk’s beak [173], with movement of the apex and basal segments and akinesia of the middle region. Reuss et al. [174] described apical akinesia with normal midventricular movement and apical hypercontractility. Kim et al. [175] described basal akinesia, normal midventricular movement, and basal hypokinesia (Table 6) [2]. Midventricular disorders are detected in 7–40% of cases, with 5% in the inverse syndrome and 7% in the other forms [40,166].
Table 6.
Atypical forms of Takotubo.
|
The origin of these differences is unclear. It may be due to a variation in autonomic innervation and/or in the distribution of adrenergic receptors within the heart due to an irregular subendocardial model [30,58]. It is thought that apical hypercontractility is due to a reduction in the density of synaptic terminals in the apex or that, after the akinesia, the apex is the first area to show a functional recovery after a major, initially global disorder [38]. The possibility of a migratory TS has also been proposed, in which apical hypercontractility constitutes one of the periods of a single syndrome [165]. Rupture of an atheromatous plaque cannot itself explain the variants [58,59,130]. Compared to classic TS, variant TS is more common in premenopausal and younger women [166], and there is greater preservation of the LEVF [166]. The atypical forms are associated with fewer risk factors, less T wave inversion, lower enzyme levels, and a rapid recovery from the dysfunction [166]. The midventricular form presents more Q waves and more hypotension than typical TS, which is associated initially with a greater degree of cardiogenic shock and heart failure [167]. Cardiac magnetic resonance imaging reveals greater involvement of segments 1–12 in the atypical forms and of segments 7–17 in the classic form [58]. There are no differences in the TIMI between the classic and midventricular forms [166], and there are also no differences in improvement [58].
Biventricular Involvement
Nyui et al. [168] were the first to describe TS with right ventricular dysfunction, and this has subsequently been corroborated by other authors [5,43,54,64,169–172]. There are no clinical differences in these patients [130,169], but there is a greater degree of systolic dysfunction and heart failure [170,172], greater dilatation of the right ventricle (RV), greater involvement of the lateral and inferior segments [172], more marked pleural effusion [172], a greater need for hemodynamic support and cardiopulmonary resuscitation [129], and a longer hospital stay [129]. Severe right apical dysfunction due to hypokinesia occurs in 14.29% of cases. Biventricular involvement is present in 26–40% of cases [130,169,171,172].
Disturbances of contractility in the right-sided cavities may be indistinguishable from those present in chronic or acute diseases such as pulmonary thromboembolism (inverted McConnell sign) [171], and there is early apical akinesia [28]. The mechanisms implicated in right-sided dysfunction are probably related to catecholamines.
Treatment
A correct diagnosis will avoid treatment of ischemic heart disease [25], which has not been shown to be of any benefit in TS and could give rise to adverse effects [7,18]. At the present time, treatment is symptomatic and, as with other cardiomyopathies, is determined by the complications occurring during the acute phase [20–29,56].
The use of intra-aortic balloon pump (IABP) support has been required [31,35,56], and even cardiopulmonary support techniques and renal replacement therapy such as continuous venovenous hemodiafiltration. The use of inotropes is controversial due to the increase in circulating catecholamines [25]. Levosimendan [88,173] may be beneficial for its inotropic and vasodilator effects. IABP is required by 8–46% of patients, less than in the ACS [41]. Up to 36.36% of patients require vasoactive drugs [35,54], and inotropes are used in 20–43.75% [15,21]. Short-term anticoagulation may be considered, at least until recovery of ventricular function. The implantation of defibrillators is controversial; they are implanted in 2.5–8.3% of cases [46,50], but the number of arrhythmogenic events registered after a year of follow-up could be zero [70].
In the case of left ventricular outflow obstruction with hemodynamic repercussion, treatment should be given with beta-blockers, alpha-adrenergic agents such as phenylephrine, and volume expansion; calcium channel blockers may be used to reduce the outflow gradient. Most important in these cases is to treat the trigger and to recognise the condition in order to avoid treatment with nitrites or inotropes [152–160,167]. The use of calcium channel blockers such as diltiazem or verapamil may be indicated if vasospasm is suspected [161]. In the case of functional mitral insufficiency, initial treatment may be conservative; 36% require IABP and valve replacement may even be necessary [68].
Long-term treatment is currently undefined [40]. It is thought that it may be appropriate in order to reduce myocardial stunning caused by catecholamines and that it would theoretically avoid recurrences. However, there is no evidence to support the use of chronic pharmacological treatment except in cases of cardiac dysfunction, despite the fact that treatment could alter the incidence of recurrence [25,174]. Experimental studies are evaluating the controversial benefit of oestradiol [175], ranolazine [176], and the reduction in the frequency of recurrence through the administration of beta-blockers [177], but beta-blocking drugs were not absolutely protective [178].
Critical Care Medicine and Takotsubo Syndrome
In 28% of cases, the apical ballooning syndrome develops in critically ill patients with no primary heart disease [179,180], but there are few studies on this subject due to a lack of diagnosis [2]. The situation of stress to which the critically ill patient is subjected acts as a trigger. Park et al. [179] studied 92 patients admitted to an intensive care unit and, through the use of echocardiography at the time of admission, with repetition a few days later, observed that 28% of patients presented echocardiographic abnormalities compatible with TS, with a reduction of the LVEF, and sepsis as the variable associated with cardiac dysfunction. This phenomenon has also been observed in critically ill patients with other diseases [36] and even after cardiopulmonary resuscitation [181]; these patients present echocardiographic signs of myocardial dysfunction, with an initially reduced LVEF that improves over time, as do the electrocardiographic disturbances. Haghi et al. [180] included 6 patients with a diagnosis of TS. All the patients presented electrocardiographic and echocardiographic disturbances with an initially reduced LVEF and normal angiography.
The true prevalence is unknown. The age is similar to that of patients outside intensive care units (63–68 years). Only 35–50% are women [36]. Clinically, the typical symptoms are not usually present, possibly due to the state of sedation-analgesia under which these patients are usually maintained, or due to the hemodynamic situation or severity of the critically ill patients. Occasionally patients refer precordial discomfort [180], although they are more likely to present pulmonary edema, ischemic changes on the ECG, sometimes detected by telemetry, arrhythmias such as ventricular tachycardia, a moderate rise in the biomarkers, or hemodynamic disturbances. The most prevalent risk factor is systemic hypertension [36]. The echocardiographic, electrocardiographic, and enzyme changes normalise over a period that varies between 1 and 6 months [36,179–181]. Mortality is somewhat lower: 16.67–52% [48,51,52], with higher figures than in patients not admitted to these units; the cardiac dysfunction possibly affects the prognosis. There is a growing need for vasoactive drugs [180]: 54.55–83.33% [36,180,181]. The etiology and pathogenesis are believed to be similar to other forms of TS [36,180,181].
The differential diagnosis must be made with stress cardiomyopathies (of the critically ill patient, catecholamine-induced, neurological, and various respiratory insufficiencies) [2,182]. The cardiac dysfunction that develops could be an expression of a previous disease or the intercurrent situation that the patient is suffering (Table 7), or procedures that the patient requires during admission to the intensive care unit (endotracheal intubation, mechanical ventilation, tracheostomy, etc), and not a true TS. The problem is in differentiating whether the disorder is due to the patient’s underlying disease, is a TS, or if the 2 are the same thing. The end result is that the association with cardiac dysfunction can worsen the prognosis. Clinically, the subtlety of the initial symptoms, the lack of suspicion, and the low level of use of bedside echocardiography, means that diagnosis will be delayed and that treatments may be used erroneously and, on occasions, even with a risk of causing harm [36].
Table 7.
Cardiac dysfunction.
Structural cardiomyopathies:
|
Prognosis and Recurrence
Complications are estimated to occur in 18.9% of cases of TS, with no racial differences [66]. Mortality varies between 0% and 12% [5,6,13,15,22,25,32,35]. There are no long-term studies that provide approximate figures. Tsuchihashi et al. [15] estimate in-hospital mortality at 1%, and mortality at 1 year of follow-up that reaches 2%. Donahue et al. [66] observed that older patients die earlier and that whites had a higher mortality than Asians (6% vs. 1.7%). Parodi et al. [68] detected a higher mortality among coronary patients (14% vs. 3%). Nuñez-Gil et al. [124] did not observe any in-hospital deaths, but they did detect a mortality of 3.2% during follow-up (35 months), although these figures are lower than for ACS (hospital mortality: 6.5%; mortality during follow-up for 35 months: 17.2%). Other authors have not analysed mortality in their studies [21,24,26,42]. Morbidity and mortality are lower in TS patients than in patients with ACS, and left ventricular function could determine the prognosis [21,42,60].
Recurrence is rare but has been reported [63,74]. Clinically, the syndrome reappears as precordial pain and, morphologically, can vary in the type of echocardiographic or ventriculographic presentation [74]. The time to recurrence varies between 3 months and 13 years [15,21,25,63]. The incidence does not exceed 13% [2,5,6,15,21–25,66,68,124,185] and, as a rule, recurrence is not observed [3,11,33,36,64,68,78,89,129]. There is also doubt about whether all recurrences are TS [183]. At the present time, reasonable doubt may still be expressed about the complete reversibility of a stress cardiomyopathy [184,186].
Footnotes
Source of support: Self financing
References
- 1.Maron BJ, Towbin JA, Thiene G, et al. Contemporary Definitions And Classification Of The Cardiomyopathies: An American Heart Association Scientific Statement From The Council On Clinical Cardiology, Heart Failure And Transplantation Committee; Quality Of Care And Outcomes Research And Functional Genomics And Translational Biology Interdisciplinary Working Groups; And Council On Epidemiology And Prevention. Circulation. 2006;113:1807–16. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 2.Bybee KA, Prasad A. Stress-Related Cardiomyopathy Syndromes. Circulation. 2008;118:397–409. doi: 10.1161/CIRCULATIONAHA.106.677625. [DOI] [PubMed] [Google Scholar]
- 3.Abe Y, Kondo M, Matsuoka R, et al. Assessment of Clinical Features in Transient Left Ventricular Apical Ballooning. J Am Coll Cardiol. 2003;41:737–42. doi: 10.1016/s0735-1097(02)02925-x. [DOI] [PubMed] [Google Scholar]
- 4.Abe Y, Kondo M. Apical Ballooning Of The Left Ventricle: A Distinct Entity? Heart. 2003;89:974–76. doi: 10.1136/heart.89.9.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bybee KA, Kara T, Prasad A, et al. Systematic Review: Transient Left Ventricular Apical Ballooning: A Syndrome That Mimics ST-Segment Elevation Myocardial Infarction. Ann Intern Med. 2004;141:858–65. doi: 10.7326/0003-4819-141-11-200412070-00010. [DOI] [PubMed] [Google Scholar]
- 6.Prasad A. Apical Ballooning Syndrome. An Important Differential Diagnosis of Acute Myocardial Infarction. Circulation. 2007;115:e56–59. doi: 10.1161/CIRCULATIONAHA.106.669341. [DOI] [PubMed] [Google Scholar]
- 7.Segovia Cubero J, Peraira Moral R. Transient apical ballooning syndrome: a transition towards adulthood. Rev Esp Cardiol. 2004;57:194–97. [in Spanish with English abstract] [PubMed] [Google Scholar]
- 8.Kawai S, Kitabatake A, Tomoike H, et al. Guidelines For Diagnosis Of Takotsubo (Ampulla) Cardiomyopathy. Circ J. 2007;71:990–92. doi: 10.1253/circj.71.990. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa K. “Takotsubo” Cardiomyopathy A Syndrome Characterized by Transient Left Ventricular Apical Ballooning that Mimics the Shape of a Bottle Used for Trapping Octopus in Japan. Intern Med. 2004;43:275–76. doi: 10.2169/internalmedicine.43.275. [DOI] [PubMed] [Google Scholar]
- 10.Inoue F, Takaoka M, Kumura H, et al. So-Called “Ampulla” Cardiomyopathy Associated with Vasospasm Compared with Acute Myocardial Infarction Showing Similar Abnormal Left Ventricular Wall Motion: Two Case Reports. J Cardiol. 2002;39:29–38. [in Japanese with English abstract] [PubMed] [Google Scholar]
- 11.Kawai S, Suzuki H, Yamaguchi H, et al. Ampulla Cardiomyopathy (“Takotsubo” Cardiomyopathy) – Reversible Left Ventricular Dysfunction: with ST Segment Elevation. Jpn Circ J. 2000;64:156–59. doi: 10.1253/jcj.64.156. [DOI] [PubMed] [Google Scholar]
- 12.Owa M, Aizawa K, Urasawa N, et al. Emotional Stress-Induced “Ampulla Cardiomyopathy”: Discrepancy Between the Metabolic and Sympathetic Innervation Imaging Performed During the Recovery Course. Jpn Circ J. 2001;65:349–52. doi: 10.1253/jcj.65.349. [DOI] [PubMed] [Google Scholar]
- 13.Yamasa T, Ikeda S, Ninomiya A, et al. Characteristic Clinical Findings of Reversible Left Ventricular Dysfunction. Intern Med. 2002;41:789–92. doi: 10.2169/internalmedicine.41.789. [DOI] [PubMed] [Google Scholar]
- 14.Movahed MR, Donohue D. Review: Transient Left Ventricular Apical Ballooning, Broken Heart Syndrome, Ampulla Cardiomyopathy, Atypical Apical Ballooning, or Tako-Tsubo Cardiomyopathy. Cardiovasc Revasc Med. 2007;8:289–92. doi: 10.1016/j.carrev.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchihashi K, Ueshima K, Uchida T, et al. Transient Left Ventricular Apical Ballooning without Coronary Artery Stenosis: A Novel Herat Syndrome Mimicking Acute Myocardial Infarction. Angina Pectoris Myocardial Infarction Investigations in Japan. J Am Coll Cardiol. 2001;38:11–18. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 16.Pavin D, Le Breton H, Daubert C. Human Stress Cardiomyopathy Mimicking Acute Myocardial Syndrome. Heart. 1997;78:509–11. doi: 10.1136/hrt.78.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharkey SW, Lesser JR, Maron MS. Stress Cardiomyopathy. J Am Coll Cardiol. 2007;49:921. doi: 10.1016/j.jacc.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Brandspiegel HZ, Marinchak RA, Rials SJ, et al. A Broken Heart. Circulation. 1998;98:1349. doi: 10.1161/01.cir.98.13.1349. [DOI] [PubMed] [Google Scholar]
- 19.Khallafi H, Chacko V, Varveralis N, et al. “Broken Heart Syndrome”: Catecholamine Surge Or Aborted Myocardial Infarction? J Invasive Cardiol. 2008;20:e9–13. [PubMed] [Google Scholar]
- 20.Gueffet JP, Langlard JM, Burban M, et al. Can One Die of Sorrow? Arch Mal Coeur Vaiss. 2001;94:1413–17. [in French with English abstract] [PubMed] [Google Scholar]
- 21.Dote K, Sato H, Tateishi H, et al. Myocardial Stunning Due to Simultaneous Multivessel Coronary Spasms: A Review of Five Cases. J Cardiol. 1991;21:203–14. [in Japanese with English abstract] [PubMed] [Google Scholar]
- 22.Desmet WJ, Adriaenssens BF, Dens JA. Apical Ballooning of the Left Ventricle: First Series in White Patients. Heart. 2003;89:1027–31. doi: 10.1136/heart.89.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hertting K, Krause K, Härle T, et al. Transient Left Ventricular Ballooning In A Community Hospital In Germany. Int J Cardiol. 2006;112:282–88. doi: 10.1016/j.ijcard.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Kurisu S, Sato H, Kawagoe T, et al. Tako-Tsubo-Like Left Ventricular Dysfunction With ST-Segment Elevation: A Novel Cardiac Syndrome Mimicking Acute Myocardial Infarction. Am Heart J. 2002;143:448–55. doi: 10.1067/mhj.2002.120403. [DOI] [PubMed] [Google Scholar]
- 25.Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and Reversible Cardiomyopathy Provoked By Stress in Women from the United States. Circulation. 2005;111:472–79. doi: 10.1161/01.CIR.0000153801.51470.EB. [DOI] [PubMed] [Google Scholar]
- 26.Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral Features Of Myocardial Stunning Due To Sudden Emotional Stress. N Eng J Med. 2005;352:539–48. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 27.Akashi YJ, Nakazawa K, Sakakibara M, et al. The Clinical Features Of Takotsubo Cardiomyopathy. Q J Med. 2003;96:563–73. doi: 10.1093/qjmed/hcg096. [DOI] [PubMed] [Google Scholar]
- 28.Donohue D, Ahsan C, Sanaei-Ardekani M, et al. Early Diagnosis Of Stress-Induced Apical Ballooning Syndrome Based On Classic Echocardiographic Findings And Correlation With Cardiac Catheterization. J Am Soc Echocardiogr. 2005;18:1423.e15–e22. doi: 10.1016/j.echo.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Guevara R, Aguinaga-Meza M, Hazin MI, et al. Takotsubo Cardiomyopathy Complicated With Acute Pericarditis And Cardiogenic Shock. J Natl Med Assoc. 2007;99:281–83. [PMC free article] [PubMed] [Google Scholar]
- 30.Hurst RT, Askew JW, Reuss CS, et al. Transient Midventricular Ballooning Syndrome. A New Variant. J Am Coll Cardiol. 2006;48:579–83. doi: 10.1016/j.jacc.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Patel HM, Kantharia BK, Morris DL, et al. Takotsubo Syndrome in African-American Women with Atypical Presentations: A Single-Center Experience. Clin Cardiol. 2007;30:14–18. doi: 10.1002/clc.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seth PS, Aurigemma GP, Krasnow JM, et al. A Syndrome of Transient Left Ventricular Apical Wall Motion Abnormality in the Absence of Coronary Disease: A Perspective from the United States. Cardiology. 2003;100:61–66. doi: 10.1159/000073040. [DOI] [PubMed] [Google Scholar]
- 33.Athanasiadis A, Vogelsberg H, Hauer B, et al. Transient Left Ventricular Dysfunction with Apical Ballooning (Tako-Tsubo Cardiomyopathy) in Germany. Clin Res Cardiol. 2006;95:321–28. doi: 10.1007/s00392-006-0380-0. [DOI] [PubMed] [Google Scholar]
- 34.Azzarelli S, Galassi AR, Amico F, et al. Clinical Features of Transient Left Ventricular Apical Ballooning. Am J Cardiol. 2006;98:1273–76. doi: 10.1016/j.amjcard.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 35.Cangella F, Medolla A, De Fazio G, et al. Stress Induced Cardiomyopathy Presenting as Acute Coronary Syndrome: Tako-Tsubo In Mercogliano, Southern Italy. Cardiovasc Ultrasound. 2007;5:36. doi: 10.1186/1476-7120-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiz Bailén M, Aguayo De Hoyos E, López Martínez A, et al. Reversible Myocardial Dysfunction, a Possible Complication in Critically Ill Patients without Heart Disease. J Crit Care. 2003;18:245–52. doi: 10.1016/j.jcrc.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Marcu C, Balf D, Donohue T. Medical Image. Takotsubo Cardiomyopathy (Left Ventricular Apical Ballooning) N Z Med J. 2005;118:1208–9. [PubMed] [Google Scholar]
- 38.Abdulla I, Kay S, Mussap C, et al. Apical Sparing In Tako-Tsubo Cardiomyopathy. Intern Med J. 2006;36:414–18. doi: 10.1111/j.1445-5994.2006.01095.x. [DOI] [PubMed] [Google Scholar]
- 39.Akashi YJ, Nakazawa K, Sakakibara M, et al. 123I-MIBG Myocardial Scintigraphy In Patients With “Takotsubo” Cardiomyopathy. J Nucl Med. 2004;45:1121–27. [PubMed] [Google Scholar]
- 40.Hansen PR. Takotsubo Cardiomyopathy: An Under-Recognized Myocardial Syndrome. Eur J Intern Med. 2007;18:561–65. doi: 10.1016/j.ejim.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Parodi G, Del Pace S, Carrabba N, et al. Incidence, Clinical Findings, And Outcome Of Women With Left Ventricular Apical Ballooning Syndrome. Am J Cardiol. 2007;99:182–85. doi: 10.1016/j.amjcard.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 42.Pillière R, Mansencal N, Digne F, et al. Prevalence of Tako-Tsubo Syndrome in a Large Urban Agglomeration. Am J Cardiol. 2006;98:662–65. doi: 10.1016/j.amjcard.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 43.Strunk B, Shaw RE, Bull S, et al. High Incidence Of Focal Left Ventricular Wall Motion Abnormalities And Normal Coronary Arteries In Patients With Myocardial Infarctions Presenting To A Community Hospital. J Invasive Cardiol. 2006;18:376–81. [PubMed] [Google Scholar]
- 44.Brenner ZR, Powers J. Takotsubo Cardiomyopathy. Heart Lung. 2008;37:1–7. doi: 10.1016/j.hrtlng.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Klinceva M, Widimský P, Pesl L, et al. Prevalence Of Stress-Induced Myocardial Stunning (Tako-Tsubo Cardiomyopathy) Among Patients Undergoing Emergency Coronary Angiography For Suspected Acute Myocardial Infarction. Int J Cardiol. 2007;120:411–13. doi: 10.1016/j.ijcard.2006.07.228. [DOI] [PubMed] [Google Scholar]
- 46.Akashi YJ, Musha H, Kida K, et al. Reversible Ventricular Dysfunction Takotsubo Cardiomyopathy. Eur J Heart Fail. 2005;7:1171–76. doi: 10.1016/j.ejheart.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 47.Bonnemeier H, Ortak J, Bode F, et al. Modulation of Ventricular Repolarization in Patients with Transient Left Ventricular Apical Ballooning: A Case Control Study. J Cardiovasc Electrophysiol. 2006;17:1340–47. doi: 10.1111/j.1540-8167.2006.00644.x. [DOI] [PubMed] [Google Scholar]
- 48.Elesber A, Lerman A, Bybee KA, et al. Myocardial Perfusion in Apical Ballooning Syndrome Correlate of Myocardial Injury. Am Heart J. 2006;152:469.e9–13. doi: 10.1016/j.ahj.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Elesber AA, Prasad A, Lennon RJ, et al. Four-Year Recurrence Rate And Prognosis Of The Apical Ballooning Syndrome. J Am Coll Cardiol. 2007;50:448–52. doi: 10.1016/j.jacc.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 50.Fazio G, Barbaro G, Sutera L, et al. Clinical Findings of Takotsubo Cardiomyopathy: Results from a Multicenter International Study. J Cardiovasc Med. 2008;9:239–44. doi: 10.2459/JCM.0b013e328216276d. [DOI] [PubMed] [Google Scholar]
- 51.Spedicato L, Zanuttini D, Nucifora G, et al. Transient Left Ventricular Apical Ballooning Syndrome: A 4-Year Experience. J Cardiovasc Med. 2008;9:916–21. doi: 10.2459/JCM.0b013e3283027f8e. [DOI] [PubMed] [Google Scholar]
- 52.Ennezat PV, Pesenti-Rossi D, Aubert JM, et al. Transient Left Ventricular Basal Dysfunction without Coronary Stenosis in Acute Cerebral Disorders: A Novel Heart Syndrome (Inverted Takotsubo) Echocardiography. 2005;22:559–602. doi: 10.1111/j.1540-8175.2005.40046.x. [DOI] [PubMed] [Google Scholar]
- 53.Giordan M, Rigatelli G, Cardaioli P, et al. Angiographic Long-Term Follow-Up of Primary Apical Ballooning of the Left Ventricle. Int J Cardiovasc Imaging. 2006;22:349–52. doi: 10.1007/s10554-005-9049-4. [DOI] [PubMed] [Google Scholar]
- 54.Bahlmann E, Schneider C, Krause K, et al. Tako-Tsubo Cardiomyopathy Characteristics in Long-Term Follow-Up. Int J Cardiol. 2008;124:32–39. doi: 10.1016/j.ijcard.2006.12.090. [DOI] [PubMed] [Google Scholar]
- 55.Buja P, Zuin G, Di Pede F, et al. Long-Term Outcome and Sex Distribution across Ages of Left Ventricular Apical Ballooning Syndrome. J Cardiovasc Med. 2008;9:905–9. doi: 10.2459/JCM.0b013e3282fec072. [DOI] [PubMed] [Google Scholar]
- 56.Bybee KA, Murphy J, Prasad A, et al. Acute Impairment of Regional Myocardial Glucose Uptake in the Apical Ballooning (Takotsubo) Syndrome. J Nucl Cardiol. 2006;13:244–50. doi: 10.1007/BF02971249. [DOI] [PubMed] [Google Scholar]
- 57.Consales G, Campiglia L, Michelagnoli G, et al. Acute Left Ventricular Dysfunction Due To Tako-Tsubo Syndrome after Induction of General Anesthesia. Minerva Anestesiol. 2007;73:655–58. [PubMed] [Google Scholar]
- 58.Haghi D, Fluechter S, Suselbeck T, et al. Cardiovascular Magnetic Resonance Findings in Typical Versus Atypical Forms of the Acute Apical Ballooning Syndrome (Takotsubo Cardiomyopathy) Int J Cardiol. 2007;120:205–11. doi: 10.1016/j.ijcard.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 59.Haghi D, Papavassiliu T, Hamm K, et al. Coronary Artery Disease in Takotsubo Cardiomyopathy. Circ J. 2007;71:1092–94. doi: 10.1253/circj.71.1092. [DOI] [PubMed] [Google Scholar]
- 60.Inoue M, Shimizu M, Ino H, et al. Differentiation between Patients with Takotsubo Cardiomyopathy and those with Anterior Acute Myocardial Infarction. Circ J. 2005;69:89–94. doi: 10.1253/circj.69.89. [DOI] [PubMed] [Google Scholar]
- 61.Merli E, Sutcliffe S, Gori M, et al. Tako-Tsubo Cardiomyopathy: New Insights into the Possible Underlying Pathophysiology. Eur J Echocardiography. 2006;7:53–61. doi: 10.1016/j.euje.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Mitsuma W, Kodama M, Ito M, et al. Serial Electrocardiographic Findings In Women With Takotsubo Cardiomyopathy. Am J Cardiol. 2007;100:106–9. doi: 10.1016/j.amjcard.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 63.Cherian J, Angelis D, Filiberti A, et al. Can Takotsubo Cardiomyopathy Be Familial? Int J Cardiol. 2007;121:74–75. doi: 10.1016/j.ijcard.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 64.Cocco G, Chu D. Stress-Induced Cardiomyopathy: A Review. Eur J Int Med. 2007;18:369–79. doi: 10.1016/j.ejim.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 65.Obón Azuara B, Ortas Nadal MR, Gutiérrez Cía I, et al. Takotsubo cardiomyopathy: transient apical dysfunction of the left ventricle. Med Intensiva. 2007;31:146–52. doi: 10.1016/s0210-5691(07)74793-9. [in Spanish with English abstract] [DOI] [PubMed] [Google Scholar]
- 66.Donohue D, Movahed MR. Clinical Characteristics, Demographics and Prognosis of Transient Left Ventricular Apical Ballooning Syndrome. Heart Fail Rev. 2005;10:311–16. doi: 10.1007/s10741-005-8555-8. [DOI] [PubMed] [Google Scholar]
- 67.Summers MR, Lennon RJ. Prasad. Pre-morbid psychiatric and cardiovascular diseases in apical ballooning syndrome (tako-tsubo/stress-induced cardiomyoapthy) potential pre-disposing factors? J Am coll Cardiol. 2010;55:700–1. doi: 10.1016/j.jacc.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 68.Parodi G, Del Pace S, Salvadori C, et al. Left Ventricular Apical Ballooning Syndrome as a Novel Cause of Acute Mitral Regurgitation. J Am Coll Cardiol. 2007;50:647–49. doi: 10.1016/j.jacc.2007.04.057. [DOI] [PubMed] [Google Scholar]
- 69.Matsuoka H, Kawakami H, Koyama Y, et al. “Takotsubo” Cardiomyopathy With A Significant Pressure Gradient In The Left Ventricle. Heart Vessels. 2000;15:203. doi: 10.1007/s003800070024. [DOI] [PubMed] [Google Scholar]
- 70.Raddino R, Pedrinazzi C, Zanini G, et al. Out-Of-Hospital Cardiac Arrest Caused By Transient Left Ventricular Apical Ballooning Syndrome. Int J Cardiol. 2008;128:e31–e33. doi: 10.1016/j.ijcard.2007.05.067. [DOI] [PubMed] [Google Scholar]
- 71.Soni A, LeLorier P. Sudden Death in Nondilated Cardiomyopathies: Pathophysiology and Prevention. Current Heart Failure Reports. 2005;2:118–23. doi: 10.1007/s11897-005-0019-x. [DOI] [PubMed] [Google Scholar]
- 72.Good CW, Hubbard CR, Harrison TA, et al. Echocardiographic guidance in treatment of cardiogenic shock complicating transient left ventricular apical ballooning syndrome. JACC Cardiovasc Imaging. 2009;2:372–74. doi: 10.1016/j.jcmg.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 73.Yaghoubi AR, Ansarin K, Hashemzadehs, et al. Tako-tsubo cardiomyopathy induced by emotional stress leading to severe mitral regurgitation, cardiogenic shock and cardiopulmonary arrest. Int J Cardiol. 2009;135:e85–86. doi: 10.1016/j.ijcard.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 74.Blessing E, Steen H, Rosenberg M, et al. Recurrence of Takotsubo Cardiomyopathy with Variant Forms of Left Ventricular Dysfunction. J Am Soc Echocardiogr. 2007;20:439.e11–e12. doi: 10.1016/j.echo.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 75.Bennett JR, McCarty D, Wilson CM, et al. Takotsubo Cardiomyopathy: An uncommon Cause of ST Segment Elevation in Intensive Care. Anaesthesia. 2007;62:279–81. doi: 10.1111/j.1365-2044.2007.04940.x. [DOI] [PubMed] [Google Scholar]
- 76.Finsterer J, Stöllberger C, Sehnal E, et al. Apical Ballooning (Takotsubo Syndrome) In Mitochondrial Disorder During Mechanical Ventilation. J Cardiovasc Med. 2007;8:859–63. doi: 10.2459/JCM.0b013e3280103d1b. [DOI] [PubMed] [Google Scholar]
- 77.Kolkebeck TE, Cotant CL, Krasuski RA. Takotsubo Cardiomyopathy: An Unusual Syndrome Mimicking an ST-Elevation Myocardial Infarction. Am J Emerg Med. 2007;25:92–95. doi: 10.1016/j.ajem.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 78.Syed FF, Asirvatham SJ, Francis J. Arrhythmia occurrence with takotsubo cardiomyopathy: a literature review. Europace. 2010 doi: 10.1093/europace/euq435. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 79.Ziegelstein RC. Acute Emotional Stress and Cardiac Arrhythmias. JAMA. 2007;298:324–29. doi: 10.1001/jama.298.3.324. [DOI] [PubMed] [Google Scholar]
- 80.Furushima H, Chinushi M, Sanada A, et al. Ventricular Repolarization Gradients In A Patient With Takotsubo Cardiomyopathy. Europace. 2008;10:1112–15. doi: 10.1093/europace/eun166. [DOI] [PubMed] [Google Scholar]
- 81.Fischer R, Schirdewan A, Kumar A, et al. Cardiac Magnetic Resonance And Cardiac Magnetic Field Mapping In A Patient With Stress-Induced Cardiomyoapthy (Tako-Tsubo) Pacing Clin Electrophysiol. 2006;29:1442–44. doi: 10.1111/j.1540-8159.2006.00561.x. [DOI] [PubMed] [Google Scholar]
- 82.Konety SH, Horwitz P, Lindower P, et al. Arrhythmias in Tako-Tsubo Syndrome-Benign or Malignant? Int J Cardiol. 2007;114:141–44. doi: 10.1016/j.ijcard.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 83.Kurisu S, Inoue I, Kawagoe T, et al. Torsade De Pointes Associated with Bradycardia and Takotsubo Cardiomyopathy. Can J Cardiol. 2008;24:640–42. doi: 10.1016/s0828-282x(08)70653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nault MA, Baranchuk A, Simpson CS, et al. Takotsubo Cardiomyopathy: A Novel “Proarrythmic” Disease. Anadolu Kardiyol Derg. 2007;(7 Suppl 1):101–3. [PubMed] [Google Scholar]
- 85.Lalonde G, Beaulieu Y. Tako-Tsubo Cardiomyopathy. Can J Cardiol. 2005;21:1213–16. [PubMed] [Google Scholar]
- 86.Lee WL, Miao LF, Chan HW, et al. Takotsubo Syndrome with Transient Complete Atrioventricular Block. Chin Med J. 2006;119:73–76. [PubMed] [Google Scholar]
- 87.Nef HM, Möllmann H, Sperzel J, et al. Temporary Third-Degree Atrioventricular Block In A Case Of Apical Ballooning Syndrome. Int J Cardiol. 2006;113:e33–e35. doi: 10.1016/j.ijcard.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 88.Lyon AR, Rees PS, Prasad S, et al. Stress (Takotsubo) Cardiomyopathy-A Novel Pathophysiological Hypothesis to Explain Catecholamine-Induced Acute Myocardial Stunning. Nat Clin Pract Cardiovasc Med. 2008;5:22–29. doi: 10.1038/ncpcardio1066. [DOI] [PubMed] [Google Scholar]
- 89.Maruyama T, Hanaoka T, Nakajima H. Acute Pericarditis In The Recovery Phase Of Transient Left Ventricular Apical Ballooning Syndrome (Takotsubo Cardiomyopathy) Intern Med. 2007;46:1857–60. doi: 10.2169/internalmedicine.46.0184. [DOI] [PubMed] [Google Scholar]
- 90.Akashi YJ, Tejima T, Sakurada H, et al. Left Ventricular Rupture Associated with Takotsubo Cardiomyopathy. Mayo Clin Proc. 2004;79:821–24. doi: 10.4065/79.6.821. [DOI] [PubMed] [Google Scholar]
- 91.Ishida T, Yasu T, Arao K, et al. Images in Cardiovascular Medicine. Bedside Diagnosis of Cardiac Rupture by Contrast Echocardiography. Circulation. 2005;112:354–55. doi: 10.1161/CIRCULATIONAHA.105.538348. [DOI] [PubMed] [Google Scholar]
- 92.Ohara Y, Hiasa Y, Hosokawa S, et al. Left Ventricular Free Wall Rupture In Transient Left Ventricular Apical Ballooning. Circ J. 2005;69:621–23. doi: 10.1253/circj.69.621. [DOI] [PubMed] [Google Scholar]
- 93.Sacha J, Maselko J, Wester A, et al. Left Ventricular Apical Rupture Caused By Takotsubo Cardiomyopathy-Comprehensive Pathological Heart Investigation. Circ J. 2007;71:982–85. doi: 10.1253/circj.71.982. [DOI] [PubMed] [Google Scholar]
- 94.Chandrasegaram MD, Celermajer DS, Wilson MK. Apical Ballooning Syndrome Complicated By Acute Severe Mitral Regurgitation With Left Ventricular Outflow Obstruction-Case Report. J Cardiothorac Surg. 2007;2:1–4. doi: 10.1186/1749-8090-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kurisu S, Inoue I, Kawagoe T, et al. Incidence and treatment of left ventricular apical thrombosis in Tako-tsubo cardiomyopathy. Int J Cardiol. 2011;146:e58–60. doi: 10.1016/j.ijcard.2008.12.208. [DOI] [PubMed] [Google Scholar]
- 96.Sharkey SW, Shear W, Hodges M, et al. Reversible Myocardial Contraction Abnormalities in Patients with an Acute Noncardiac Illness. Chest. 1998;114:98–105. doi: 10.1378/chest.114.1.98. [DOI] [PubMed] [Google Scholar]
- 97.Prasad A, Lerman A, Rihal CS. Apical Ballooning Syndrome (Tako-Tsubo Or Stress Cardiomyopathy): A Mimic Of Acute Myocardial Infarction. Am Heart J. 2008;155:408–17. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 98.Kurisu S, Inoue I, Kawagoe T, et al. Documentation of Dynamic Electrocardiographic Changes Shortly After the Onset of Tako-Tsubo Cardiomyopathy. Int J Cardiol. 2007;119:258–60. doi: 10.1016/j.ijcard.2006.07.141. [DOI] [PubMed] [Google Scholar]
- 99.Sankri-Tarbichi AG, Mathew PK, Matos M, et al. Stress-Related Cardiomyopathy. Heart Lung. 2007;36:43–46. doi: 10.1016/j.hrtlng.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 100.Ito K, Sugihara H, Kawasaki T, et al. Assessment of Ampulla (Takotsubo) Cardiomyopathy with Coronary Angiography, Two-Dimensional Echocardiography and 99mTc-Tetrofosmin Myocardial Single Photon Emission Computed Tomography. Ann Nucl Med. 2001;15:351–55. doi: 10.1007/BF02988242. [DOI] [PubMed] [Google Scholar]
- 101.Ogura R, Hiasa Y, Takahashi T, et al. Specific Findings of the Standard 12-Lead ECG in Patients with “Takotsubo” Cardiomyopathy: Comparison with the Findings of Acute Anterior Myocardial Infarction. Circ J. 2003;67:687–90. doi: 10.1253/circj.67.687. [DOI] [PubMed] [Google Scholar]
- 102.Sharkey SW, Lesser JR, Menon M, et al. Spectrum And Significance Of Electrocardiographic Patterns, Troponin Levels, And Thrombolysis In Myocardial Infarction Frame Count In Patients With Stress (Tako-Tsubo) Cardiomyopathy and Comparison To Those In Patients With ST-Elevation Anterior Wall Myocardial Infarction. Am J Cardiol. 2008;101:1723–28. doi: 10.1016/j.amjcard.2008.02.062. [DOI] [PubMed] [Google Scholar]
- 103.Bybee KA, Motieti A, Syed IS, et al. Electrocardiography Cannot Reliably Differentiate Transient Left Ventricular Apical Ballooning Syndrome from Anterior ST-Segment Elevation Myocardial Infarction. J Electrocardiol. 2007;40:38e1–6. doi: 10.1016/j.jelectrocard.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 104.Maréchaux S, Fornes P, Petit S, et al. Pathology of Inverted Takotsubo Cardiomyopathy. Cardiovasc Pathol. 2008;17:241–43. doi: 10.1016/j.carpath.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 105.Roongsritong C, Warraich I, Bradley C. Common Causes Of Troponin Elevations In The Absense Of Acute Myocardial Infarction: Incidence And Clinical Significance. Chest. 2004;125:1877–84. doi: 10.1378/chest.125.5.1877. [DOI] [PubMed] [Google Scholar]
- 106.Apple FS, Quist HE, Doyle PJ, et al. Plasma 99th Percentile Reference Limits For Cardiac Troponin And Creatine Kinase MB Mass For Use With European Society Of Cardiology/American College Of Cardiology Consensus Recommendations. Clin Chem. 2003;49:1331–36. doi: 10.1373/49.8.1331. [DOI] [PubMed] [Google Scholar]
- 107.Madhavan M, Borlaug BA, Lerman A, et al. Stress hormone and circulating biomarker profile of apical ballooning syndrome (takotsubo cardiomyopathy): insights into the clinical significance of B-type natriuretic peptide and troponin levels. Heart. 2009;95:1436–41. doi: 10.1136/hrt.2009.170399. [DOI] [PubMed] [Google Scholar]
- 108.Ferguson JL, Beckett GJ, Stoddart M, et al. Myocardial Infarction Redefined: The New ACC/ESC Definition, Based On Cardiac Troponin, Increases The Apparent Incidence Of Infarction. Heart. 2002;88:343–47. doi: 10.1136/heart.88.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang WH, Francis GS, Morrow DA, et al. National Academy Of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical Utilization Of Cardiac Biomarker Testing In Heart Failure. Circulation. 2007;116:e99–109. doi: 10.1161/CIRCULATIONAHA.107.185267. [DOI] [PubMed] [Google Scholar]
- 110.Nef HM, Möllmann H, Weber M, et al. Release Pattern of Cardiac Biomarkers in Left Ventricular Apical Ballooning. Int J Cardiol. 2007;115:128–29. doi: 10.1016/j.ijcard.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 111.Pilgrim TM, Wyss TR. Takotsubo Cardiomyopathy or Transient Left Ventricular Apical Ballooning Syndrome: A Systematic Review. Int J Cardiol. 2008;124:283–92. doi: 10.1016/j.ijcard.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 112.Harris TB, Ferrucci L, Tracy RP, et al. Associations of Elevated Interleukin-6 and C-reactive protein Levels with Mortality in the Elderly. Am J Med. 1999;106:506–12. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 113.Dhoble A, Abdelmoneim SS, Bernier M, et al. Transient Left Ventricular Apical Ballooning And Exercise Induced Hypertension During Treadmill Exercise Testing: Is There A Common Hypersympathetic Mechanism? Cardiovasc Ultrasound. 2008;6:37. doi: 10.1186/1476-7120-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marchionni N, Chechi T, Falai M, et al. Myocardial Stunning Associated With A Myocardial Bridge. Int J Cardiol. 2002;82:65–67. doi: 10.1016/s0167-5273(01)00580-0. [DOI] [PubMed] [Google Scholar]
- 115.Metzl MD, Altman EJ, Spevack DM, et al. A Case of Takotsubo Cardiomyopathy Mimicking An Acute Coronary Syndrome. Nat Clin Pract Cardiovasc Med. 2006;3:53–56. doi: 10.1038/ncpcardio0414. [DOI] [PubMed] [Google Scholar]
- 116.Ohba Y, Takemoto M, Nakano M, et al. Takotsubo Cardiomyopathy with Left Ventricular Outflow Tract Obstruction. Int J Cardiol. 2006;107:120–22. doi: 10.1016/j.ijcard.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 117.Shah DP, Sugeng L, Goonewardena SN, et al. Images in Cardiovascular Medicine. Takotsubo Cardiomyopathy. Circulation. 2006;113:e762. doi: 10.1161/CIRCULATIONAHA.105.570234. [DOI] [PubMed] [Google Scholar]
- 118.Takizawa M, Kobayakawa N, Uozumi H, et al. A Case of Transient Left Ventricular Ballooning with Pheochromocytoma, Supporting Pathogenetic Role Of Catecholamines In Stress-Induced Cardiomyopathy Or Takotsubo Cardiomyopathy. Int J Cardiol. 2007;114:e15–17. doi: 10.1016/j.ijcard.2006.07.125. [DOI] [PubMed] [Google Scholar]
- 119.Yasu T, Tone K, Kubo N, et al. Transient Mid-Ventricular Balloooning Cardiomyopathy: A New Entity of Takotsubo Cardiomyopathy. Int J Cardiol. 2006;110:100–1. doi: 10.1016/j.ijcard.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 120.Kumar S, Mostow N, Grimm RA. Quick Resolution of Takotsubo Cardiomyopathy: A Brief Review. Echocardiography. 2008;25:1117–20. doi: 10.1111/j.1540-8175.2008.00775.x. [DOI] [PubMed] [Google Scholar]
- 121.Wani S, Glatz K, Suter Y, et al. “Apical Ballooning” – What Is The Cause? J Invasive Cardiol. 2008;20:599–602. [PubMed] [Google Scholar]
- 122.Burri MV, Nanda NC, Lloyd SG, et al. Assessment Of Systolic And Diastolic Left Ventricular and Left Atrial Function Using Vector Velocity Imaging In Takotsubo Cardiomyopathy. Echocardiography. 2008;25:1138–44. doi: 10.1111/j.1540-8175.2008.00819.x. [DOI] [PubMed] [Google Scholar]
- 123.Bahlmann E, Schneider C, Krause K, et al. Tako-Tsubo Cardiomyopathy (Apical Ballooning) With Parvovirus B19 Genome in Endomyocardial Biopsy. Int J Cardiol. 2007;116:e18–21. doi: 10.1016/j.ijcard.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 124.Núñez-Gil IJ, Fernández-Ortiz A, Pérez-Isla L, et al. Clinical and Prognostic Comparison between Left Ventricular Transient Dyskinesia and a First Non-ST-Segment Elevation Acute Coronary Syndrome. Coron Artery Dis. 2008;19:449–53. doi: 10.1097/MCA.0b013e32830eab74. [DOI] [PubMed] [Google Scholar]
- 125.Hoyt J, Lerman A, Lennon R, et al. Left anterior descending artery length and coronary atherosclerosis in apical ballooning syndrome (takotsubo/stress induced cardiomyopathy) Int J Cardiol. 2010;145:112–15. doi: 10.1016/j.ijcard.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 126.Gianni M, Dentali F, Grandi AM, et al. Apical Ballooning Syndrome or Takotsubo Cardiomyopathy: A Systematic Review. Eur Heart J. 2006;27:1523–29. doi: 10.1093/eurheartj/ehl032. [DOI] [PubMed] [Google Scholar]
- 127.Tarkin JM, Khetyar M, Kaski JC. Management of Tako-Tsubo Syndrome. Cardiovasc Drugs Ther. 2008;22:71–77. doi: 10.1007/s10557-007-6074-7. [DOI] [PubMed] [Google Scholar]
- 128.Mitchell SA, Crone RA. Takotsubo Cardiomyopathy: A Case Report. J Am Soc Echocardiogr. 2006;19:1190.e9–10. doi: 10.1016/j.echo.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 129.Elesber AA, Prasad A, Bybee KA, et al. Transient Cardiac Apical Ballooning Syndrome: Prevalence and Clinical Implications of Right Ventricular Involvement. J Am Coll Cardiol. 2006;47:1082–83. doi: 10.1016/j.jacc.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 130.Haghi D, Suselbeck T, Borggrefe M. Guidelines for Diagnosis of Takotsubo (Ampulla) Cardiomyopathy. Circ J. 2007;71:1664. doi: 10.1253/circj.71.1664. [DOI] [PubMed] [Google Scholar]
- 131.Vallès E, Pujadas S, Guindo J, et al. Delayed-Contrast Enhancement Cardioresonance in Transient Left Ventricular Apical Ballooning. Int J Cardiovasc Imaging. 2007;23:243–47. doi: 10.1007/s10554-006-9128-1. [DOI] [PubMed] [Google Scholar]
- 132.Mahrholdt H, Goedecke C, Wagner A, et al. Cardiovascular Magnetic Resonance Assessment of Human Myocarditis: A Comparison to Histology and Molecular Pathology. Circulation. 2004;109:1250–58. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 133.Haghi D, Fluechter S, Suselbeck T, et al. Delayed Hyperenhancement In A Case Of Takotsubo Cardiomyopathy. J Cardiovasc Magn Reson. 2005;7:845–47. doi: 10.1080/10976640500295482. [DOI] [PubMed] [Google Scholar]
- 134.Kurisu S, Inoue I, Kawagoe T, et al. Myocardial Perfusion and Fatty Acid Metabolism in Patients with Tako-Tsubo-Like Ventricular Dysfunction. J Am Coll Cardiol. 2003;41:743–48. doi: 10.1016/s0735-1097(02)02924-8. [DOI] [PubMed] [Google Scholar]
- 135.Uchida Y, Nanjo S, Fujimoto S, et al. Scintigraphic Studies on the Etiology of Ampulla Cardiomyopathy. J Cardiol. 2008;51:121–30. doi: 10.1016/j.jjcc.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 136.Ito K, Sugihara H, Kinoshita N, et al. Assessement of Takotsubo Cardiomyopathy (Transient Left Ventricular Apical Ballooning) Using 99mTc-Tetrosfosmin, 123I-BMIPP, 123I-MIBG and 99mTc-PYP Myocardial SPECT. Ann Nucl Med. 2005;19:435–45. doi: 10.1007/BF02985570. [DOI] [PubMed] [Google Scholar]
- 137.Sato A, Aonuma K, Nozato T, et al. Stunned Myocardium in Transient Left Ventricular Apical Ballooning: A Serial Study of Dual I-123 BMIPP And Tl-201 SPECT. J Nucl Cardiol. 2008;15:671–79. doi: 10.1016/j.nuclcard.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 138.Yilmaz Y. Apical Ballooning Syndrome: A Metabolic Form of Cardiomyopathy? Med Sci Monit. 2008;14(6):HY9–12. [PubMed] [Google Scholar]
- 139.Obunai K, Misra D, Van Tosh A, et al. Metabolic Evidence of Myocardial Stunning In Takotsubo Cardiomyopathy: A Positron Emission Tomography Study. J Nucl Cardiol. 2005;12:742–44. doi: 10.1016/j.nuclcard.2005.06.087. [DOI] [PubMed] [Google Scholar]
- 140.Di Carli MF, Prcevski P, Singh TP, et al. Myocardial Blood Flow, Function, And Metabolism In Repetitive Stunning. J Nucl Med. 2000;41:1227–34. [PubMed] [Google Scholar]
- 141.Dorbala S, Di Carli MF. Metabolic Imaging of Myocardial Stunning. Heart Metab. 2003;19:18–22. [Google Scholar]
- 142.Nef HM, Möllamann H, Akashi J, et al. Mechanisms of stress (Takotsubo) cardiomyopathy. Nat Rev Cardiol. 2010;7:187–93. doi: 10.1038/nrcardio.2010.16. [DOI] [PubMed] [Google Scholar]
- 143.Stein AB, Tang XL, Guo Y, et al. Delayed Adaptation Of The Heart To Stress: Late Preconditioning. Stroke. 2004;35(11 Suppl 1):2676–79. doi: 10.1161/01.STR.0000143220.21382.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ueyama T, Kasamatsu K, Hano T, et al. Emotional Stress Induces Transient Left Ventricular Hypocontraction In The Rat Via Activation Of Cardiac Adrenoceptors: A Possible Animal Model Of “Tako-Tsubo” Cardiomyopathy. Circ J. 2002;66:712–13. doi: 10.1253/circj.66.712. [DOI] [PubMed] [Google Scholar]
- 145.Simoes MV, Marín-Neto JA, Maciel BC. Variable Regional Left Ventricular Dysfunction In Takotsubo Cardiomyopathy Syndrome. Echocardiography. 2007;24:893. doi: 10.1111/j.1540-8175.2007.00548.x. [DOI] [PubMed] [Google Scholar]
- 146.Kawano H, Okada R, Yano K. Histological Study on the Distribution of Autonomic Nerves in the Human Heart. Heart Vessels. 2003;18:32–39. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- 147.Kaski JC. Cardiac Syndrome X in Women: The Role of Oestrogen Deficiency. Heart. 2006;92(Suppl 3):iii5–9. doi: 10.1136/hrt.2005.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Johnson BD, Shaw LJ, Buchthal SD, et al. Prognosis in Women with Myocardial Ischemia in the Absence of Obstructive Coronary Disease: Results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:2993–99. doi: 10.1161/01.CIR.0000130642.79868.B2. [DOI] [PubMed] [Google Scholar]
- 149.Demir H, Kahraman G, Isgoren S, et al. Evaluation Of Post-Stress Left Ventricular Dysfunction and its Relationship with Perfusion Abnormalities Using Gated SPECT in Patients With Cardiac Syndrome X. Nucl Med Commun. 2008;29:208–14. doi: 10.1097/MNM.0b013e3282f52c49. [DOI] [PubMed] [Google Scholar]
- 150.Ueyama T, Kasamatsu K, Hano T, et al. Catecholamines And Estrogen Are Involved In The Pathogenesis Of Emotional Stress-Induced Acute Heart Attack. Ann N Y Acad Sci. 2008;1148:479–85. doi: 10.1196/annals.1410.079. [DOI] [PubMed] [Google Scholar]
- 151.Galiuto L, De Caterina AR, Porfidia A, et al. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in apical balloning or tako-tsubo syndrome. Eur Heart J. 2010;31:1319–27. doi: 10.1093/eurheartj/ehq039. [DOI] [PubMed] [Google Scholar]
- 152.Bielecka-Dabrowa A, Mikhailidis DP, Hannam S, et al. Takotsubo cardiomyopathy-The current state of knowledge. Int J Cardiol. 2010;142:120–25. doi: 10.1016/j.ijcard.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 153.Page SP, Pantazis A, Elliott PM. Acute Myocardial Ischemia Associated with Latent Left Ventricular Outflow Tract Obstruction in the Absence of Left Ventricular Hypertrophy. J Am Soc Echocardiog. 2007;20:772.e1–4. doi: 10.1016/j.echo.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 154.Thorne KD, Kerut EK, Moore CK. Apical Ballooning “Tako-Tsubo” Syndrome Associated with Transient Left Ventricular Outflow Tract Obstruction. Echocardiography. 2007;24:770–72. doi: 10.1111/j.1540-8175.2007.00464.x. [DOI] [PubMed] [Google Scholar]
- 155.Peñas-Lado M, Barriales-Villa R, Goicolea J. Transient Left Ventricular Apical Ballooning and Outflow Tract Obstruction. J Am Coll Cardiol. 2003;42:1143–44. doi: 10.1016/s0735-1097(03)00892-1. [DOI] [PubMed] [Google Scholar]
- 156.Makaryus AN, Meraj P, Rosman D. Dynamic Left Ventricular Outflow Tract Obstruction Induced By Dobutamine Stress Echocardiography Leading To Myocardial Ischemia And Infarction. Int J Cardiovasc Imaging. 2006;22:763–69. doi: 10.1007/s10554-006-9102-y. [DOI] [PubMed] [Google Scholar]
- 157.Krasnow N. Subaortic Septal Bulge Simulates Hypertrophic Cardiomyopathy By Angulation Of The Septum with Age, Independent Of Focal Hypertrophy. An Echocardiographic Study. J Am Soc Echocardiogr. 1997;10:545–55. doi: 10.1016/s0894-7317(97)70009-9. [DOI] [PubMed] [Google Scholar]
- 158.Previtali M, Repetto A, Scuteri L. Dobutamine Induced Severe Midventricular Obstruction and Mitral Regurgitation in Left Ventricular Apical Ballooning Syndrome. Heart. 2005;91:353. doi: 10.1136/hrt.2004.037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cabrera-Bueno F, Gómez-Doblas JJ, Muñoz-García A, et al. Effort Angina, Normal Coronary Angiogram, and Dynamic Left Ventricular Obstruction. J Am Soc Echocardiogr. 2007;20:415–20. doi: 10.1016/j.echo.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 160.Desmet W. Dynamic LV Obstruction in Apical Ballooning Syndrome: The Chicken or the Egg. Eur J Echocardiogr. 2006;7:1–4. doi: 10.1016/j.euje.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 161.Ibáñez B, Navarro F, Córdoba M, et al. Tako-Tsubo Transient Left Ventricular Apical Ballooning: Is Intravascular Ultrasound The Key To Resolve The Enigma? Heart. 2005;91:102–4. doi: 10.1136/hrt.2004.035709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ibánez B, Navarro F, Farré J, et al. Tako-tsubo syndrome associated with a long course of the left anterior descending coronary artery along the apical diaphragmatic surface of the left ventricle. Rev Esp Cardiol. 2004;57:209–216. doi: 10.1016/s0300-8932(04)77092-x. [in Spanish with English abstract] [DOI] [PubMed] [Google Scholar]
- 163.Pison L, De Vusser P, Mullens W. Apical Ballooning in Relatives. Heart. 2004;90:e67. doi: 10.1136/hrt.2004.046813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sharkey SW, Maron BJ, Nelson P, et al. Adrenergic receptor polymorphisms in patients with stress (tako-tsubo) cardiomyopathy. J Cardiol. 2009;53:53–57. doi: 10.1016/j.jjcc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 165.Cortese B, Robotti S, Puggioni E, et al. Transient Left Ventricular Apical Ballooning Syndrome: All That Glitters Is Not Apical. J Cardiovasc Med. 2007;8:934–36. doi: 10.2459/JCM.0b013e3280122345. [DOI] [PubMed] [Google Scholar]
- 166.Kurowski V, Kaiser A, Von Hof K, et al. Apical And Midventricular Transient Left Ventricular Dysfunction Syndrome (Tako-Tsubo Cardiomyopathy): Frequency, Mechanisms, And Prognosis. Chest. 2007;132:809–16. doi: 10.1378/chest.07-0608. [DOI] [PubMed] [Google Scholar]
- 167.Yoshioka T, Hashimoto A, Tsuchihashi K, et al. Clinical Implications Of Midventricular Obstruction And Intravenous Propanolol Use In Transient Left Ventricular Apical Ballooning (Tako-Tsubo Cardiomyopathy) Am Heart J. 2008;155:526e1–7. doi: 10.1016/j.ahj.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 168.Nyui N, Yamanaka O, Nakayama R, et al. “Tako-Tsubo” Transient Ventricular Dysfunction: A case report. Jpn Circ J. 2000;64:715–19. doi: 10.1253/jcj.64.715. [DOI] [PubMed] [Google Scholar]
- 169.Song ZZ, Ma J. Right Ventricular Involvement in Takotsubo Cardiomyopathy. Eur Heart J. 2007;28:1037–38. doi: 10.1093/eurheartj/ehl571. [DOI] [PubMed] [Google Scholar]
- 170.López-Candales A, Rajagopalan N, Saxena N, et al. Right Ventricular Systolic Function Is Not The Sole Determinant Of Tricuspid Annular Motion. Am J Cardiol. 2006;98:973–77. doi: 10.1016/j.amjcard.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 171.Haghi D, Suselbeck T, Poerner T, et al. A Novel Regional Right Ventricular Wall-Motion Abnormality Observed in a Case of Acute Pulmonary Embolism (Reverse McConnell Sign) J Am Soc Echocardiogr. 2005;18:75–77. doi: 10.1016/j.echo.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 172.Haghi D, Athanasiadis A, Papavassiliu T, et al. Right ventricular involvement in Takotsubo cardiomyopathy. Eur Heart J. 2006;27:2433–39. doi: 10.1093/eurheartj/ehl274. [DOI] [PubMed] [Google Scholar]
- 173.Padayachee L. Levosimendan: The Inotrope of Choice in Cardiogenic Shock Secondary to Takotsubo Cardiomyopathy? Heart, Lung and Circulation. 2007;16(Suppl 3):S65–70. doi: 10.1016/j.hlc.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 174.Abu-Fanne R, Rott D, Klein M, et al. Recurrent Apical Ballooning Despite Treatment With Verapamil. Cardiology. 2007;108:210–13. doi: 10.1159/000096779. [DOI] [PubMed] [Google Scholar]
- 175.Ueyama T, Hano T, Kasamatsu K, et al. Estrogen Attenuates The Emocional Stress-Induced Cardiac Response In The Animal Model Of Tako-Tsubo (Ampulla) Cardiomyopathy. J Cardiovasc Pharmacol. 2003;42(Suppl 1):S117–19. doi: 10.1097/00005344-200312001-00024. [DOI] [PubMed] [Google Scholar]
- 176.Hale SL, Kloner RA. Ranolazine, An Inhibitor Of The Late Sodium Channel Current, Reduces Postischemic Myocardial Dysfunction In The Rabbit. J Cardiovasc Pharmacol Ther. 2006;11:249–55. doi: 10.1177/1074248406294607. [DOI] [PubMed] [Google Scholar]
- 177.Abdulla I, Ward MR. Tako-Tsubo Cardiomyopathy: How Stress Can Mimic Acute Coronary Occlusion. Med J Aust. 2007;187:357–60. doi: 10.5694/j.1326-5377.2007.tb01281.x. [DOI] [PubMed] [Google Scholar]
- 178.Sharkey SW, Windenburg DC, Lesser JR, et al. Natural history and expansive clinical profile of stress (Tako-Tsubo) cardiomyopathy. J Am Coll Cardiol. 2010;55:333–41. doi: 10.1016/j.jacc.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 179.Park JH, Kang SJ, Song JK, et al. Left Ventricular Apical Ballooning Due To Severe Physical Stress In Patients Admitted To The Medical ICU. Chest. 2005;128:296–302. doi: 10.1378/chest.128.1.296. [DOI] [PubMed] [Google Scholar]
- 180.Haghi D, Fluechter S, Suselbeck T, et al. Takotsubo Cardiomyopathy (Acute Left Ventricular Apical Ballooning Syndrome) Occurring In The Intensive Care Unit. Intensive Care Med. 2006;32:1069–74. doi: 10.1007/s00134-006-0111-z. [DOI] [PubMed] [Google Scholar]
- 181.Ruiz-Bailén M, Aguayo de Hoyos E, Ruiz-Navarro S, et al. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation. 2005;66:175–81. doi: 10.1016/j.resuscitation.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 182.Chockalingam A, Mehra A, Dorairajan S, et al. Acute left ventricular dysfunction in the critically ill. Chest. 2010;138:198–207. doi: 10.1378/chest.09-1996. [DOI] [PubMed] [Google Scholar]
- 183.Vydt T, Dubois C, Papas C, et al. Once Apical Ballooning, Always Apical Ballooning? Int J Cardiol. 2008;127:e132–33. doi: 10.1016/j.ijcard.2007.04.134. [DOI] [PubMed] [Google Scholar]
- 184.Barcin C, Kursaklioglu H, Kose S, et al. Takotsubo Cardiomyopathy In A Patient With Addison Disease: Is Apical Ballooning Always Reversible? Int J Cardiol. 2010;138:e15–17. doi: 10.1016/j.ijcard.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 185.Previtali M, Repetto A, Camporotondo R, et al. Clinical characteristics and outcome of left ventricular ballooning syndrome in a European population. Am J Cardiol. 2011;107:120–25. doi: 10.1016/j.amjcard.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 186.Ruiz-Bailén M, Romero-Bermejo FJ, Rucabado-Aguilar L, et al. Myocardial dysfunction in the critically ill patient: Is it really reversible? Int J Cardiol. 2010;145(3):615–16. doi: 10.1016/j.ijcard.2010.09.032. [DOI] [PubMed] [Google Scholar]


