Summary
Background
There is no simple and practical way to monitor sleep patterns in patients in acute care units. We designed this study to assess sleep patterns, energy expenditure and circadian rhythms of patients’ skin temperature in the coronary care unit (CCU) utilizing a new portable device.
Material/Methods
The SenseWear Armband (SWA) was used to record sleep duration, distribution over 24 hr, energy expenditure and the circadian rhythms of skin temperature in 46 patients with acute coronary syndrome (ACS) for the first 24 hr in the CCU and upon transfer to the ward. An advanced analysis was used to extract and compare data associated with the above variables in the two settings.
Results
Patients in the CCU had a reduced night’s sleep duration (5.6±2.2 hr) with more frequent and significantly shorter night sessions (p=0.015) than patients in the ward. Energy expenditure and METs (metabolic equivalents of a task) were significantly lower in the CCU than in the ward. However, the midline-estimating statistic of rhythm (MESOR) and acrophase for skin temperature did not exhibit any significant difference between the two settings.
Conclusions
Patients with ACS have sleep fragmentation and shorter nocturnal sleep duration in the CCU compared to the ward. On the other hand, there was no difference in the circadian rhythms of skin temperature between patients in the CCU and the general wards.
Keywords: acute coronary syndrome, acute myocardial infarction, circadian rhythm, sleep, energy expenditure, temperature
Background
In critically ill patients, alterations in the normal sleep pattern have been described to include sleep deprivation, poor sleep quality and disruption of the normal circadian rhythm [1,2]. These alterations are important to recognize, as they may have adverse biological and psychological effects [3–7]. Important factors that can contribute to sleep disruption include the underlying disease process, therapeutic interventions, diagnostic procedures, medications, disturbed light-dark cycle and noise generated in the acute care unit (ACU) environment [8–10]. Although a number of studies have objectively addressed sleep in acutely ill patients, most of them have included heterogeneous groups of patients with different underlying illnesses and have only monitored patients’ sleep at night, without performing daytime monitoring. One of the major obstacles facing investigators studying sleep patterns in the ACUs is the difficulty of performing full polysomnography in the ACU environment and the need for certain expertise. Hence, investigators started looking for an alternative method to monitor sleep in acutely ill patients.
The SenseWear Pro Armband™ (SWA) is a portable device that has been used to study the circadian pattern of sleep, skin temperature and energy expenditure. We hypothesized that the pattern of sleep, skin temperature and energy expenditure will be disturbed in patients with acute coronary syndrome (ACS) during coronary care unit (CCU) stay compared to the pattern in the general ward.
Therefore, we utilized a new portable validated device, the SWA, to study sleep patterns, energy expenditure and the circadian rhythms of skin temperature of patients admitted to the CCU with ACS. We also sought to investigate changes in sleep duration, distribution over 24 hours (circadian pattern) and energy expenditure upon discharge to the general medical ward.
Material and Methods
Study group
The study group consisted of 60 consecutive patients admitted to the CCU between September 2008 and June 2009 with the diagnosis of ACS. This included the first-ever acute ST segment elevation myocardial infarction (STEMI) patients, in which classical ECG changes were found along with elevated serum troponin or creatinine phosphokinase with an MB fraction of >10%. It also included patients with acute non-ST segment elevation myocardial infarction (NSTEMI), which was diagnosed based on elevated serum troponin or creatinine phosphokinase with an MB fraction of >10% without ST segment elevation. The study group also included patients with unstable angina (UA), which was diagnosed, based on the presence of ischemic symptoms that were new, were accelerated or occurred at rest in addition to classical ECG findings; cardiac enzymes, including troponin, were not elevated in UA. Demographic and clinical data were obtained at the time of admission to the CCU. Co-morbid medical conditions including diabetes mellitus, hypertension, hyperlipidemia, cerebrovascular accident, asthma, chronic lung diseases and psychiatric disorders were recorded. Patients studied in the CCU were accommodated in a common suite containing 5 beds. The study was approved by our institutional review board, and the study objectives and the methods were explained to the patients. In addition, written informed consent was obtained. Patients were excluded from the study if they were morbidly obese (BMI ≥35), had a history of sleep disorders, psychiatric or neurological disorders, sepsis or chronic lung diseases, or were on mechanical ventilation, sedatives or inotropes. The study group did not include any patients with alcoholism. Fourteen patients were later excluded from the study for the following reasons: 6 patients were transferred out of the CCU during the 24-hour study period, 2 required inotropes, 1 required mechanical ventilation, and the data for 5 patients were not collected by the SWA due to low battery power.
Study protocol
According to the manufacturer’s instructions, all study patients were asked after admission to the CCU to wear the SWA on the upper right arm over the triceps muscle at the midpoint between the acromion and olecranon processes. The SWA was removed only during cardiac catheterization and showering. Patients received their usual medications as per the CCU protocol. There was no interference with standard nursing care or procedures. At the end of the 72-hour period or upon the patient’s transfer out of the CCU, the SWA was removed and placed again on the patient for at least 24 hours immediately after being discharged into the general ward. The use of sedatives and hypnotics was not allowed during the follow-up period. Nighttime sleep was defined as sleep that occurs between 21:00 and 06:00. If the patient slept outside of this period, it was recorded as daytime sleep (Nap). Total sleep time (TST) was defined as the sum of nighttime sleep and any naps. If the wake time between 2 sleep periods was less than 60 min, the 2 periods were counted as 1 sleep session.
The noise levels in the CCU and in the female and male general wards were measured over 24 hours for 3 consecutive days using a Type 2222 Precision Integrated Sound Level Meter and a Type 2307 Level Recorder (Brüel & Kjær, Denmark). At the same time, the light level was measured every 2 hours using a Spectral Star Light Meter LX-1(JAPAN), which was placed on the patient’s pillow. The peak light levels during both daytime (06:00–18:00) and nighttime (18:00–06:00) were recorded. Ambient temperature was measured every 6 hours for 1 week with a thermistor.
Data collection and analysis
Armband
The SenseWear Pro Armband™ (SWA) (Body Media, Pittsburgh, PA) used in this study is a portable device 8.8×5.6×2.1 cm in size and 82 g in weight. The SWA has been used to study the circadian pattern of sleep and skin temperature [11]. Its sensors measure skin and near body temperature, galvanic skin response, heat flux and body acceleration (movement). These data and the patient demographic characteristics (sex, age, height, weight) were processed by proprietary algorithms to report the total and active energy expenditure, metabolic equivalents of a task (METs), the duration and amount of physical activity, body position, sleep efficiency and sleep duration [12]. An MET is defined as the amount of oxygen consumed while sitting at rest and is equal to 3.5 ml O2 per kg body weight per min; it is equivalent to a metabolic rate consuming 1 kilocalorie per kilogram of body weight per hour [13]. A thermistor-based sensor located on the backside of the SWA was used to measure skin temperature with an accuracy of ±0.1°C within the range of 0–70°C. The measured skin temperature is continuously and linearly indicative of core activities in the body because proximal skin temperature has been shown to follow the same circadian rhythm as rectal temperature [14,15]. Calibration of the sensor was performed at room temperature (23–25°C), and the data obtained were stored for offset correction on a scale of 0–50°C and at a resolution of 0.018°C per A/D tick. The near-body ambient temperature sensor measures the air temperature immediately around the armband, and it therefore reflects changes in environmental conditions. Monitoring of sensors took place 32 times per second, and data were collected over a period of 60 seconds [16]. The galvanic skin response, which indicates evaporative heat loss, was measured using 2 hypoallergenic stainless steel electrodes. The proprietary heat flux sensor in the SWA measures the amount of heat dissipated by the body. Minute-by-minute data from the SWA were analyzed by algorithms using the Body Media InnerView Research Software (version 5.1) provided by Body Media, Inc. [17]. These algorithms have been validated in many studies and have been compared to metabolic carts, which are the gold standard for measuring energy expenditure. Validation of resting and active energy expenditure has been performed against double-labeled water [18] and metabolic carts [12,15], and they have been shown to exhibit a high degree of correlation. The accelerometer in the SWA measures motion using a 2-axis micro-electronic mechanical sensor, which allows the assessment of sleep and wakefulness. The SWA has been validated for studying sleep because it has a moderate to high sensitivity and specificity, which allows it to identify sleep and wakefulness via its body media algorithm [19,20]. The American Academy of Sleep Medicine (AASM) has stated that the use of devices that monitor body movements through an accelerometer can be a useful adjunct in the diagnosis of circadian rhythm disorders [21].
Statistical analysis
The collected data were downloaded from each armband to a PC, and an advanced analysis technique was used to extract and analyze the different variables for each patient using Body Media InnerView Professional 6.1. For heat flux, skin temperature, near-body temperature, energy expenditure, and METs, data were analyzed and averages were calculated as follows: overall weekly average, overall hourly average for each day, and hourly average for each week. In order to obtain the best estimates of the overall acrophase for skin temperature and energy expenditure, the 24-hour cosinor model (where M = MESOR; A = Amplitude; Φ = Acrophase; T=24 h) was used [11]. This model was carried out for patients during their stay in the CCU and again in the ward. Data in the tables are expressed as means ± standard deviation (SD). For continuous variables, the t-test was used for comparisons. If the normality test failed, the Mann-Whitney rank-sum test was used. Results were considered statistically significant if P values were ≤0.05. The Statistical Package for Social Sciences (SPSS 16.0) and MS Excel 2007 were used for analysis and presentation.
Results
We studied 46 consecutive patients; 39 (85%) were males and 14 (30%) were smokers, with ACS observed within the first 24 hours of admission to the CCU. The admitting diagnoses were acute myocardial infarction (MI) in 37 patients (80%) and unstable angina (UA) in 9 patients (20%). NSTEMI was diagnosed in 15 patients, and STEMI was diagnosed in 22 patients. Of the STEMI group, the site of infarction was anterior or antro-lateral in 10 patients and inferior or infero-posterior in 12 patients. The mean age of the patients was 58.7±8.6 yrs, and the mean body mass index (BMI) was 26.7±5.4 Kg/m2. Ejection fraction (EF) data were available for 41 patients. The ejection fraction was >45% in 18 patients, 30–45% in 14 patients, and <30% in the remaining 9 patients. Co-morbid medical conditions present in the study group included hypertension in 16 patients, diabetes mellitus in 20 patients and hyperlipidemia in 18 patients. Table 1 shows the sleep characteristics and energy expenditure of all patients in the CCU. The TST was 6.9±2.3 hours divided into 6.8±3.4 sessions. Night sleep constituted 83% of total sleep time. Energy expenditure was 1.2 ± 0.2 kcal/min, and the METs were 1.0±0.1. The sex and smoking history of each patient with MI and unstable angina did not have a significant impact on the TST or nap time, skin temperature, energy expenditure or METs. On the other hand, TST + nap duration was significantly lower in patients with lower ejection fractions (EF >45% =7.84±2.43 h; EF 30±45% =6.36±1.9 h and EF <30% =5.58±2.45 h, P value=0.041).
Table 1.
Demographics and sleep characteristics of patients admitted to the CCU.
| Variable (n=46) | Mean ± SD |
|---|---|
| Male | 39 (85%) |
| Smoker | 14 (30%) |
| Number of patients staying only in the CCU | 18 (39%) |
| Number of patients staying in both the CCU & the ward | 28 (61%) |
| Age (years) | 58.7±8.6 |
| BMI (Kg/m2) | 26.7±5.4 |
| TST (hr) | 6.9±2.3 |
| Nighttime sleep duration (hr) | 5.8±2.0 |
| Nighttime sleep sessions | 2.3±0.6 |
| Nighttime sleep duration (hr)/session | 2.7±1.2 |
| NAP duration (hr) | 1.2±1 |
| Number of NAP sessions | 1.6±1 |
| NAP duration (hr)/session | 0.8±0.5 |
| Heat flux | 62.2±13 |
| Skin temperature (°C) | 32.7±0.6 |
| Near body temperature (°C) | 32.7±0.6 |
| Energy expenditure (kcal/min) | 1.2±0.2 |
| METs | 1±0.1 |
METs – metabolic equivalents of task; CCU – coronary care unit.
Follow-up monitoring was done for 28 of the 46 patients (21 males) after transfer to the general ward because the remaining 18 were discharged from the CCU directly to home after 72 hours. Table 2 shows a comparison between sleep characteristics and energy expenditure for these 28 patients in the CCU and the general wards. There was no significant difference between nighttime sleep in the CCU and the wards (5.6±2.2 h and 6.2±2.0 h, respectively). Similarly, no significant difference was found in the time spent in naps. However, nighttime sleep sessions were more frequent and shorter in the CCU than in the ward (2.4 h vs. 3.5 h P=0.015). The energy expenditure and METs were significantly less in the CCU than in the wards. Skin and near body temperature were significantly lower in the CCU than in the wards. Table 3 shows the MESOR and acrophase of skin temperature and energy expenditure in the CCU and in the wards. The MESOR for skin temperature was 32.79°C and 33.06°C in the CCU and the wards, respectively. The acrophase for skin temperature in CCU was at 13.3±9.5 h, and it did not differ significantly from that in the ward at 10.6±9.6 hours. The distribution of sleep over 24 hours (Figure 1) demonstrates that most sleep sessions took place during the night in both the CCU (Figure 1A) and the general ward (Figure 1B). On the other hand, the MESOR and the amplitude of energy expenditure were significantly lower in the CCU than in the general ward.
Table 2.
Comparison between sleep characteristics and energy expenditure in the CCU and the wards.
| Variable | CCU n=28 | Ward n=28 | p value |
|---|---|---|---|
| TST (hr) | 6.66±2.43 | 7.49±2.42 | NS |
| Nighttime sleep duration (hr) | 5.64±2.21 | 6.23±2.03 | NS |
| Night sleep sessions | 2.47±0.51 | 2.13±0.81 | 0.056 |
| Night sleep (hr)/session | 2.40±1.11 | 3.53±2.30 | 0.015 |
| NAP duration (hr) | 1.26±1.06 | 1.26±1.09 | NS |
| Nap sessions | 1.54±1.04 | 1.40±1.14 | NS |
| NAP duration (hr)/session | 0.76±0.48 | 0.67±0.50 | NS |
| Total sleep sessions | 7.39±3.48 | 8.79±5.43 | NS |
| Night sleep + NAP duration (hr)/session | 1.71±0.66 | 2.34±1.11 | 0.004 |
| Heat flux | 61.93±13.21 | 63.22±13.8 | NS |
| Skin temperature (°C) | 32.76±0.61 | 33.04±0.47 | 0.011 |
| Near body temperature (°C) | 32.73±0.63 | 33.01±0.47 | 0.013 |
| Energy expenditure (kcal/min) | 1.19±0.18 | 1.24±0.19 | 0.000 |
| METs | 1.00±0.13 | 1.05±0.15 | 0.000 |
TST – total sleep time; METs – metabolic equivalents of task.
Table 3.
Cosinor Model results for skin temperature and energy expenditure in the CCU and the wards.
| CCU | Ward | P-value | |
|---|---|---|---|
| Skin temperature (°C) | |||
| MESOR | 32.79±0.79 | 33.06±0.59 | 0.104 |
| Amplitude | 0.66±0.3 | 0.62±0.33 | 0.715 |
| Acrophase (24 hr) | 13.31±9.53 | 10.58±9.58 | 0.437 |
| Energy expenditure (kcal/min) | |||
| MESOR* | 1.22±0.19 | 1.28±0.2 | 0.007 |
| Amplitude* | 0.05±0.03 | 0.1±0.08 | 0.005 |
| Acrophase (24 hr) | 11.85±7.24 | 9.24±7.46 | 0.335 |
Figure 1.
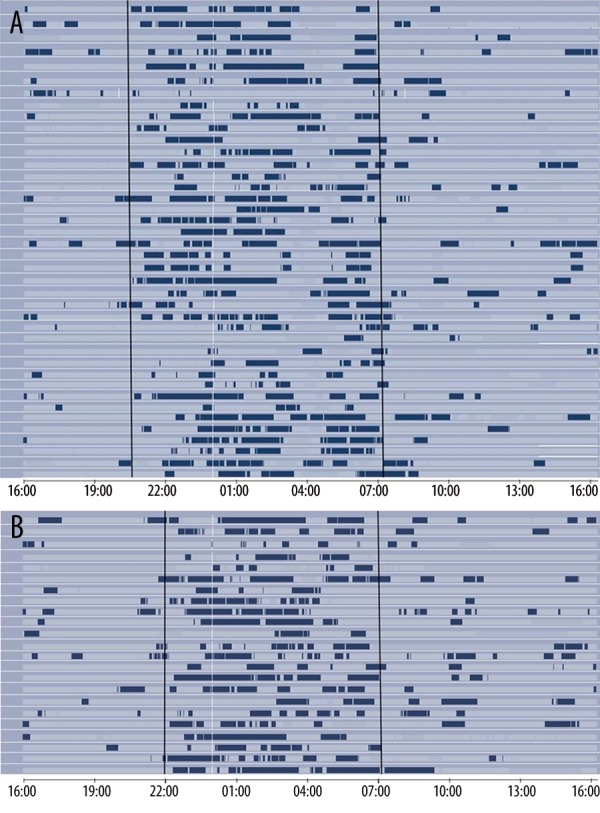
Distribution of sleep over the first 24 hr in the CCU (A) and the general ward (B).
The average noise level was highest in the CCU (70 decibels), followed by the male ward (65 decibels). The least amount of noise was in the female ward (55 decibels) (Table 4). Peak light level during the daytime in the CCU was 134 lux; during the nighttime, the peak light level was 1 lux. In the male ward, peak light levels during the day and night were lower compared to the female ward (57 lux and 16 lux vs. 38 lux and 9 lux, respectively). Lights were turned off at 22:00 in the CCU, and the wards as per the hospital policy. The mean ambient temperature in the CCU was 23.0±0.4°C, compared to 24.3±0.3°C in the ward. The humidity level was 30% in both the CCU and the wards.
Table 4.
Noise levels in the CCU and the wards.
| Attributes | Maximum (dB) | Average (dB) | Minimum (dB) |
|---|---|---|---|
| CCU | 85 | 70 | 50 |
| Male ward | 88 | 65 | 40 |
| Female ward | 85 | 55 | 25 |
CCU – coronary care unit.
Discussion
This is the first study utilizing the SWA portable device to examine the sleep and circadian patterns of temperature in patients with ACS in the CCU. We studied the sleep patterns, circadian rhythms of temperature and energy expenditure in a homogeneous group of 46 patients with ACS during their CCU stay and after discharge to the general ward. We objectively demonstrated that patients in the CCU have partial sleep deprivation along with fragmented sleep.
The nighttime sleep of patients in the CCU was less than the standard normative values (7–7.9 h) [22]. This deficit in sleep time was compensated for by the addition of naps. However, whether a nap has the same physiologic advantages as that obtained during night sleep is uncertain [23]. Many investigators have demonstrated the adverse effects of poor sleep continuity and partial sleep deprivation on the various biological and psychological processes in the body, including immune function, the hormonal and metabolic systems and neuro-cognitive abilities [3–6]. Furthermore, longer naps taken as a replacement for shortened night sleep were found to be less effective in achieving their restorative function [24,25]. Similarly, other studies have shown a reduction of TST in the period following an acute MI; multiple etiological explanations have been proposed, including the acute illness itself, its associated psycho-physiological changes and different environmental factors [26,27]. Recently, animal studies have suggested that structural changes that occur in the central nervous system post-MI – particularly in the limbic system, hypothalamus and brainstem – can be partly responsible for sleep deprivation [28]. Another contributing factor could be the increase in inflammatory cytokines noted in the brain following AMI [29]. On the other hand, the symptoms of depression and perceived distress during AMI were found to be associated with acute stress disorder, which in turn may have an important impact on sleep duration [30].
During their CCU stay, patients spent 1.2±1.0 h in naps. This contributed to only 17% of their TST. In an earlier study of 12 patients who had recently experienced an acute MI, Broughton and Baron estimated the amount of daytime sleep to be around 8–10 hours [2]. The difference between our results and those of Broughton and Baron could be due to the increased awareness regarding minimizing environmental and staff-related nighttime sleep disturbances in the CCU over the years.
Nighttime sleep sessions in the CCU were more frequent and shorter than in the ward. This indicates the presence of fragmented sleep in the CCU, possibly with more awakenings. Indeed, a study using polysomnography on 50 patients with ACS revealed significantly lower sleep efficiency and a higher arousal index in the first few days following ACS [31]. In another study this was shown to be transient and normalized by the ninth post-infarction night [2]. Furthermore, Redeker et al., using wrist-mounted actigraphy, found the combination of age, sex, New York Heart Association functional class, and pre-hospitalization sleep loss to be a strong predictor of sleep efficiency and duration of non-sleep time in 33 patients with acute MI or UA [32]. In our study group, sleep duration in the CCU was shorter in patients with a lower EF.
The contribution of environmental factors to sleep disruption and frequent awakenings in the CCU has been described previously [33,34]. However, environmental noise was found to be the cause of only 17% of awakenings in the intensive care unit (ICU) [35]. The Environmental Protection Agency recommends that the peak noise level should not exceed 35 dB at night and 45 dB during the day [36]. The peak noise level in our CCU was higher than the recommended level and higher than the peak level in the general wards. These higher noise levels in intensive care areas have been reported previously, with staff conversation being the main source of recorded noise [34,35,37]. Light is the primary zeitgeber or cue responsible for setting the circadian clock. Disturbances in the light-dark cycle are the primary causes of sleep disruption. There is evidence to suggest that a dose- response relationship between light level and the resetting of the circadian clock exists in humans [38]. The recorded light level in our study was low during both night and day in the CCU and the ward. This is probably related to the location of light sources away from the head of the bed and the frequent use of curtains to maintain patient privacy, which was more obvious in the female ward readings; however, it is still possible that there was a transient increase in light levels during acute care activities in the night as our recordings were intermittent once every 2 hours.
Body temperature is a frequently measured marker of circadian rhythm because body temperature rises during the day and decreases at night. This is believed to be mainly due to circadian changes in the rate of heat loss through the extremities as a result of melatonin-mediated cutaneous vasodilation [39–41]. In our study we recorded the patients’ skin temperature over 24 hours in the CCU and later in the ward. This monitoring revealed no significant alteration of temperature acrophase, which was recorded around midday in both the CCU and the ward. These data contrast with the findings of 2 retrospective studies that reported circadian rhythm disturbances in critically ill patients in the ICU [42,43]. However, our patients were less sick, maintained a normal level of consciousness and had a different disease process.
Overall energy expenditure and METs were found to be significantly lower in the CCU than in the ward. This may reflect a decrease in the metabolism of body cell mass, and it could be related to the strict bed rest advised during the acute phase of ACS. However, other factors related to the metabolic effects of an acute MI need to be explored further because this is the first time that the energy expenditure in ACS patients has been measured in the CCU.
A limitation to this study was the lack of recording of patient care interactions, which have been found to be a significant cause of arousals and awakenings in critical care units [35,44]. Such interactions could therefore contribute to the observed difference in night awakenings. Another possible contributing factor that was not addressed in our study is the presence of sleep-disordered breathing (SDB), which is highly prevalent in patients with ACS, resulting in frequent arousals [32]; however, SDB is less likely to be the cause of increased awakenings in the CCU because sleep fragmentation improved within days after transfer to the ward [31].
Conclusions
The SWA is a promising and convenient tool that allows for the study of sleep patterns, circadian rhythms and metabolic changes over 24 hours in patients with ACS in the CCU and later in the ward. Patients with ACS have sleep fragmentation and shorter nocturnal sleep duration in the CCU compared to the ward. However, there was no difference in the patients’ circadian rhythms of skin temperature between the CCU and the ward. Future studies should utilize the SWA to assess the effect of sleep promotion interventions on sleep patterns in patients in acute care units.
Abbreviations
- CCU
coronary care unit
- SWA
SenseWear armband
- ACS
acute coronary syndrome
- METs
metabolic equivalents of a task
- MESOR
midline-estimating statistic of rhythm
- ACU
acute care unit
- STEMI
ST segment elevation myocardial infarction
- NSTEMI
non-ST segment elevation myocardial infarction
- UA
unstable angina
- TST
total sleep time
Footnotes
Competing interest section
The authors have no competing interest to report.
Source of support: This work was supported by a grant from the College of Medicine Research Center (CMRC) and the University Sleep Disorders Center, King Saud University
References
- 1.Cooper AB, Thornley KS, Young GB, et al. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117(3):809–18. doi: 10.1378/chest.117.3.809. [DOI] [PubMed] [Google Scholar]
- 2.Broughton R, Baron R. Sleep patterns in the intensive care unit and on the ward after acute myocardial infarction. Electroencephalogr Clin Neurophysiol. 1978;45(3):348–60. doi: 10.1016/0013-4694(78)90187-6. [DOI] [PubMed] [Google Scholar]
- 3.Bryant PA, Trinder J, Curtis N. Sick and tired: Does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4(6):457–67. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- 4.Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350(16):1629–38. doi: 10.1056/NEJMoa020266. [DOI] [PubMed] [Google Scholar]
- 5.Jackson JC, Hart RP, Gordon SM, et al. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med. 2003;31(4):1226–34. doi: 10.1097/01.CCM.0000059996.30263.94. [DOI] [PubMed] [Google Scholar]
- 6.Forest G, Godbout R. Attention and memory changes. In: Kushida CA, editor. Sleep Deprivation: Basic Science, Physiology, and Behavior. New York: Marcel Dekker; 2005. pp. 199–222. [Google Scholar]
- 7.Chrusciel P, Goch A, Banach M, et al. Circadian changes in the hemostatic system in healthy men and patients with cardiovascular diseases. Med Sci Monit. 2009;15(10):RA203–8. doi: 10.12659/msm.878203. [DOI] [PubMed] [Google Scholar]
- 8.Meyer TJ, Eveloff SE, Bauer MS, et al. Adverse environmental conditions in the respiratory and medical ICU settings. Chest. 1994;105(4):1211–16. doi: 10.1378/chest.105.4.1211. [DOI] [PubMed] [Google Scholar]
- 9.Weinhouse GL, Schwab RJ. Sleep in the critically ill patient. Sleep. 2006;29(5):707–16. doi: 10.1093/sleep/29.5.707. [DOI] [PubMed] [Google Scholar]
- 10.BaHammam A. Sleep in acute care units. Sleep Breath. 2006;10(1):6–15. doi: 10.1007/s11325-005-0044-8. [DOI] [PubMed] [Google Scholar]
- 11.BaHammam A, Alrajeh M, Albabtain M, et al. Circadian pattern of sleep, energy expenditure, and body temperature of young healthy men during the intermittent fasting of Ramadan. Appetite. 2010;54(2):426–29. doi: 10.1016/j.appet.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Jakicic JM, Marcus M, Gallagher KI, et al. Evaluation of the SenseWear Pro Armband to assess energy expenditure during exercise. Med Sci Sports Exerc. 2004;36(5):897–904. doi: 10.1249/01.mss.0000126805.32659.43. [DOI] [PubMed] [Google Scholar]
- 13.Jette M, Sidney K, Blumchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13(8):555–65. doi: 10.1002/clc.4960130809. [DOI] [PubMed] [Google Scholar]
- 14.Krauchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol. 1994;267(3 Pt 2):R819–29. doi: 10.1152/ajpregu.1994.267.3.R819. [DOI] [PubMed] [Google Scholar]
- 15.Malavolti M, Pietrobelli A, Dugoni M, et al. A new device for measuring resting energy expenditure (REE) in healthy subjects. Nutr Metab Cardiovasc Dis. 2007;17(5):338–43. doi: 10.1016/j.numecd.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Teller A. A platform for wearable physiological computing. Interacting with Computers. 2004;16(5):917–37. [Google Scholar]
- 17.Malavolti M, Poli M, Pietrobelli A, et al. Body composition and nutritional habits in professional ballet dancers. Int J Body Compos Res. 2005;3:63–68. [Google Scholar]
- 18.Mignault D, St-Onge M, Karelis AD, et al. Evaluation of the Portable HealthWear Armband: a device to measure total daily energy expenditure in free-living type 2 diabetic individuals. Diabetes Care. 2005;28(1):225–27. doi: 10.2337/diacare.28.1.225-a. [DOI] [PubMed] [Google Scholar]
- 19.Germain A, Buysse D, Kupfer DJ. Preliminary validation of a new device for studying sleep. Utah Sleep; Presented at (Sleep 2006) 20th Anniversary meeting of the Associated Professional Sleep Societies (APSS); June 17–22, 2006; Salt Lake City. 2006. p. A1078. [Google Scholar]; Patel, Slivka WA, Sciurba FC. Validation of a wearable body monitoring. 2006. [Google Scholar]
- 20.Miwa H, Sasahara S-i, Matsui T. Roll-over Detection and Sleep Quality Measurement using a Wearable Sensor. Engineering in Medicine and Biology Society 29th Annual International Conference of the IEEE; 2007. pp. 1507–10. [DOI] [PubMed] [Google Scholar]
- 21.Littner M, Hirshkowitz M, Davila D, et al. Practice parameters for the use of auto-titrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome. An American Academy of Sleep Medicine report. Sleep. 2002;25(2):143–47. doi: 10.1093/sleep/25.2.143. [DOI] [PubMed] [Google Scholar]
- 22.Ferrara M, De Gennaro L. How much sleep do we need? Sleep Med Rev. 2001;5(2):155–79. doi: 10.1053/smrv.2000.0138. [DOI] [PubMed] [Google Scholar]
- 23.Hardin KA. Sleep in the ICU: potential mechanisms and clinical implications. Chest. 2009;136(1):284–94. doi: 10.1378/chest.08-1546. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi M, Watanabe M, Hori T. The effects of a 20 min nap in the mid-afternoon on mood, performance and EEG activity. Clin Neurophysiol. 1999;110(2):272–79. [Google Scholar]
- 25.Stampi C, Mullington J, Rivers M, et al. Ultra short sleep schedules: sleep architecture and recuperative value of 80-, 50- and 20-min naps. In: Horne J, editor. Sleep. Vol. 90. Pontenagel Press; Bochum: 1990. pp. 71–74. [Google Scholar]
- 26.BaHammam A. Sleep quality of patients with acute myocardial infarction outside the CCU environment: a preliminary study. Med Sci Monit. 2006;12(4):CR168–72. [PubMed] [Google Scholar]
- 27.Schiza SE, Simantirakis E, Bouloukaki I, et al. Sleep patterns in patients with acute coronary syndromes. Sleep Med. 2010;11(2):149–53. doi: 10.1016/j.sleep.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Bah TM, Laplante F, Wann BP, et al. Paradoxical sleep insomnia and decreased cholinergic neurons after myocardial infarction in rats. Sleep. 2010;33(12):1703–10. doi: 10.1093/sleep/33.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rana I, Stebbing M, Kompa A, et al. Microglia activation in the hypothalamic PVN following myocardial infarction. Brain Res. 2010;1326:96–104. doi: 10.1016/j.brainres.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 30.Roberge MA, Dupuis G, Marchand A. Acute stress disorder after myocardial infarction: prevalence and associated factors. Psychosom Med. 2008;70(9):1028–34. doi: 10.1097/PSY.0b013e318189a920. [DOI] [PubMed] [Google Scholar]
- 31.BaHammam A, Al-Mobeireek A, Al-Nozha M, et al. Behaviour and time-course of sleep disordered breathing in patients with acute coronary syndromes. Int J Clin Pract. 2005;59(8):874–80. doi: 10.1111/j.1742-1241.2005.00534.x. [DOI] [PubMed] [Google Scholar]
- 32.Redeker NS, Tamburri L, Howland CL. Prehospital correlates of sleep in patients hospitalized with cardiac disease. Res Nurs Health. 1998;21(1):27–37. doi: 10.1002/(sici)1098-240x(199802)21:1<27::aid-nur4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 33.Aaron JN, Carlisle CC, Carskadon MA, et al. Environmental noise as a cause of sleep disruption in an intermediate respiratory care unit. Sleep. 1996;19(9):707–10. doi: 10.1093/sleep/19.9.707. [DOI] [PubMed] [Google Scholar]
- 34.Gabor JY, Cooper AB, Crombach SA, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167(5):708–15. doi: 10.1164/rccm.2201090. [DOI] [PubMed] [Google Scholar]
- 35.Freedman NS, Gazendam J, Levan L, et al. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163(2):451–57. doi: 10.1164/ajrccm.163.2.9912128. [DOI] [PubMed] [Google Scholar]
- 36.Information on Levels of Environmental Noise Requisite to Protect Public Health and Welfare with an Adequate Margin of Safety. U.S. Environmental Protection Agency; Washington, DC: Mar, 1974. Report No. 550/9-74-004. [Google Scholar]
- 37.Allaouchiche B, Duflo F, Debon R, et al. Noise in the postanaesthesia care unit. Br J Anaesth. 2002;88(3):369–73. doi: 10.1093/bja/88.3.369. [DOI] [PubMed] [Google Scholar]
- 38.Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379(6565):540–42. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- 39.Cagnacci A. Influences of melatonin on human circadian rhythms. Chronobiol Int. 1997;14(2):205–20. doi: 10.3109/07420529709001156. [DOI] [PubMed] [Google Scholar]
- 40.Krauchi K. How is the circadian rhythm of core body temperature regulated? Clin Auton Res. 2002;12(3):147–49. doi: 10.1007/s10286-002-0043-9. [DOI] [PubMed] [Google Scholar]
- 41.Carranza-Lira S, Garcia Lopez F. Melatonin and climactery. Med Sci Monit. 2000;6(6):1209–12. [PubMed] [Google Scholar]
- 42.Tweedie IE, Bell CF, Clegg A, et al. Retrospective study of temperature rhythms of intensive care patients. Crit Care Med. 1989;17(11):1159–65. doi: 10.1097/00003246-198911000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Nuttall GA, Kumar M, Murray MJ. No difference exists in the alteration of circadian rhythm between patients with and without intensive care unit psychosis. Crit Care Med. 1998;26(8):1351–55. doi: 10.1097/00003246-199808000-00019. [DOI] [PubMed] [Google Scholar]
- 44.Tamburri LM, DiBrienza R, Zozula R, Redeker NS. Nocturnal care interactions with patients in critical care units. Am J Crit Care. 2004;13(2):102–12. quiz 14–15. [PubMed] [Google Scholar]


