Summary
Background
Tularemia is a zoonotic infection, and the causative agent is Francisella tularensis. A first-line therapy for treating tularemia is aminoglycosides (streptomycin or, more commonly, gentamicin), and treatment duration is typically 7 to 10 days, with longer courses for more severe cases.
Material/Methods
We evaluated 11 patients retrospectively. Failure of the therapy was defined by persistent or recurrent fever, increased size or appearance of new lymphadenopathies and persistence of the constitutional syndrome with elevation of the levels of the proteins associated with the acute phase of infection.
Results
We observed fluctuating size of lymph nodes of 4 patients who were on the 7th day of empirical therapy. The therapy was switched to streptomycin alone and continued for 14 days. The other 7 patients, who had no complications, were on cefazolin and gentamycin therapy until the serologic diagnosis. Then we evaluated them again and observed that none of their lymph nodes regressed. We also switched their therapy to 14 days of streptomycin. After the 14 days on streptomycin therapy, we observed all the lymph nodes had recovered or regressed. During a follow-up 3 weeks later, we observed that all their lymph nodes had regressed to the clinically non-significant dimensions (<1 cm).
Conclusions
All patients were first treated with gentamicin, but were than given streptomycin after failure of gentamicin. This treatment was successful in all patients. The results of our study suggest that streptomycin is an effective choice of first-line treatment for pediatric oropharyngeal tularemia patients.
Keywords: oropharyngeal tularemia, treatment failure, gentamicin, streptomycin
Background
Tularemia is a zoonotic infection. In humans the disease is usually severe and occasionally fatal. The causative agent, Francisella tularensis, is a Gram-negative, aerobic bacterium. Currently, there are 4 recognized subspecies (tularensis, holarctica, mediasiatica and novicida) [1]. Outbreaks of tularemia have been observed in many regions of the world. The highly virulent subspecies tularensis is restricted almost completely to North America. In contrast, F. tularensis subsp. holarctica, which causes a milder form of the disease, is found in the entire northern hemisphere, especially in Scandinavia, central Europe, Russia and Asia [2]. F. tularensis spreads through the bite of infected animals, direct contact with infected tissue, or contaminated soil, inhalation of aerosolized organisms, and ingestion of contaminated meat or water [3]. In humans there are several tularemia syndromes, mostly dependent on the route of entry of the bacterium. Ulceroglandular and glandular tularemia are usually acquired by a bite from an arthropod vector or handling of infected animals. Oculoglandular tularemia occurs as a result of a transfer of bacterium through the conjunctiva. Oropharyngeal or gastrointestinal tularemia is acquired by oral intake of contaminated food or water. Pulmonary tularemia appears after inhalation of bacteria. The most severe form, typhoidal tularemia, has a less clear route of acquisition [4–6]. Oropharyngeal infection is a common presentation in Turkey and in other Eastern European countries, attributed to the consumption of contaminated water and food [7].
Antibiotic treatment is necessary for all forms, and for decades therapy consisted of aminoglycosides and tetracyclines, or chloramphenicol in the early days. However, tetracycline or chloramphenicol therapy increases the risk of a relapse. Moreover, the adverse effect of tetracycline in children may be of concern [8,9].
Tularemia is less frequent among children compared to adults, and pediatric cases are often misdiagnosed. We aimed to describe the clinical characteristics and therapeutic options of 11 pediatric patients at our center who were diagnosed with tularemia.
Material and Methods
Eleven patients diagnosed with tularemia were evaluated retrospectively. They were referred to Cumhuriyet University Hospital, Turkey, from January 2009 through March 2010, with persistent fever, sore throat, and massive swelling of the cervical lymph nodes, despite at least 5 days’ therapy with β-lactam/macrolide antibiotics for a pre-diagnosed upper respiratory tract infection.
All patients with suspected tularemia were physically examined and by using a clinical history protocol the following data were collected: symptoms, physical signs, and results of laboratory tests. We obtained detailed information about the site of infection, day of onset, water characteristics, eating of hunted animals, rodent bites or contacts, eating food without appropriate cleaning and cooking, travel to an endemic region, insect bites, contact with animals, working in poultry houses, and clinical symptoms. The sanitary conditions and the water sources of these villages were examined by the local office of the Ministry of Health.
Blood specimens were obtained at admission to the hospital from all 11 patients with suspected tularemia. Blood specimens were transported immediately to the national reference laboratories for tularemia testing. The microagglutination test was used for serological diagnosis. The sera with antibody titers equal to or higher than 1/160 were accepted as positive for F. tularensis in all cases. The diagnosis of tularemia was made with the positivity of antibodies and compatible clinical findings [10,11]. Due to the high virulence of F. tularensis, special precautions are necessary for culturing. Since the recommended security level is not currently available for isolation of F. tularensis in our laboratory, blood cultures, throat smears, and lymph node aspirates were not studied in our center.
Failure of therapy was defined by the presence of 1 of the following findings: persistent or recurrent fever, increased size or appearance of new lymphadenopathies and persistence of the constitutional syndrome with the elevation of the levels of the proteins associated with the acute phase of infection [12].
Results
A total of 11 patients (4 females and 7 males) were diagnosed with tularemia. The mean age (Mean ±SD) of the patients was 12.5±2.9 (8–17) years.
None of the patients had a previous clinical history of tularemia. No patient had a history of tick bite or eating of game (wild animal meat). However, all were living in rural regions and consuming non-chlorinated water. The most important epidemiological finding was that poultry farm work was the most common livelihood in this region, and increased rodent activity around poultry houses and villages had been reported by residents during the previous year.
The most frequent symptoms were sore throat (82%) and fever (82%). Lymphadenopathy (100%) and pharyngeal hyperemia (82%) were the most frequent signs (Figure 1). The lymphadenopathies were localized in the cervical region. Clinical characteristics, treatments, and outcomes for the patients are shown in Table 1.
Figure 1.
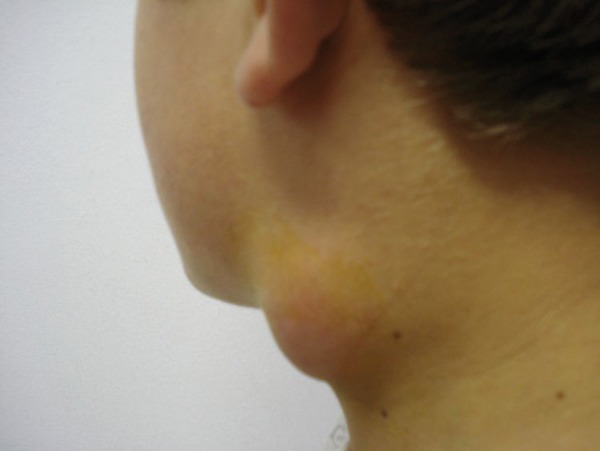
Lymph node enlargement in a case of oropharyngeal tularemia.
Table 1.
Clinical characteristics of patients.
| No/age(y)/sex | Clinical form | MA titre | Symptoms | Signs | OS-GT | LAP Drainage | Outcome |
|---|---|---|---|---|---|---|---|
| 1/12/M | Oropharyngeal | 1/1280 | Fever, Sore throat, Swelling on the neck | LAP, Pharynx hyperemia, Tonsillopharyngitis | 16 | − | Recovered |
| 2/10/M | Oropharyngeal | 1/1280 | Fever, Sore throat, Swelling on the neck, Myalgia | LAP, Pharynx hyperemia, Tonsillopharyngitis | 14 | − | Recovered |
| 3/13/F | Oropharyngeal | 1/640 | Fever, Sore throat, Swelling on the neck | LAP, Pharynx hyperemia, Tonsillopharyngitis | 14 | + | Recovered |
| 4/15/M | Oropharyngeal | 1/1280 | Fever, Sore throat, Swelling on the neck | LAP, Pharynx hyperemia, Tonsillopharyngitis | 17 | + | Recovered |
| 5/17/M | Oropharyngeal | 1/1280 | Fever, Sore throat, Swelling on the neck | LAP, Pharynx hyperemia, Tonsillopharyngitis | 15 | + | Recovered |
| 6/14/F | Oropharyngeal | 1/320 | Swelling on the neck | LAP | 18 | − | Recovered |
| 7/8/M | Oropharyngeal | 1/1280 | Fever, Sore throat, Swelling on the neck | LAP, Pharynx hyperemia, Tonsillopharyngitis | 12 | + | Recovered |
| 8/17/F | Oropharyngeal | 1/1280 | Fever, Sore throat, Swelling on the neck | LAP, Pharynx hyperemia, Tonsillopharyngitis | 14 | − | Recovered |
| 9/10/M | Oropharyngeal | 1/640 | Fever, Sore throat, Swelling on the neck | LAP, Pharynx hyperemia, Tonsillopharyngitis | 13 | − | Recovered |
| 10/12/M | Oropharyngeal | 1/1280 | Fever, Sore throat, Swelling on the neck | LAP, Pharynx hyperemia, Tonsillopharyngitis | 13 | − | Recovered |
| 11/10/F | Oropharyngeal | 1/1280 | Swelling on the neck | LAP | 12 | − | Recovered |
F – female; M – male; MA – microagglutination; LAP – lymphadenopathy; OS-GT – duration between onset of symptoms and starting of gentamicin treatment (days).
The results from the patients’ laboratory tests were normal, except for an increase in the serum levels of erythrocyte sedimentation rate, C-reactive protein and leukocytosis. Serological diagnosis was made on the basis of the following findings: the antibody titer against F. tularensis was found to be 1/320 for 1 patient’s sera, 1/640 for 2 patients’ sera, and 1/1280 for 8 patients’ sera. We didn’t perform a second test because all our patients’ antibody titers were over 1/160. Serologic tests for salmonella, brucella, toxoplasma, cytomegalovirus, Epstein-Barr virus, hepatitis A/B/C, human immunodeficiency virus, and parvovirus B19 were all negative. Purified protein derivative tests were all negative. Aerobic and anaerobic blood culture, urine culture and throat swab culture were also all negative for all the patients. Based on all these findings we diagnosed them as having oropharyngeal tularemia.
Before the diagnosis of tularemia, 11 patients had been using β-lactam/macrolide antibiotics, which were prescribed by the local physician. After the hospitalization, we prescribed cefazolin sodium (100 mg/kg/day, 3 doses) and gentamycin (7.5 mg/kg/day, 3 doses) empirically. After the serologic confirmation of tularemia, we continued the therapy, using only gentamycin. The median of duration between onset of symptoms and starting the gentamicin treatment was 14 (12–18) days (Table 1). We observed the fluctuating size of the lymph nodes of the 4 patients who were on the 7th day of empirical cefazolin and gentamycin therapy. Three of these patients were boys, and their mean age was 13.3±3.9 (8–17 years). Their serum white blood count, sedimentation rate, and CRP levels increased at this time. The nodes were surgically drained and then the therapy was switched to streptomycin (30 mg/kg/day, 2 doses) alone and continued for 14 days. The other 7 patients who had no complications were on cefazolin and gentamycin therapy for 7–10 days until the serologic diagnosis was confirmed. After the confirmation, we evaluated them again and observed that none of their lymph nodes regressed. Three of them had enlarged lymph nodes, and their serum white blood count, sedimentation rate, and CRP levels were still at high levels, so we also switched their therapy to 14 days of streptomycin. After the 14 days on streptomycin therapy, we evaluated the patients again, and we observed all the lymph nodes had recovered or regressed, and their laboratory parameters had regressed to the normal limits. At that time, all the patients were discharged. During a follow-up 3 weeks later, we observed that all their lymph nodes had regressed to clinically non-significant dimensions (<1 cm). None of the patients died, and all the patients recovered without complications.
Discussion
The first documented tularemia epidemic, affecting 150 patients, occurred in 1936 in the Trakya region of Turkey. This was followed by other epidemics, and sporadic cases were observed in different regions of Turkey [13–15]. Most of the reported tularemia cases in Turkey in the last 20 years have been oropharyngeal and related to the consumption of contaminated water [14–16]. No previous tularemia cases were reported in the Sivas province, in the central Anatolia region. These cases are the first tularemia cases reported in Sivas, Turkey.
The key to successful treatment of tularemia is early diagnosis and initiation of appropriate antibiotic therapy. Because F. tularensis infections are rare, no randomized studies have compared either treatment regimens or duration of therapy [17,18].
Aminoglycosides (streptomycin or, more commonly, gentamicin) are first-line therapies. Treatment duration is typically 7 to 10 days, with longer courses for more severe cases [18]. In the United States, where the highly virulent F. tularensis subsp. Tularensis prevails, antibiotic treatment with streptomycin or gentamicin for 7–14 days is the main treatment employed [19]. A meta-analysis review of treated tularemia cases revealed that treatment with streptomycin resulted in a 97% cure rate in 224 individuals treated [8]. Successful in 86% (31/36) of treated cases, gentamicin is considered to be an acceptable alternative to streptomycin. In 6 individuals treated with another aminoglycoside, tobramycin, only half were successfully treated, so tobramycin is not recommended as an alternative [20].
Early diagnosis and application of the relevant treatment in advance (within the first 3 weeks) is much more effective to resolve the infection and prevent lymph node suppuration [14]. In our series, the gentamicin treatment started within 3 weeks of the disease onset; however, it failed in all patients and did not prevent lymph node suppuration. Eventually surgical drainage was needed in 4 patients.
Several other antibiotics have been shown to have good efficacy in the treatment of tularemia and may be used as alternatives if aminoglycosides are contraindicated or cannot be tolerated [20]. Alternative antimicrobials with activity against F. tularensis include ciprofloxacin, imipenem-cilastatin, doxycycline, and chloramphenicol [17,18].
In 1 study, tetracycline therapy was successful in 44 of 50 (88%) patients and resulted in no treatment failures, but 12% of individuals relapsed. Clinical relapses have been reported after treatment with either tetracyclines or chloramphenicol [8]. Because recrudescence of tularemia has been reported following discontinuation of therapy with the bacteriostatic antibiotics tetracycline and chloramphenicol – these antibiotics should be continued for at least 14 days [8]. But when alternatives exist, doxycycline should not be used in children younger than 8 years because tetracyclines are known to stain dental enamel [21].
Ciprofloxacin has not been approved in children younger than 12 years because of possible cartilage damage [17], but clinical experience using these safely in children is increasing [22–24]. Evidence of the efficacy of fluoroquinolones in the treatment of tularemia is increasing. In a review of 79 tularemia cases treated with fluoroquinolones between 1966 and 2000, 86% of infected individuals were successfully treated, and only 14% relapsed, failed therapy, or had to discontinue treatment because of adverse effects [25]. In a tularemia outbreak in Spain, ciprofloxacin had the highest treatment success rate and the fewest adverse events [26]. Ciprofloxacin has also been used to successfully treat a child who had failed gentamicin therapy [27].
Risi et al. [28] described a patient with ulceroglandular tularemia who initially responded to therapy with gentamicin but then clinically relapsed, so therapy was switched to ciprofloxacin for 28 days, and the patient was clinically cured. Therefore gentamycine, as a member of the aminoglycosides, is a drug of choice in the treatment of tularemia; however, alternative therapies are sometimes necessary.
If optimal therapy is not initiated within a few days of illness, defervescence may be delayed, and suppuration may progress in spite of effective treatment [29]. Therapeutic failure was observed for all of our patients after 10-days of gentamycin therapy, and fluctuating lymph nodes were seen at 4 of these patients. These findings led us to believe gentamycin to be ineffective for tularemia therapy. The patients’ recovery after 14 days of streptomycin therapy, including the regression of the lymphadenopathies and the laboratory findings, caused us to change our therapy of choice to streptomycin because of the loss of the signs and symptoms and because the recovery of the lymph nodes without any suppuration means the goal of the therapy was achieved [14,15].
Conclusions
In conclusion, oropharyngeal tularemia should be thought of as an alternative diagnosis for tonsillopharyngitis patients resistant to beta-lactam and macrolide antibiotics therapy. We recommend prior streptomycin therapy as an effective choice of treatment for pediatric oropharyngeal tularemia patients.
Acknowledgements
The authors thank Refik Saydam Hygiene Center of Ankara, Turkey for testing the serum samples.
Footnotes
Conflict of interest
No conflict of interest to declare.
Source of support: Departmental sources
References
- 1.Marinov KT, Georgieva ED, Ivanov IN, Kantardjiev TV. Characterization and genotyping of strains of Francisella tularensis isolated in Bulgaria. J Med Microbiol. 2009;58:82–85. doi: 10.1099/jmm.0.003426-0. [DOI] [PubMed] [Google Scholar]
- 2.Sjörstedt A. Tularemia: History, Epidemiology, Pathogen Physiology and Clinical Manifestations. Ann N Y Acad Sci. 2007;1105:1–29. doi: 10.1196/annals.1409.009. [DOI] [PubMed] [Google Scholar]
- 3.Sandström G, Sjöstedt A, Forsman M, et al. Characterization and classification of strains of Francisella tularensis isolated in the central Asian focus of the Soviet Union and in Japan. J Clin Microbiol. 1992;30:172–75. doi: 10.1128/jcm.30.1.172-175.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor JP, Istre GR, McChesney TC, et al. Epidemiological characteristics of human tularemia in the southwest-central states. 1981–1987. Am J Epidemiol. 1991;133:1032–38. doi: 10.1093/oxfordjournals.aje.a115812. [DOI] [PubMed] [Google Scholar]
- 5.Christova I, Velinov T, Kantardjiev T, Galev A. Tularemia outbreak in Bulgaria. Scand J Infect Dis. 2004;36:785–89. doi: 10.1080/00365540410021199. [DOI] [PubMed] [Google Scholar]
- 6.Celebi S, Hacimustafaoglu M, Gedikoglu S. Tularemia in children. Indian J Pediatr. 2008;75:1129–32. doi: 10.1007/s12098-008-0180-9. [DOI] [PubMed] [Google Scholar]
- 7.Tärnvik A, Priebe HS, Grunow R. Tularemia in Europe: an epidemiological overview. Scand J Infect Dis. 2004;36:350–55. doi: 10.1080/00365540410020442. [DOI] [PubMed] [Google Scholar]
- 8.Enderlin G, Morales L, Jacobs RF, Cross JT. Streptomycin and alternative agents for the treatment of tularemia: review of the literature. Clin Infect Dis. 1994;19:42–47. doi: 10.1093/clinids/19.1.42. [DOI] [PubMed] [Google Scholar]
- 9.Sawyer WD, Dangerfield HG, Hogge AL, Crozier D. Antibiotic prophylaxis and therapy of airborne tularemia. Bacteriol Rev. 1966;30:542–50. doi: 10.1128/br.30.3.542-550.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uhari M, Syrjälä H, Salminen A. Tularemia in children caused by Francisella tularensis biovar palaearctica. Pediatr Infect Dis J. 1990;9:80–83. doi: 10.1097/00006454-199002000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Koskela P, Herva E. Immunity against Francisella tularensis in northern Finland. Scand J Infect Dis. 1982;14:195–99. doi: 10.3109/inf.1982.14.issue-3.07. [DOI] [PubMed] [Google Scholar]
- 12.Tärnvik A, Sandström G, Sjöstedt A. Infrequent manifestations of tularaemia in Sweden. Scand J Infect Dis. 1997;29:443–46. doi: 10.3109/00365549709011851. [DOI] [PubMed] [Google Scholar]
- 13.Karadenizli A, Gürcan Ş, Kolaylı F, Vahaboğlu H. Outbreak of tularemia in Golcuk, Turkey in 2005: report of five cases and an overview of the literature from Turkey. Scand J Infect Dis. 2005;37:712–16. doi: 10.1080/00365540510012125. [DOI] [PubMed] [Google Scholar]
- 14.Helvaci S, Gedikoğlu S, Akalin H, Oral HB. Tularemia in Bursa, Turkey: 205 cases in ten years. Eur J Epidemiol. 2000;16:271–76. doi: 10.1023/a:1007610724801. [DOI] [PubMed] [Google Scholar]
- 15.Celebi G, Baruönü F, Ayoğlu F, et al. Tularemia, a reemerging disease in northwest Turkey: epidemiological investigation and evaluation of treatment responses. Jpn J Infect Dis. 2006;59:229–34. [PubMed] [Google Scholar]
- 16.Willke A, Meric M, Grunow R, et al. An outbreak of oropharyngeal tularaemia linked to natural spring water. J Med Microbiol. 2009;58:112–16. doi: 10.1099/jmm.0.002279-0. [DOI] [PubMed] [Google Scholar]
- 17.Burkhardt JE, Walterspiel JN, Schaad UB. Quinolone arthropathy in animals versus children. Clin Infect Dis. 1997;25:1196–204. doi: 10.1086/516119. [DOI] [PubMed] [Google Scholar]
- 18.Nigrovic LE, Wingerter SL. Tularemia. Infect Dis Clin North Am. 2008;22:489–504. doi: 10.1016/j.idc.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Eliasson H, Broman T, Forsman M, Bäck E. Tularemia: Current epidemiology and disease management. Infect Dis Clin N Am. 2006;20:289–311. doi: 10.1016/j.idc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Dennis DT, Inglesby TV, Henderson DA, et al. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–73. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 21.Kashyap AS, Sharma HS. Discolouration of permanent teeth and enamel hypoplasia due to tetracycline. Postgrad Med J. 1999;75:772. [PubMed] [Google Scholar]
- 22.Chalumeau M, Tonnelier S, D’Athis P, et al. Fluoroquinolone safety in pediatric patients: a prospective, multicenter, comparative cohort study in France. Pediatrics. 2003;111:714–19. doi: 10.1542/peds.111.6.e714. [DOI] [PubMed] [Google Scholar]
- 23.Forsythe CT, Ernst ME. Do fluoroquinolones commonly cause arthropathy in children. CJEM. 2007;9:459–62. doi: 10.1017/s1481803500015517. [DOI] [PubMed] [Google Scholar]
- 24.Johansson A, Berglund L, Gothefors L, et al. Ciprofloxacin for treatment of tularemia in children. Pediatr Infect Dis J. 2000;19:449–53. doi: 10.1097/00006454-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Amsden JR, Warmack S, Gubbins PO. Tick-borne bacterial, rickettsial, spirochetal, and protozoal infectious diseases in the United States: a comprehensive review. Pharmacotherapy. 2005;25:191–210. doi: 10.1592/phco.25.2.191.56948. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Castrillon JL, Bachiller-Luque P, et al. Tularemia epidemic in northwestern Spain: clinical description and therapeutic response. Clin Infect Dis. 2001;33:573–76. doi: 10.1086/322601. [DOI] [PubMed] [Google Scholar]
- 27.Risi GF, Pombo DJ. Relapse of tularemia after aminoglycoside therapy: case report and discussion of therapeutic options. Clin Infect Dis. 1995;20:174–75. doi: 10.1093/clinids/20.1.174. [DOI] [PubMed] [Google Scholar]
- 28.Syrjälä H, Schildt R, Räisäinen S. In vitro susceptibility of Francisella tularensis to fluoroquinolones and treatment of tularemia with norfloxacin and ciprofloxacin. Eur J Clin Microbiol Infect Dis. 1991;10:68–70. doi: 10.1007/BF01964409. [DOI] [PubMed] [Google Scholar]


