Summary
Background
Subjective tinnitus is an auditory perception that is not caused by external stimulation, its source being anywhere in the auditory system. Furthermore, evidence exists that exposure to noise alters cochlear micromechanics, either directly or through complex feed-back mechanisms, involving the medial olivocochlear efferent system. The aim of this study was to assess the role of the efferent auditory system in noise-induced tinnitus generation.
Material/Methods
Contralateral sound-activated suppression of TEOAEs was performed in a group of 28 subjects with noise-induced tinnitus (NIT) versus a group of 35 subjects with normal hearing and tinnitus, without any history of exposure to intense occupational or recreational noise (idiopathic tinnitus-IT). Thirty healthy, normally hearing volunteers were used as controls for the efferent suppression test.
Results
Suppression of the TEOAE amplitude less than 1 dB SPL was considered abnormal, giving a false positive rate of 6.7%. Eighteen out of 28 (64.3%) patients of the NIT group and 9 out of 35 (25.7%) patients of the IT group showed abnormal suppression values, which were significantly different from the controls’ (p<0.0001 and p<0.045, respectively).
Conclusions
The abnormal activity of the efferent auditory system in NIT cases might indicate that either the activity of the efferent fibers innervating the outer hair cells (OHCs) is impaired or that the damaged OHCs themselves respond abnormally to the efferent stimulation.
Keywords: tinnitus, otoacoustic emissions, efferent auditory system, suppression, noise-induced hearing loss
Background
Tinnitus is a sound, audible to an individual, which does not have an evident external origin, although occasionally it has an externally detectable component; in the latter case it is termed objective tinnitus, in the former case subjective tinnitus [1]. Until now it has not been possible to demonstrate any physiological correlates of subjective tinnitus. The source of the “tinnitogenic” activity may be anywhere in the auditory system (the cochlea, eighth nerve, brainstem and the cerebral cortex) [2]. Reports on the epidemiology of tinnitus indicate that noise-induced hearing loss (NIHL) is the most common cause of tinnitus [3]. Furthermore, it has been well-established that noise exposure produces morphological and functional changes at various levels of the auditory system and, therefore, both peripheral and central mechanisms may lead to tinnitus generation. In noise exposure, the most extensive morphological changes occur in the cochlea. There is well-documented evidence that noise exposure alters cochlear micromechanics, either directly or through complex feed-back mechanisms [4–6].
Abnormally elevated spontaneous discharge rates within single auditory neurons have been documented in OHC damage due to noise exposure [7] and this altered spontaneous activity may be perceived as tinnitus. Although the functional significance of the efferent auditory system is not fully understood, there exists a large body of evidence that the medial olivocochlear system is of particular importance in the modulation of the cochlear activity controlling the micromechanical properties of the OHCs [8,9]. The medial olivocochlear bundle is mainly inhibitory and it has been suggested that dysfunction of the efferent auditory system, at any level from auditory cortex to cochlea, may be a basis for tinnitus generation [10].
The amplitude of transiently evoked otoacoustic emissions (TEOAEs) is reduced, whenever simultaneous contralateral sound stimulation is applied [11–14]; this is known as contralateral TEOAE suppression. This phenomenon is believed to be mediated via the efferent auditory system [14] and in this context the OAE responses may be useful indices in the clinical assessment of the medial olivocochlear system.
The aim of this study was to assess the role of the efferent auditory system on the generation of tinnitus in 2 different but etiologically homogeneous groups (the etiology refers to the hearing threshold levels and the mechanisms that probably cause tinnitus): (i) subjects with NIHL and tinnitus and (ii) subjects with normal hearing, suffering from tinnitus and with no documented history of noise exposure (idiopathic tinnitus-IT). These 2 groups were investigated in order to extrapolate evidence for possible different pathogenetic mechanisms in tinnitus generation, avoiding any biases due to etiologic heterogeneity.
Material and Methods
Subjects
Sixty-three (63) adults were enrolled in the study, reporting intense tinnitus for at least 3 months prior to admission and inclusion to the study, without any middle ear or retrocochlear pathology. Thirty (30) adult normal hearing subjects were used as controls. The enrolled subjects were divided into 3 groups, A, B and C as follows:
Group A consisted of 28 patients (25 males and 3 females) with noise-induced tinnitus (NIT), according to history of chronic noise exposure and audiometric findings of NIHL. Their age ranged from 22 to 58 years (mean=35.95, SD=11.4). Eighteen patients (18) complained of unilateral tinnitus (16 left-sided), whereas the other 10 could not detect the side of tinnitus or did not complain of tinnitus “in the head”. This group will be referred to as the “noise-induced tinnitus (NIT) group”.
Group B consisted of 35 age-matched individuals to Group A (9 males and 26 females) with idiopathic tinnitus (IT) with normal hearing, no previous history of otologic disease or ear surgery or cranio-cerebral injury and without established chronic exposure to intense noise, either occupational or recreational. Their age ranged from 20 to 64 years (mean=37.09, SD=11.68). Twenty-four (24) subjects complained of unilateral tinnitus (15 left-sided) and 11 of bilateral tinnitus or tinnitus “in the head”. This group will be referred to as the “idiopathic tinnitus (IT) group”.
Group C consisted of 30 healthy adult volunteers with normal hearing (12 males, 18 females) with a mean age of 29.5 years and SD=9.32. This group served as the control group for the efferent suppression test.
The members of groups A and B were chosen so that the mean group age and the corresponding standard deviation remained within a close range (i.e., the groups were characterized by similar age distributions).
Measurements
Pure-tone audiometric (PTA) threshold levels were measured in a sound-treated booth with a GSI 61 clinical audiometer (Grason-Stadler, USA), for a frequency range from 0.5–8 kHz (3 and 6 kHz included). Acoustic impedance audiometry was performed in all subjects with a middle ear analyzer GSI 33 II (Grason – Stadler, USA) in order to exclude middle ear and retrocochlear pathology.
TEOAEs were recorded in a sound-treated booth according to previously published protocols [15] by means of an ILO 92 apparatus (Otodynamics Ltd) running software version 5.6. Responses were elicited by non-linear click stimuli of an amplitude approximately 80 dB SPL (78 to 83 dB SPL). The TEOAE recordings were terminated as soon as 260 (“quiet”) responses were obtained. The responses were considered acceptable when the TEOAE reproducibility (the correlation between the ILO traces A & B) was ≥60% and the stimulus stability > than 80%. TEOAEs were considered to be present if their amplitude was of at least 3 dB SPL above noise level, across the frequency range of 0.8 to 2 kHz in the NIT cases of group A and a range from 0.8 to 4 kHz for the IT cases of group B.
The TEOAE suppression test was performed according to the protocol reported by Williams et al [16] and Prasher et al. [17]. A contralateral white noise stimulus of 50 dB SL was generated by a GSI 61 audiometer and was it presented to the ear via the Telephonics TDH-50P earphones. Linear click stimuli of 60 dB SPL (≤2 dB SPL) were used to elicit TEOAEs. Sixty “quiet” responses (sweeps) were collected in the absence of contralateral noise, alternating with 60 responses collected with contralateral noise, up to a total of 300 responses for each period (with or without noise). The degree of suppression was estimated as the difference between the amplitude (in dB SPL) of the total response with and without contralateral noise [17].
Spontaneous Otoacoustic Emissions (SOAEs) was recorded from all tested subjects using the default ILO-92 protocol.
Statistics
A Kolmogorov-Smirnov test was performed to assess normal distribution of the numerical data. An independent samples t-test was used to compare the group means and an ANOVA (one-way analysis of variance) analysis was used to compare means across all groups. To compare the statistical significance of percentile differences between groups, the Pearson chi-square test was used. A P <0.05 was considered as statistically significant.
Results
Control group C
All subjects presented normal hearing thresholds (<20 dB HL) and normal impedance audiometry. The TEOAE amplitude suppression ranged from 6.9 to 0.8 dB SPL (mean=2.26, SD=1.12), showing great intra-individual variability. Suppression values greater than 1 dB were established in 28 out of 30 subjects (93.3%), whereas in 2 subjects (6.7%) the TEOAE amplitude was suppressed by 0.8 dB SPL. No significant difference in the suppression values was found between left and right ears (Table 1).
Table 1.
Results of the efferent suppression of the controls (group C).
| TEOAE suppression | Left ear | Right ear | Total (n=60) |
|---|---|---|---|
| Mean ±SD | 2.2±0.96 | 2.37±1.26 | 2.26±1.12 |
| Max | 5.1 | 6.9 | 6.9 |
| Min | 0.8 | 0.8 | 0.8 |
| Suppression <1 dB SPL | 2 | 2 | 4 |
| Suppression ≥1 dB SPL | 28 | 28 | 56 |
Considering the value of 1 dB SPL as a criterion of the lowest “normal” value of TEOAE amplitude suppression [17], the data from the control group suggest that this criterion gives a false positive rate of 6.67% and a specificity of 93%, which is in agreement with previously reported data [17].
Group A (NIT)
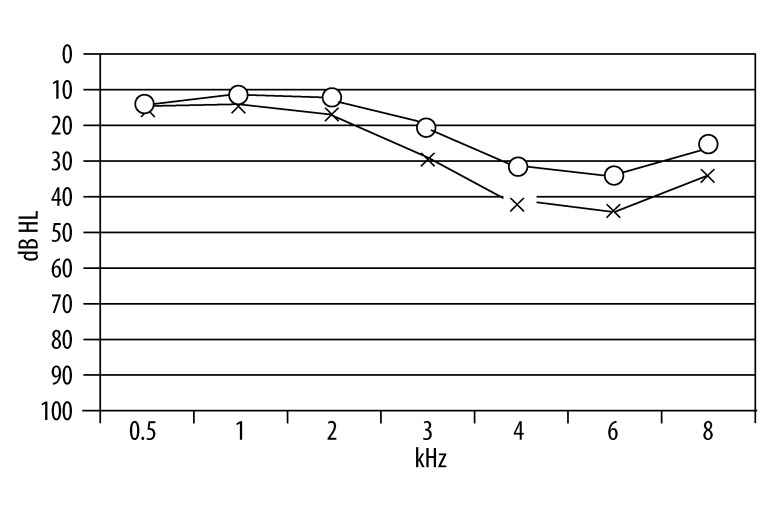
According to the behavioral data, all 28 subjects suffered from noise-induced threshold shifts (>20 dB HL) at 3.0, 4.0, 6.0 and 8.0 kHz. Figure 1 illustrates the mean behavioral thresholds from this group. The compliance, as estimated by tympanometry testing, indicated normal middle ear function in all subjects, and the acoustic reflexes (ipsilateral and contralateral) were elicited with normal thresholds, thus retrocochlear pathology was excluded.
Figure 1.
Mean behavioral thresholds of NIT subjects.
Acceptable TEOAE responses (see criteria in the methods section) were recorded from both ears in all subjects, except for the left ear of 1 subject, presumably due to the degree of NIHL. The suppression test was performed on a total of 55 (i.e., 56-1) ears. The TEOAE responses were characterized by the typical features of NIHL (of amplitude and frequency-range reduction). The TEOAE amplitude ranged from 19.4 to 1.2 dB SPL (mean=11.62, SD=4.09) and the corresponding reproducibility ranged from 98% to 63% (mean=88.65, SD=8.48). SOAE recordings were feasible in only 1 subject, bilaterally (3.5%).
The suppression of the TEOAE amplitude ranged from 3.6 to −0.7 dB SPL (mean=1.29, SD=0.88). The difference between the mean amplitude suppression in this group and the control group C was statistically significant (Table 2).
Table 2.
TEOAE suppression values in the two tinnitus groups and in the control group C.
| TEOAE suppression | Group NIT (n=55 ears) | Group IT (n=70 ears) | Controls (n=60 ears) |
|---|---|---|---|
| Mean ±SD | 1.29±0.88* | 1.7±0.96* | 2.26±1.12 |
| Max value | 3.6 | 5.3 | 6.9 |
| Min value | −0.7 | −0.5 | 0.8 |
indicates a significant difference between the tinnitus group compared to the control group. The obtained p values were p<0.001 and p<0.05, respectively.
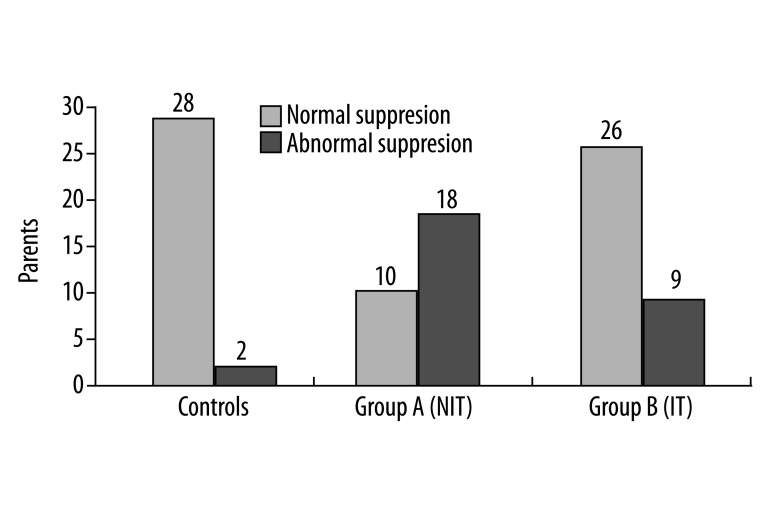
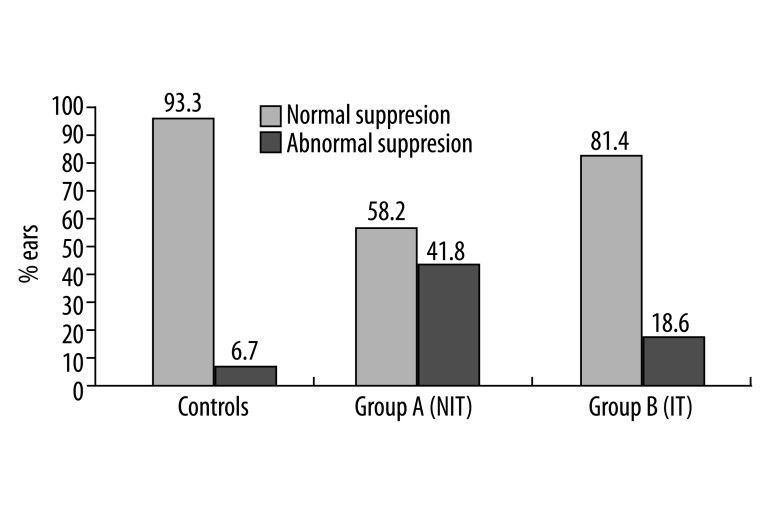
Furthermore, 18 out of 28 subjects (64.28%) showed abnormal suppression (<1 dB SPL), 5 of them bilaterally. Abnormal TEOAE suppression was observed in 23 out of the 55 tested ears (41.8%). The number of subjects and ears showing suppression less than 1 dB SPL in the NIT group differed significantly from those in the control group (Table 3 and Figures 2, 3).
Table 3.
Percentage of individuals and ears having abnormal TEOAE suppression, in the two tinnitus groups as compared to the controls.
| Individuals | Ears | |||
|---|---|---|---|---|
| Abnormal suppression | (%) | Abnormal suppression | % | |
| Controls | 2/30 | 6.7 | 4/60 | 6.7 |
| Group A (NIT) | 18/28 | 64.3 | 23/55 | 41.8* |
| Group B (IT) | 9/35 | 25.7 | 13/70 | 18.6* |
indicates a significant difference of the percentage (%) of abnormal suppression in NIT and IT group as compared to the controls’. Observed values were p<0.0001 and p<0.045, respectively). A significant difference (p<0.008) of the percentage of abnormal emission suppression, between the two tinnitus groups was also observed.
Figure 2.
Number of subjects exhibiting abnormal TEOAE suppression in NIT (group A) and IT (group B) subjects, as compared to controls.
Figure 3.
Percentage (%) of ears exhibiting abnormal TEOAE suppression in NIT (group A) and IT (group B) subjects, as compared to controls.
The ears with abnormal suppression of TEOAEs coincided with the ears in which tinnitus was identified, in 15 out of 18 subjects (83.3%). In detail: (i) 4 out of 5 subjects, complaining of tinnitus in both ears, showed abnormal suppression bilaterally; (ii) 10 subjects showed abnormal suppression on the side (ear) where tinnitus was identified, whereas the TEOAEs of the non-tinnitus ears were normally suppressed; and (iii) a subject suffering from unilateral tinnitus showed abnormal suppression bilaterally, but abnormality was significantly greater on the tinnitus side (0.9 vs. 0.5) (Table 4).
Table 4.
Efferent dis-inhibition and tinnitus laterality in the two tinnitus groups.
| Group A (NIT) | ||||
|---|---|---|---|---|
| Abnormal TEOAE suppression | ||||
| Lateral side | Contralateral. side | Bilaterally | ||
| Tinnitus unil. | 10/18 (55.6%) | 3/18 (16.7%) | 1/18 (5.5%) | |
| Tinnitus bilat. | 0 | 0 | 4/18 (22.2%) | |
| Group B (IT) | ||||
| Abnormal TEOAE suppression | ||||
| Lateral side | Contralat. side | Bilateral | Unilateral | |
| Tinnitus unil. | 3/9 (33.3%) | 2/9 (22.2%) | 2/9 (22.2%) | |
| Tinnitus bilat. | 0 | 0 | 0 | 2/9 (22.2%) |
Group B (IT)
Normal behavioral thresholds (≤20 dB HL) were obtained from all subjects across the frequency range from 0.25 to 8 kHz (3 and 6 kHz included). All subjects had normal compliance and all except 3 presented normal ipsilateral and contralateral acoustic reflexes. Further investigation with ABR and MRI of these 3 subjects failed to demonstrate any retrocochlear pathology, so these 3 cases were included in the analyses.
Normal TEOAE recordings were collected from both ears of all subjects. The TEOAE amplitude ranged from 18.8 to 2.9 dB SPL (mean=12.48, SD=3.65) and the reproducibility from 98% to 68% (mean=89.68, SD=6.93).
TEOAE suppression was present in both ears of all 35 subjects, thus a total of 70 ears were studied. Suppression values ranged from 5.3 to −0.5 dB SPL (mean=1.7, SD=0.96). Significant differences were found in the mean suppression values between the tinnitus groups (B and A) and controls (C) (Table 2). Nine out of the 35 subjects (25.7%) exhibited abnormal suppression (<1 dB SPL), 4 of them bilaterally. Abnormal suppression was observed in 13 out of the 70 studied ears (18.57%) (Table 3, Figures 2, 3). This percentage was of borderline significance in comparison to group C (P<0.045), and significantly different from the abnormal suppression percentage observed in group A.
For this group our observations have indicated that: (i) 5 subjects showed abnormal suppression ipsilaterally to the tinnitus ear (44.4%) and 2 of them, reporting tinnitus in both ears, failed to suppress TEOAEs bilaterally; (ii) 2 subjects exhibited abnormal suppression to the ear contralateral to the tinnitus, and another 2, despite of having bilateral tinnitus, showed unilateral abnormal suppression; (iii) tinnitus and efferent disinhibition were at the same ear in 7 out of 9 subjects (77.7%) (Table 4).
SOAEs were obtained from 17 out of 35 subjects (48.57%), in 9 of them bilaterally. Multiple SOAEs were recorded from 8 subjects. Regarding any possible relation between SOAEs and the abnormal function of the efferent auditory pathway (suggested by the extent of the emission’s suppression), SOAEs were obtained only from 2 out of the 9 subjects who exhibited abnormal emission suppression.
Discussion
The outer hair cells (OHCs) are predominantly innervated by neurons of the medial olivo-cochlear system (a branch of the efferent auditory system), which mainly arise from the contralateral superior olivary complex of the brainstem [17–19]. The auditory afferent fibers terminate in the cochlear nuclei, which is connected with both the ipsilateral and contralateral superior olivary complex. Therefore a neural pathway exists from the cochlea, via the auditory afferents, to the olivary complex and then to the contralateral cochlea via the medial efferent system.
It has been shown that the TEOAE amplitude can be suppressed by sound stimulation of the contralateral ear [11,12]. This phenomenon occurs as a consequence of an inhibitory neural activity mediated by the medial efferent auditory system, causing alterations to the OHCs’ micro-mechanical properties [14]. In this context, otoacoustic emissions can be envisioned as clinical tool for the non-invasive and objective assessment of the efferent neural activity.
Reports on tinnitus epidemiology indicate that NIHL is the most common cause of tinnitus. According to Axelsson [3], in 411 consecutive tinnitus patients, tinnitus was attributed to NIHL in 28% of cases. Noise exposure was the most frequently suspected cause of tinnitus in 42% of cases, as stated by Penner et al. [20].
In the present study 3 groups of subjects were compared, controlling where possible numerous experimental factors (age, degree of hearing loss, presence of spontaneous emissions, repeatability of measurements and experimental errors, etc.)1. For some factors, such as age, different interpretations exist in the literature. Data from previous studies [20–22] have suggested that age interacts with the function of the medial olivocochlear efferent system, while Quaranta et al. [23] suggest lack of any interaction effects. The precise relationship between age and suppression is a subtlety beyond the scope of this work. In the presented data-sets the mean age differences between groups were not significant, and although the OAEs suppression was found to be reduced in the elderly, it remained within normal limits (i.e., >1 dB SPL). In terms of spontaneous otoacoustic emissions and their effect on suppression, the experimental design of this study did not allow an investigation of these effects, considering that the majority of subjects in group A (NIT cases) did not present any SOAEs. These findings verify data from a previous study by Ceranic et al. [24] showing that the prevalence of SOAEs in patients with tinnitus and noise-induced hearing loss was very low (17.6%).
The data presented suggest that a large percentage of the noise-induced tinnitus subjects (NIT-group A) lack efferent suppression, while patients suffering from idiopathic tinnitus (IT-group B) grossly maintained the inhibitory function of the efferent system. Furthermore, tinnitus and efferent disinhibition shared the same side in 77.8% of the subjects of the NIT group, whereas in the IT group this percentage was 55.6%.
These findings seem to be partly in agreement with those reported previously by Attias et al. [25], who investigated the reduction of the TEOAE amplitude as a function of increased contralateral noise in 5 different groups: normal hearing non-tinnitus subjects with no history of noise exposure, normal hearing subjects suffering from tinnitus, subjects with NIHL and no tinnitus and subjects suffering from NIHL and tinnitus. According their data, the TEOAE amplitude of both non-tinnitus groups decreased in a similar manner as a function of the increased intensity of the contralateral white noise and did not differ significantly at any intensity. The TEOAEs from the tinnitus ears tended to increase, especially at low intensities of contralateral noise, or decrease slightly.
Other studies using TEOAE suppression have showed that contralateral auditory stimulation is less effective in tinnitus patients [26–28]. Lind [29] studied the TEOAE contralateral suppression in 20 subjects with unilateral tinnitus but could not establish any difference in suppression between tinnitus and non-tinnitus ears. In addition, a number of studies have suggested that for tinnitus no general conclusions can be drawn from the global testing of the medial olivocochlear system using TEOAE suppression [30,31]. These contradictory findings might be the consequence of several factors such as: (i) testing of tinnitus patients presenting various etiologies, which affect different sites across the auditory pathway; or (ii) using small sample sizes, which do not permit global conclusions.
The significantly higher percentage of efferent disinhibition in subjects with NIT, as compared to normal hearing non-noise-exposed tinnitus sufferers, might indicate that either the OHCs themselves respond abnormally to the efferent stimulation, or that the activity of the efferent fibers innervating the OHCs is impaired, possibly due to alterations at the level of the inferior colliculi and/or the auditory cortex. A combination of these 2 mechanisms cannot be excluded in the effect of SOAEs on normal ears.
Little is known about the underlying physiologic mechanisms that cause tinnitus in individuals with sensorineural hearing impairment. One theory, the “edge effect”, supported by several authors [2,32–35] suggests that the diffuse nature of OHC innervation (only 1 fiber per 20–30 OHCs) could generate some effects that could be responsible for tinnitus. These effects could take place on the basilar membrane on a number of locations, either at the edge between an area where inner hair cells (IHCs) and OHCs are intact and an adjacent area where they are damaged, or in a zone of discordance between lesions affecting IHCs and OHCs. Such lesions typically occur after exposure to noise. The local damage to OHCs and/or IHCs would produce a decrease in auditory information from this lesioned area, which in turn would decrease the inhibition that the medial olivocochlear system normally exerts on the hair cells. Due to diffused efferent innervation of OHCs, this inhibitional decrease would affect not only the damaged OHCs, but also neighboring normally functioning OHCs located at the periphery of the lesioned zone. The basal activity of IHCs to which these intact OHCs are related would therefore be increased, forming a starting point of a peripheral signal which, after being reinforced by central processing in auditory pathways, could result in the perception of tinnitus (functional dissociation of OHCs and IHCs) [2,29]. There is a strong possibility that the theory of “edge effect” could account for the generation of NIT. This is supported by a study conducted by Chery-Croze et al. [36], suggesting that the frequency area associated with tinnitus may escape efferent inhibition.
Although noise mainly affects the cochlea, it also results in morphological and physiological changes in the central auditory system [35]. Cochlear lesions alter the activity in the auditory nerve and increase the excitability of the cochlear nucleus, inferior colliculus [37,38] and geniculate body [39].
These changes may be of relevance to tinnitus generation, since the inferior colliculi are obligatory synapses for both the afferent and efferent auditory pathway [40], thereby causing an imbalance between the excitatory and inhibitory mechanisms. Furthermore, regional cochlear damage also produces a tonotopic reorganization of the auditory cortex. The damage to the cochlea leads to an expansion of the cortical representation of a restricted frequency-band, adjacent to the region of the cochlear loss [41,42]. Such a plasticity of frequency selectivity may alter perceptual function and/or the cortical efferent activity and therefore may contribute to the generation of tinnitus. Attias et al [43] reported that the amplitude of auditory event-related potentials (ERPs) were significantly lower in NIT patients than in controls. This may indicate attenuated or “abnormal” auditory central processing in NIT patients. Since the efferent auditory system plays an important role in both stimuli processing and sensory modulation, it seems that NIT may be associated with a dysfunction of efferent pathways at a cortex level.
In the present study, the NIT subjects of group A exhibited a very low prevalence of SOAEs (3.5%), a finding that is in agreement with data from Ceranic et al. [24], but not in agreement with the study conducted by Prasher et al. [44]. In the latter 73.1% of the noise-exposed subjects with tinnitus presented multiple SOAEs. It is not clear whether these subjects suffered permanent or temporary threshold shifts due to noise exposure. In our study no relation between efferent disinhibition in tinnitus subjects and the presence of SOAEs could be established.
In another study on tinnitus due to head injury [10], a significantly high percentage (65%) of abnormal TEOAEs suppression and high prevalence of multiple SOAEs (100%) was found in tinnitus patients compared to normal subjects and to subjects after head injury but without any tinnitus complains. In this context, it seems that a dysfunction of the medial efferent system plays an important role in tinnitus generation in etiologically different groups of people with tinnitus.
Conclusions
The efferent system seems to play an important role, at least in noise-induced tinnitus generation.
The abnormal activity of the efferent auditory system in tinnitus subjects might indicate that either the activity of the efferent fibers innervating OHCs is impaired or that the OHCs themselves respond abnormally to the efferent stimulation. A combination of these 2 mechanisms cannot be excluded.
Acknowledgements
The authors would like to thank Drs L. Collet, K. Markou, and M.G. Tsalighopoulos for generous comments on a previous version of the manuscript.
Footnotes
The precise relationship (multiple interaction effects) between these factors – age, suppression levels and degree of hearing loss – was outside the scope of the present work and requires future investigations.
Source of support: Departmental sources
References
- 1.Hinchcliffe R, King PF. Medigolegal aspects of tinnitus: Medicolegal position and current state of knowledge. J Audiol Med. 1992;1:38–58. [Google Scholar]
- 2.Jastreboff PF. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res. 1990;8:221–54. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- 3.Axelsson A. Causes of tinnitus. Proceedings, IV International Tinnitus Seminar; Bordeaux. 1990. pp. 275–77. [Google Scholar]
- 4.Nageris BL, Attias J, Raveh E. Test-retest tinnitus characteristics in patients with noise-induced hearing loss. Am J Otolaryngol. 2010;31(3):181–84. doi: 10.1016/j.amjoto.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Saunders JC, Deer SP, Schneider ME. The anatomical consequences of acoustic injury. Review and tutorial. J Acoust Soc Am. 1985;78:833–60. doi: 10.1121/1.392915. [DOI] [PubMed] [Google Scholar]
- 6.Borg E, Canlon B, Engström B. Noise-induced hearing loss: Literature review and experiments in rabbits. Scan Audiol. 1995;24:1–147. [PubMed] [Google Scholar]
- 7.Duan ML, Ulfendahl M, Ahlberg A, et al. Protection and treatment of sensorineural hearing disorders caused by exogenous factors: Experimental findings and potential clinical applications. Hear Res. 2002;169:169–98. doi: 10.1016/s0378-5955(02)00484-7. [DOI] [PubMed] [Google Scholar]
- 8.Liberman MC, Dodds LW. Acute ultrastructure changes in acoustic trauma: Serial-section reconstruction of stereocilia and cuticular plates. Hear Res. 1987;26:45–64. doi: 10.1016/0378-5955(87)90035-9. [DOI] [PubMed] [Google Scholar]
- 9.Collet L, Veuillet E, Bene J, Morgon A. Effects of contralateral white noise on click-evoked emissions in normal and sensorineural ears: Towards an exploration of the medial olivocochlear system. Audiol. 1995;31:1–7. doi: 10.3109/00206099209072897. [DOI] [PubMed] [Google Scholar]
- 10.Kujawa SG, Fallon M, Bobbin RP. Time-varying alterations in the f2-f1 DPOAE response to continuous primary stimulation I: response characteristics and contribution of olivocochlear efferents. Hear Res. 1995;85:142–54. doi: 10.1016/0378-5955(95)00041-2. [DOI] [PubMed] [Google Scholar]
- 11.Ceranic BJ, Prasher DK, Raglan E, Luxon L. Tinnitus after head injury: evidence from otoacoustic emissions. J Neurol Neurosurg Psychiatry. 1998;65:523–29. doi: 10.1136/jnnp.65.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collet L, Kemp DT, Veuillet E, et al. Effect of contralateral auditory stimuli on active cochlear micro-mechanical properties in human subjects. Hear Res. 1990;43:251–62. doi: 10.1016/0378-5955(90)90232-e. [DOI] [PubMed] [Google Scholar]
- 13.Ryan S, Kemp DT, Hinchcliffe R. The influence of contralateral acoustic stimulation on click-evoked otoacoustic emissions in humans. Br J Audiol. 1991;25:391–97. doi: 10.3109/03005369109076614. [DOI] [PubMed] [Google Scholar]
- 14.Moulin A, Collet L, Duclaux R. Contralateral auditory stimulation alters distortion products in humans. Hear Res. 1992;65:193–210. doi: 10.1016/0378-5955(93)90213-k. [DOI] [PubMed] [Google Scholar]
- 15.Hatzopoulos S, Petrucelli J, Morlet T, Martini A. Otoacoustic Emission Protocols revised. Data from adult subjects. Intern J Audiol. 2002;42:339–47. doi: 10.3109/14992020309101327. [DOI] [PubMed] [Google Scholar]
- 16.Williams EA, Brookes GB, Prasher DK. Effects of contralateral acoustic stimulation on otoacoustic emissions following vestibular neurectomy. Scand Audiol. 1993;22:197–203. doi: 10.3109/01050399309047469. [DOI] [PubMed] [Google Scholar]
- 17.Prasher D, Ryan S, Luxon L. Suppression of Transiently Evoked Otoacoustic Emissions and Neuro-Otology. Br J Audiol. 1994;28:247–54. doi: 10.3109/03005369409086574. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen G. The olivary pendule and other fibrous projections of the superior olivary complex. J Comp Neurol. 1946;84:141–219. doi: 10.1002/cne.900840204. [DOI] [PubMed] [Google Scholar]
- 19.Warr WB, Guinan JJ., Jr Efferent innervation of the organ of Corti: Two separate systems. Brain Res. 1979;173:152–55. doi: 10.1016/0006-8993(79)91104-1. [DOI] [PubMed] [Google Scholar]
- 20.Penner MJ. An estimate of the prevalence of tinnitus caused by spontaneous otoacoustic emissions. Arch Otolaryngol Head Neck Surg. 1990;116:418–23. doi: 10.1001/archotol.1990.01870040040010. [DOI] [PubMed] [Google Scholar]
- 21.Castor X, Veuillet E, Morgon A, Collet L. Influence of aging on active cochlear micromechanical properties and on the medial olivocochlear system in humans. Hear Res. 1994;77:1–8. doi: 10.1016/0378-5955(94)90248-8. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Frisina DR, Frisina RD. Effects of age on contralateral suppression of distortion product otoacoustic emissions in human listeners with normal hearing. Audiol Neurootol. 2002;7:348–57. doi: 10.1159/000066159. [DOI] [PubMed] [Google Scholar]
- 23.Parthasarathy TK. Aging and contralateral suppression effects on transient evoked otoacoustic emissions. J Am Acad Audiol. 2001;12:80–85. [PubMed] [Google Scholar]
- 24.Quaranta N, Debole S, Di Girolamo S. Effect of ageing on otoacoustic emissions and efferent suppression in humans. Audiology. 2001;40:308–12. [PubMed] [Google Scholar]
- 25.Ceranic BJ, Prasher DK, Luxon L. Presence of tinnitus indicated by variable spontaneous otoacoustic emissions. Audiol Neurootol. 1998;3(5):332–44. doi: 10.1159/000013803. [DOI] [PubMed] [Google Scholar]
- 26.Attias J, Bresloff I, Furman V. The influence of the Efferent Auditory System on Otoacoustic Emissions in Noise Induced Tinnitus: Clinical relevance. Acta Otolaryngol (Stockh) 1996;116:534–39. doi: 10.3109/00016489609137885. [DOI] [PubMed] [Google Scholar]
- 27.Veuillet E, Collet L, Duclaux R. Effects of contralateral acoustic stimulation on active cochlear micromechanical properties in human subjects: dependence on stimulus variables. J Neurophysiol. 1991;65:724–35. doi: 10.1152/jn.1991.65.3.724. [DOI] [PubMed] [Google Scholar]
- 28.Geven LI, de Kleine E, Free RH, van Dijk P. Contralateral suppression in tinnitus patients. Otol Neurotol. 2011;32(2):315–21. doi: 10.1097/MAO.0b013e3181fcf180. [DOI] [PubMed] [Google Scholar]
- 29.Lind O. Transient-evoked otoacoustic emissions and contralateral suppression in patients with unilateral tinnitus. Scand Audiol. 1996;25:167–72. doi: 10.3109/01050399609048000. [DOI] [PubMed] [Google Scholar]
- 30.Chery-Croze S, Collet L, Morgon A. Is the test of medial efferent system function a relevant investigation in tinnitus? Br J Audiol. 1994;28:13–25. doi: 10.3109/03005369409077909. [DOI] [PubMed] [Google Scholar]
- 31.Chery-Croze S, Truy E, Morgon A. Contralateral suppression of transiently evoked otoacoustic emissions and tinnitus. In: Collet L, Grandori F, editors. Advances in otoacoustic emissions. III. 1994. pp. 83–95. (“Suppression effects of otoacoustic emissions”. Casa Editrice Stefanoni-Lecco). [Google Scholar]
- 32.Graham RL, Hazel JW. Contralateral Suppression of transient otoacoustic emissions: intraindividual variability in tinnitus and normal subjects. In: Collet L, Grandori F, editors. Advances in otoacoustic emissions. III. 1994. pp. 56–70. (“Suppression effects of otoacoustic emissions”. Casa Editrice Stefanoni-Lecco). [DOI] [PubMed] [Google Scholar]
- 33.Kemp DT. Physiologically active cochlear micromechanics – one source of tinnitus. In: Evered D, Lawrenson G, editors. Tinnitus: Ciba Foundation Symposium. Vol. 85. London: Pitman; 1981. pp. 54–81. [DOI] [PubMed] [Google Scholar]
- 34.Hazell JWP. In: Feldman H, editor. A cochlear model of tinnitus; Proceedings, 3rd International Tinnitus Seminar; Muenster Karlsruhe: Harsch Verlag; 1987. pp. 121–28. [Google Scholar]
- 35.Wilson JP. Theory of tinnitus generation. In: Hazel JWP, editor. Tinnitus. Edinburgh: Churchill Livingstone; 1987. pp. 20–45. [Google Scholar]
- 36.Chery-Croze S, Moulin A, Collet L, Morgon A. Medial olivocochlear system and tinnitus. Acta Otolaryngol (Stockh) 1993;113:285–90. doi: 10.3109/00016489309135810. [DOI] [PubMed] [Google Scholar]
- 37.Salvi RJ, Powers NL, Saunders SS, et al. Enhancement of evoked response amplitude and single unit activity after noise exposure. In: Dancer AL, Henderson D, Salvi RJ, Hamernick RP, editors. Noise-induced hearing loss. St. Louis: Mosby Year Book; 1992. pp. 156–71. [Google Scholar]
- 38.Willot JF, Lu S-V. Noise-induced hearing loss can alter neural coding and increase excitability in the central nervous system. Science. 1982;216:1331–32. doi: 10.1126/science.7079767. [DOI] [PubMed] [Google Scholar]
- 39.Salvi RJ, Ahroon WA. Tinnitus and neural activity. J Speech Hear Res. 1983;26:629–32. doi: 10.1044/jshr.2604.629. [DOI] [PubMed] [Google Scholar]
- 40.Gerken GM. Central denervation hypersensitivity in the auditory system of the cat. J Acoust Soc Am. 1979;66:721–27. doi: 10.1121/1.383222. [DOI] [PubMed] [Google Scholar]
- 41.Kimiskidis VK, Lalaki P, Papagiannopoulos S, et al. Sensorineural hearing loss and word deafness caused by a mesencephalic lesion: clinico-electrophysiological correlations. Otol Neurotol. 2004;25:178–82. doi: 10.1097/00129492-200403000-00017. [DOI] [PubMed] [Google Scholar]
- 42.Schwaber MK, Garraghty PE, Kaas JH. Neuroplasticity of the adult primary auditory cortex following cochlear hearing loss. Am J Otol. 1993;3:252–58. [PubMed] [Google Scholar]
- 43.Attias J, Urbach D, Gold S, Shemesh Z. Auditory event related potentials in chronic tinnitus patients with noise induced hearing loss. Hear Res. 1993;71:106–13. doi: 10.1016/0378-5955(93)90026-w. [DOI] [PubMed] [Google Scholar]
- 44.Prasher D, Ceranic B, Sulkowski W, Guzek W. Objective evidence for tinnitus from spontaneous emission variability. In: Henderson D, Prasher D, Kopke R, et al., editors. Noise Induced Hearing Loss: Basic Mechanisms, Prevention and Control. London: Noise Research Publications; 2001. pp. 471–83. [PubMed] [Google Scholar]