Summary
Background
As reported in our previous studies, the complexity of physiologic time series is a sensitive measure of muscle fatigability. This study compared the differences between 2 different analyses following 4 weeks of core stability exercises (CSE) in subjects with and without chronic low back pain (LBP). We examined whether the observed Shannon (information) entropy, as compared with median frequency (MF), was able to differentiate fatigability of the thoracic and lumbar parts of the erector spinae (ES) muscles following the intervention.
Material/Methods
In total, 32 subjects participated in this study. There were 13 subjects in the CSE intervention group (average age 50.4±9.1 years) and 19 subjects in the control group (average age 46.6±9.1 years). The CSE group performed the specific exercise intervention, but the control group was asked to maintain their current activity and/or exercise levels. The endurance of the back muscles was determined by using a modified version of the isometric fatigue test as originally introduced by Sorensen.
Results
Pain level decreased significantly for all subjects (F=25.29, p=0.001), but there was no difference between groups (F=0.42, p=0.52). The MF was not different between groups following treatment (F=0.81, p=0.37). Although there was no entropy level changes following treatment (F=0.01, p=0.93), the interactions between muscles and groups following treatment were significant (F=7.25, p=0.01). The entropy level decreased in both thoracic ES muscles following intervention in the exercise group, while remaining the same in the control group.
Conclusions
Although the change in pain level was not different between groups, the Shannon entropy measure more sensitively differentiated the exercise intervention than did MF. In addition, the results also suggested that complexity is related to muscle fatigue, which corresponds to the values of entropy between groups. Further studies are needed to investigate the effectiveness of nonlinear time series of EMG data for fatigability.
Keywords: electromyography, low back pain, nonlinear time series, complexity, fatigue, core stability exercise, median frequency
Background
People with low back pain (LBP) often have reduced muscle strength and endurance, which may compromise the functional capacity of spinal stability and flexibility. As a result, clinicians commonly assess muscle fatigability in subjects with LBP, which is one of the most common types of musculoskeletal pain [1,2].
Several studies have investigated the difference between entropic measures and frequency of power spectrum for surface electromyography (EMG) to non-invasively evaluate localized muscular fatigue [3–7]. Previously, we confirmed that the entropy measurement for characterizing neuromuscular alterations is reliable [8]; however, the sensitivity of entropy it is still unknown. It is important to study entropy in order to objectively assess localized muscle fatigability and power spectrum measurements when considering pain level.
Several studies also have identified a difference between easily fatigued back muscles and LBP based on endurance tests [9–11]. However, there is a lack of research regarding erector spinae (ES) muscle fatigue following specific exercise interventions in subjects with chronic LBP. Core stability exercises (CSE) may improve co-contraction of the trunk muscles which could restore stability to the spine and, theoretically, may protect it from biomechanical stresses and further injuries [12].
Core strengthening of trunk muscles included the paraspinal and gluteal muscles in the back, the diaphragm as the roof, and the pelvic floor and hip girdle musculature as the bottom [13]. It has been reported that back extensor and abdominal strength are still considered the gold standard for analyzing a predisposition to back injuries [14–16]. Therefore, our study investigated ES muscles following CSE in order to evaluate the core muscles as a unit to stabilize the limbs.
There is a connection between localized muscular fatigue and EMG spectral analyses for back muscle endurance following intervention [17–19]. The plateau value of the entropy was lower for subjects with LBP than for individuals in the control group. In addition, the entropy associated with the LBP subjects saturates at very short times – 2 orders of magnitude shorter than for the healthy subjects [4]. Pathology/dysfunction is associated with less variability between the entropy time dependence by subjects with and without LBP [3–5].
Subjects with LBP have less endurance and thus smaller median frequency (MF) during sustained muscle contractions [19–21]. The signal from surface EMG is the instantaneous algebraic summation of action potentials from muscle fibers, and its power spectrum can be estimated from a fast Fourier transform of the signal. Therefore, the MF of the EMG power spectrum is sensitive to physiological manifestations of muscle fatigue as an alternative assessment tool to identify muscle fatigue [20,21]. However, there is a lack of research comparing this tool with other nonlinear measurements based on pain level.
During a fatiguing contraction, a compression of the power spectrum of the EMG signal to lower frequencies is typically observed [22]. This phenomenon is measured during a contraction as a decrease in the MF of the EMG signal. Individuals with better endurance exhibit a less precipitous decay rate of the MF [21]. Thus, it would be important to compare the results between Shannon entropy levels of the EMG and MF of the spectral quantities following intervention to enhance outcome measurements. However, there have been few investigations of a specific therapeutic intervention that considers pain level changes as well as other objective measurements in subjects with chronic LBP.
Therefore, the purpose of this study was to assess the effects of 4 weeks of CSE intervention based on entropic measures of thoracic and lumbar ES muscle fatigability compared with MF based on power spectral analyses in subjects with chronic LBP. We hypothesized that the entropic measures of muscle fatigability would be more sensitive than MF following CSE intervention.
Material and Methods
Selection of subjects
Volunteers for this study were subjects who presented with LBP, met study inclusion criteria, and experienced a disturbing impairment or abnormality in the functioning of the low back for more than a 2-month duration [23]. Subjects were eligible to participate if they: 1) were 21 years of age or older and 2) had LBP for more than 2 months without pain referral into the lower extremities. Individuals were excluded from participation if they: 1) had a diagnosed psychological illness that might interfere with the study protocol, 2) had experienced overt neurological signs (sensory deficits or motor paralysis), or 3) were pregnant. Subjects were withdrawn from the study if they requested to withdraw. Those subjects who met study inclusion criteria received information regarding the purpose and methods of the study and signed a copy of the Institutional Review Board-approved consent form. After inclusion in the study, the subjects were randomized into 1 of 2 groups using a computer-generated random list. In total, 32 subjects were enrolled in the study (15 females and 17 males). Eligible participants were assessed and randomly allocated to either a CSE or control group. Of the 32 subjects, 13 (41%) were assigned to the SCE treatment and 19 (49%) to the control group.
Pain level
Subject disability was inferred from self-reported scores on the Million pain interference visual analogue scale (MVAS), which was given to each subject during the initial and final testing sessions. The MVAS includes 15 questions, which are answered by marking a point on a 100 mm line anchored at each end. Responses range from 0–100 mm, where 100 represents maximum disability.
EMG measurement
The EMG measurements were obtained from the participants and repeated under identical conditions after 4 weeks. No subjects underwent any traumatic event or injury between the 2 different measurement days.
In this study, the endurance of the back muscles was determined by using a modified version of the isometric fatigue test as originally introduced by Sorensen [2]. The subjects’ upper bodies were positioned with their iliac crests at the edge of the table; their lower bodies were secured at the ankles and hamstring level using seatbelt straps. Subjects held their arms across their chests with each hand placed on the opposite shoulder while standard verbalized encouragement was given throughout the test (Figure 1).
Figure 1.
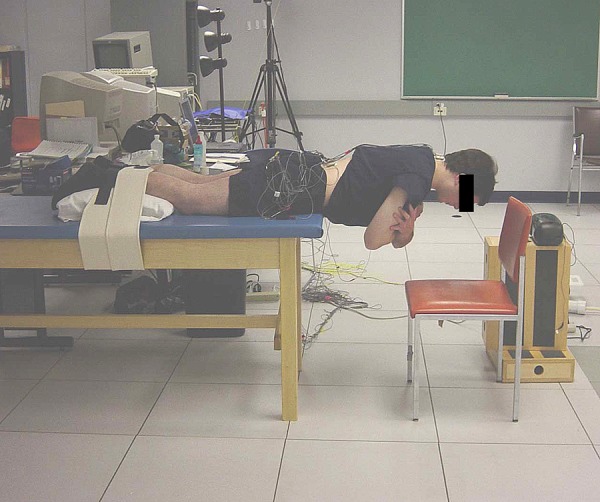
Modified Sorensen test for fatigue measure. The subject is shown with EMG electrodes attached over the thoracic and lumbar erector spinae (ES) muscles of the low back. During this test, subjects lay prone and lift their trunks off the table for one minute.
The EMG electrodes were placed bilaterally over the greatest convexity of the thoracic ES at the T10-T11 level and the lumbar ES muscles at the lumbar 4–5 levels with a 5 cm distance between electrodes of each pair. The electrode sites and the distance of the electrodes were carefully determined for each subject according to Zipp [24]. The EMG signals were pre-amplified at the skin (gain 35×) and further amplified downstream (bandwidth 20–4000 Hz; model D-100 pre-amplifier and model ENG 55 driver amplifier, Therapeutics Unlimited, Iowa City, Iowa) with the total system adjusted for each subject to allow maximal amplification without saturation of the analogue-to-digital converter. The EMG signal was fed through a low-pass filter (cut-off frequency 480 Hz at 6 dB per octave) and subsequently passed to a BNC connector board (BNC 2080, National Instruments, Austin, Texas). The signal also interfaced with a 12-bit analogue-to-digital converter (AT-MIO-16E-10; National Instruments) that amplified 100 times and sampled each channel at 1024 Hz. The digitized data were stored on computer disks for subsequent analysis.
Using standard Fast Fourier Transform of the EMG data, the power spectrum for each 1-second time interval was obtained. The MF of the signal, <f>, was calculated from the spectrum for each 1-second time interval.
The EMG signals from the isometric fatigue test were transformed into their frequency spectrum using a wavelet analysis. The MF of the spectrum was then recorded. After dividing the range of X(t) into 500 equal-sized bins, a histogram was used to determine the probability distribution pj,t. The entropy is calculated from:
where Pj is the probability for outcome number ‘j’ of a given experiment. The above equation is the standard formulation of uncertainty as it has the following features: (i) the lowest entropy (S=0) corresponds to 1 of the outcomes being certain (ie, probability 1) and the others never occurring (ie, probability 0); (ii) the largest value for the entropy, S=lnM, is achieved when all outcomes are equally likely [all probabilities are equal to each other Pj=1/M]; and (iii) S is additive over partitions of the outcomes [4].
The variance of the EMG signal during 1-second time intervals was calculated. The variance remained constant and did not exhibit any significant time dependence during the 1-minute test. However, the variance showed a sharp peak at the beginning and/or end of the test period for some subjects. Because of this peak, the raw EMG time series, yi, was averaged during a 10 ms moving window: xi=(yi+yi+1+…+yi+9)/10. The reduced time series consisted of approximately 6000 values xi (in units of millivolts), where subscript i represents 0.01s time increments. As a result, the short-time behavior [t<10 ms] of the signal was averaged out.
Since the variance showed no systematic time dependence, the EMG time series is consistent with a stationary random process. However, more stringent tests are needed to confirm these results. The description of the EMG signal as a random walk (Brownian motion) is based on an interpretation of the signal xn at time n as random jumps at discrete times. It follows that the sum X(t)=xs+xs+1+…+xs+t is the displacement between times s and s+t. The mean square displacement is defined as (t)= 〈[X(t)−<X(t)>]2〉. Here, the bracket < > indicates the average with respect to initial times.
The Shannon (information) entropy of the time series quantifies the degree of “noisiness” of a signal. After dividing the range of X(t) into 100 equal-sized bins, we determined the probabilities, Pi, from the histogram. The entropy was calculated as S=−∑ Pi lnPi. Following standard practice, entropy was reported in arbitrary units. If the displacement X follows a Gaussian distribution, the entropy is approximately proportional to the logarithm of the variance (e.g., S(t) ~ln [Δ (t)]). The entropy S versus time t exhibits a plateau for t >10 ms. This plateau value of S(t) was referred to as the entropy. The detailed entropy calculation process is fully described in previous studies [3–5,25]. Our previous data were partially used in this study in order to monitor the effects of the exercise intervention.
The CSE protocol
Those subjects who were assigned to the CSE group came into the lab once a week for 4 weeks in addition to performing the exercises at home daily for 20 minutes. The subjects were supervised in the lab in order to ensure that the exercises were performed correctly. To ensure adherence, the subjects kept an exercise log and phone calls were made to each subject at least once a week. The intensity of the exercises was at the subject’s tolerance level, and the subjects were encouraged to report any problems immediately. Participants in the control group were asked to maintain their current activities and/or exercise levels during the study.
In Table 1 the CSE approach utilized in this study is commonly advocated in the rehabilitation of LBP patients [19,26]. The exercise program consisted of 5 different types of exercises, such as upper body extension in prone position, alternate arm and leg lift in quadruped position, alternate arm and leg lift in prone position, and diagonal curl-up and straight curl-up in supine position. The quadruped exercises, performed from an all-fours position with the arms and legs extending reciprocally, is used to recruit the trunk and hip extensors.
Table 1.
The core stability exercise (CSE) program.
| Type of exercise | Description |
|---|---|
| 1. Upper body extension | With pillow supporting abdomen, clasp hands behind back and lift body off floor. Keep chin tucked while lifting in prone position. |
| 2. Alternate arm and leg lift | Keep knee locked and lift leg 8–10 inches from floor along with opposite arm in quadruped position. |
| 3. Alternate arm and leg extension on all fours | Raise opposite arm and leg in quadruped position. Do not arch neck. |
| 4. Diagonal curl-up | Keeping arms folded across chest, tilt pelvis to flatten back in supine position. Lift head and shoulders from floor while rotating to one side. |
| 5. Curl-up | With arms at sides, tilt pelvis to flatten back in supine position. Raise shoulders and head from floor. Use arms to support trunk if necessary. |
Statistical analysis
Descriptive statistics were used to compare the mean and standard deviation of each muscle group as well as subject characteristics. The entropy levels of the EMG signals and MF for the thoracic and lumbar ES muscles were compared. The repeated measure analysis of variance (ANOVA) was used to examine any significant differences between power spectrum analysis for MF and entropy levels of the EMG signals based on nonlinear time series before and after the intervention.
Regarding the design of experiments and ANOVA, a main effect is the effect of an independent variable on a dependent variable averaging across the levels of any other independent variables [27]. Therefore, the level of pain was considered with regards to both outcome variables (entropy level and MF).
The entropy values of the EMG and MF based on the power spectrum were analyzed to compare the differences between pre- and post-exercise intervention. The MathCad package (MathSoft, Cambridge, MA) was used for this analysis, which was loaded onto a PC running the Windows XP operating system. For all statistical tests, type I error rate was set at 0.05.
Preliminary power analyses associated with comparing the 2 independent treatment groups, conducted under the assumptions of setting type I error rate at 0.05 with 2-tailed testing and assuming effect sizes of 0.2, 0.4, 0.6, 0.7, 0.8, and 0.9, produced estimated power values of 0.10, 0.26, 0.50, 0.63, 0.75, and 0.84, respectively. Since these power estimates would be associated with follow-up tests of simple effects (eg, exploring the nature of a treatment by time interaction for outcomes), these power estimates are potentially conservative on 2 counts. First of all, the sample sizes are smaller than would be involved in effects that take advantage of multiple observations per subject; and second, the effect size is likely to be larger than estimated due to the expected and reasonably large correlations in outcomes within patients across time.
Results
The subjects’ demographics are summarized in Table 2. Thirty-two subjects who are right hand dominant completed this study. There were 13 subjects in the CSE intervention group (average age of 50.4±9.1 years) and 19 subjects in the control group (average age of 46.6±9.1 years). Overall, there was no sex difference between groups (χ2=1.17, p=0.25). The subjects in the exercise group were slightly taller (172.8±7.5 cm) than the control subjects (167.8±7.8 cm), but height was not significantly different between groups (t=1.78, p=0.09). Body weight was not significantly different between groups (t=1.68, p=0.11), nor was the number of months since pain onset (t=0.11, p=0.91).
Table 2.
Summary of subject demographics with group differences.
| Variable | Exercise | Control | Statistic | p |
|---|---|---|---|---|
| N | 13 | 19 | ||
|
| ||||
| Age (yrs) | ||||
| Range | 37–63 | 26–59 | ||
| Mean ± SD | 50.4±9.1 | 46.6±9.1 | t=1.17 | 0.25 |
|
| ||||
| Sex | ||||
| Female | 5 | 10 | χ2 = 0.62 | 0.49 |
| Male | 8 | 9 | ||
|
| ||||
| Height (cm) | ||||
| Range | 155–187 | 159–184 | t=1.78 | 0.09 |
| Mean ± SD | 172.87±7.51 | 167.87±7.82 | ||
|
| ||||
| Body weight (kg) | t=1.68 | 0.11 | ||
| Range | 52–82 | 50–79 | ||
| Mean ± SD | 70.53±8.03 | 64.80±10.51 | ||
|
| ||||
| Number of months since pain onset | ||||
| Range | 4–24 | 4–17 | ||
| Mean ± SD | 10.9±7.1 | 11.1±5.3 | t=0.11 | 0.91 |
p<0.05.
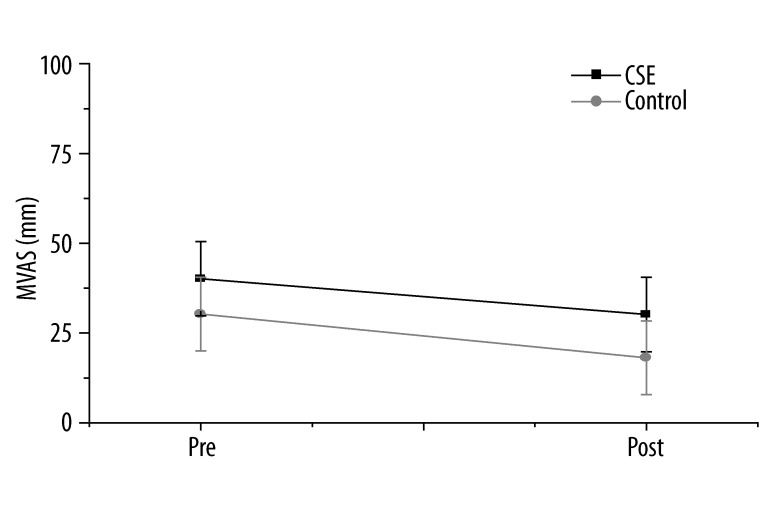
Before the intervention, the pain level was 41.11±12.8 for the CSE group and 32.85±11.9 for the control group (Figure 2). However, following intervention, the pain level was 31.94±14.2 for the CSE group and 20.93±11.59 for the control group. The pain level decreased significantly for the control group (t=4.24, p=0.001) as well as for the CSE group (t=3.08, p=0.01). However, the results of the repeated measure analysis indicated that there was no difference in pain reduction between groups (F=0.42, p=0.52).
Figure 2.
Pain changes based on million visual analogue scale (MVAS) following intervention. Pain in the low back was measured using a horizontal analogue scale, with options ranging from 0 to 100 mm, in which 100 mm reflected the worst pain imaginable. The pain level decreased significantly (F=25.29, p=0.001), but there was no difference between groups (F=0.42, p=0.52).
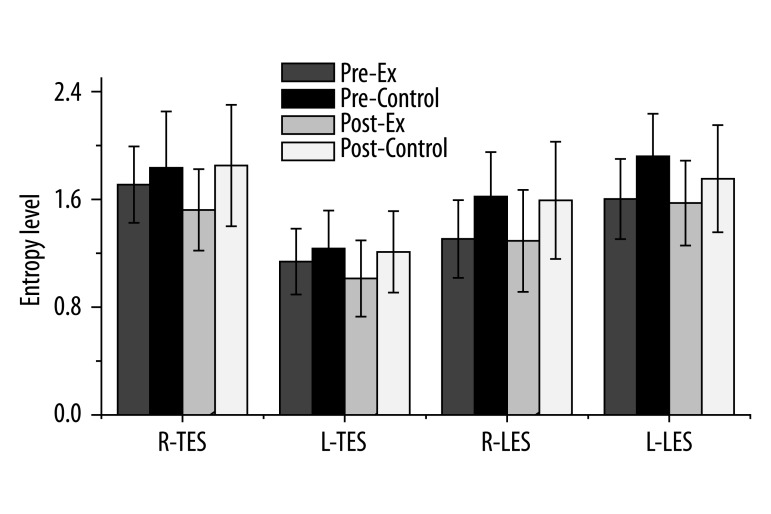
Figure 3 indicates the Shannon entropy differences for the thoracic and lumbar ES muscles. Although there was no entropy level change following treatment (F=0.01, p=0.93), the interactions were significant between muscles and intervention following treatment (F=7.25, p=0.01). The entropy level of the ES muscle decreased following intervention in the exercise group, while it remained constant in the control group. Prior to the intervention, the right thoracic ES was 1.70±0.28 for the CSE group and 1.83±0.41 for the control group. Following the intervention, however, the right thoracic ES was 1.52±0.30 for the CSE group and 1.85±0.45 for the control group. Regarding the left thoracic ES, the entropy level was 1.13±0.24 for the CSE group and 1.23±0.28 for the control group. Following the intervention, however, the left thoracic ES was 1.01±0.28 for the CSE group and 1.21±0.30 for the control group.
Figure 3.
Shannon entropy measurement for thoracic and lumbar ES muscles. Although there was no entropy level change following treatment (F=0.01, p=0.93), the interactions between muscles and intervention following treatment were significant (F=7.25, p=0.01). The entropy levels of the ES muscles decreased following intervention in the exercise group, but remained the same in the control group. (R: right, L: left, TES: thoracic erector spinae muscle, LES: lumbar erector spinae muscle).
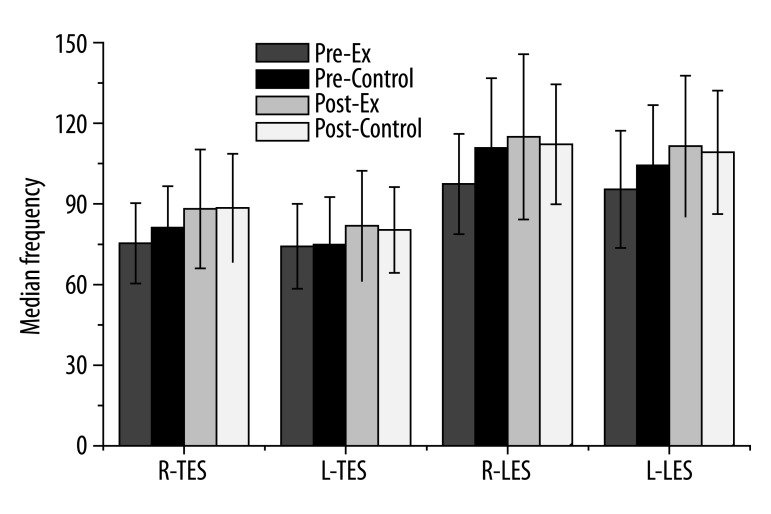
Figure 4 indicates the MF measurements for the thoracic and lumbar ES muscles. There was no MF change following treatment (F=0.81, p=0.37); however, there were significant MF differences among muscles (F=39.36, p=0.0001). Both sides of the lumbar ES muscles revealed significantly higher MF values compared with the other muscles. Prior to the intervention, the MF of the right thoracic ES muscle was lower (75.33±14.94 for CSE group vs. 81.22±15.36 for control group). However, following the intervention, the MF of the right thoracic ES muscle was higher (88.14±22.13 for CSE group vs. 88.55±20.10 for control group). The MF of the left thoracic ES muscle prior to the intervention was lower (74.24±15.75 for CSE group vs. 74.86±17.71 for control group). However, following the intervention, the MF was higher (81.88±20.45 for CSE group vs. 80.31±15.93 for control group) for the left thoracic ES muscle.
Figure 4.
The median frequency (MF) measurements for thoracic and lumbar ES muscles. There was no change in the MF following treatment (F=0.81, p=0.37); however, there were significant differences among the muscles (F=39.36, p=0.0001). Both sides of the LES muscles revealed significantly higher MF values compared with the other muscles. (R: right, L: left, TES: thoracic erector spinae muscle, LES: lumbar erector spinae muscle).
Discussion
The purpose of this study was to assess the effects of 4 weeks of CSE based on entropic measures of thoracic and lumbar ES muscle fatigability compared with MF based on power spectral analyses in subjects with chronic LBP. Following the intervention, the level of pain reported by all subjects decreased significantly; however, there was no difference in pain reduction between groups. This result indicated that subjective measures based on the MVAS pain scale might not be sufficiently sensitive to differentiate the changes. The Shannon entropy levels of the EMG and MF based on power spectrum analysis were also not significantly different between groups following the intervention.
The Shannon entropy measurement, on the other hand, demonstrated significant interactions between muscles and groups following treatment. The results of this interaction indicated that the joint effects based on muscle, group, and intervention were discernibly larger and significant among those explanatory variables [28]. When 2 or more explanatory variables are considered simultaneously, one could ask whether their joint effect is significant on the response variable. In our study, the factorial design was used in order to investigate the main effect, which is what the independent variables elicit when averaged out over each other as well as interaction effects among those variables.
The term interaction has a very precise statistical meaning and refers to how the effect on the response of 1 explanatory variable depends on the level of 1 or more other explanatory variables [28]. The general definition of interaction implies that if there is no interaction among 2 explanatory variables, then the effect of 1 explanatory variable is constant or remains the same across all levels or values of the other. If the effect of 1 factor depends on the level of another factor, the 2 factors involved are said to interact, and the contrast involving all these levels is called their interaction [28]. The results of our study indicated that the entropy levels of the EMG signals demonstrated significant interaction between muscles and groups following treatment. Therefore, the effect of the entropy level has an interaction with group and depends on another explanatory variable, which is entropy change among muscles following treatment.
The MF increased following the completion of the CSE program for both groups. The increased MF results indicated enhanced endurance following the intervention; however, there was no significant difference between groups. People with chronic LBP often have decreased muscle endurance, which may compromise the functional capacity of the spine and increase the likelihood of re-injury [29]. Numerous studies have also identified an association between LBP and easily fatigued back muscles based on back muscle endurance [10,11,30–32]. However, the MF changes based on power spectrum analysis were not sensitive enough to determine whether 4 weeks of the CSE intervention is capable of altering patterns of localized muscle fatigue in chronic LBP.
Regarding the thoracic and lumbar ES muscles, the response of the MF change was significantly different. The thoracic part of the ES muscle may have played an important role in spinal endurance for subjects with LBP even though there was no significant relationship between the MF of the ES muscles. As our previous study indicated, the increased fatigability of the thoracic part needs to be emphasized [33]. Therefore, rehabilitation strategies for subjects with LBP might effectively create a stabilizing moment at the lumbar part in order to maintain spinal stability.
The results of the study indicated that there was no statistical difference following the intervention to affect entropy levels of the EMG signals or the MF based on power spectrum. The CSE involves the co-contraction of muscles, which may restore stability to the spine and theoretically protect it from biomechanical stresses and further injuries [34]. According to Janda, the ES muscles stiffen more than the abdominal muscles in subjects with LBP [35]. A 4-week exercise intervention significantly reduced LBP without group difference. The overall change in entropy levels of the EMG signals and the MF were not significantly different given the limited exercise period. The differences may not be detected either because the measurement was not sensitive enough or because physiologic changes did not occur [36] during the short period of time of the intervention.
Another important finding of our study indicated that the entropy levels of the EMG signals demonstrated significant interaction between muscles and groups following treatment; however, the MF did not demonstrate this interaction. The significant interaction effect of the entropy between muscles and groups following treatment for muscle endurance during the 1-minute back extension test supports the characteristics of the recorded signals that occurs with fatigue [38]. Exercises for graded activity programs can be used to increase trunk muscle endurance and decrease pain [38,39]. Undoubtedly, other muscles participated in the load sharing during the testing as well as when subjects performed the intervention exercises. The attachment of the lumbar ES muscles, rather than the thoracic ES muscles, results in an effective lever arm for lumbar stabilization. Therefore, the lumbar ES muscle is more effective in creating a stabilizing moment over the lumbar vertebral segments during the test [40,41].
Our previous study indicated that control subjects revealed significantly larger entropy levels of the EMG than the subjects with LBP [5]. Thus, the results of the current study consistently demonstrated a connection between physiological “health” and complexity [6,42]. We also explored the use of entropy derived from time series as an alternative quantitative measure of EMG signals that can be used in a clinical assessment [5]. It was evident that the entropy levels of the EMG decreased following the intervention.
The results of our current study indicated that the entropy levels of the right thoracic and left lumbar muscles were higher even though there were no statistical differences in the entropy levels. As indicated in Figure 2, the right thoracic and left lumbar muscles demonstrated higher entropy levels. This pattern might be necessary to maintain the Sorensen testing position; therefore, further investigation based on the handedness of the subjects is warranted, since muscle development could be related to LBP. Although there was no significant entropy level difference, the entropy level of the thoracic ES was higher on the right side than on the left side. Both sides of the thoracic and lumbar trunk muscles were also different, and this difference was significant when the exercise intervention was considered.
Therefore, the results of this study indicated that Shannon entropy might be a valuable tool for use in measuring the difference of outcomes following the exercise intervention. Future studies should include a larger sample size and include control groups in order to generalize the results. Follow-up, randomized controlled trials to more fully investigate treatment effects, and factors that might mediate these effects, should also be pursued.
Conclusions
The average pain levels reported by the subjects decreased significantly following 4 weeks of the CSE program. However, the subjective pain level change measurement tool was not statistically different between groups.
This study investigated back muscle fatigability following exercise intervention in subjects with chronic LBP. The Shannon entropy measure was more sensitive than the MF for the exercise intervention.
Although there was no entropy level change following treatment, the interactions between muscles and groups following treatment were significant. The entropy level decreased in both thoracic ES muscles following intervention in the CSE group, while it remained the same in the control group.
Footnotes
Source of support: This research was supported by 2009 National Agenda Project funded by Korea Research Council of Fundamental Science & Technology (P-09-JL-LU63-COI)
References
- 1.Frymoyer JW, Cats-Baril WL. An overview of the incidences and costs of low back pain. Orthop Clin North Am. 1991;22(2):263–71. [PubMed] [Google Scholar]
- 2.Arokoski JP, et al. Back and abdominal muscle function during stabilization exercises. Arch Phys Med Rehabil. 2001;82(8):1089–98. doi: 10.1053/apmr.2001.23819. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman M, Zurcher U, Sung P. Entropy of Electromyography Time Series. Physica A Statistical Mechanics and its Applications. 2007;386(2):698–707. [Google Scholar]
- 4.Sung PS, Zurcher U, Kaufman M. Nonlinear analysis of electromyography time series as a diagnostic tool for low back pain. Med Sci Monit. 2005;11(1):CS1–5. [PubMed] [Google Scholar]
- 5.Sung PS, Zurcher U, Kaufman M. Comparison of spectral and entropic measures for surface electromyography time series: A pilot study. J Rehabil Res Dev. 2007;44(4):599–610. doi: 10.1682/jrrd.2006.10.0132. [DOI] [PubMed] [Google Scholar]
- 6.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of biological signals. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71(2 Pt 1):021906. doi: 10.1103/PhysRevE.71.021906. [DOI] [PubMed] [Google Scholar]
- 7.Aho AJ, et al. Facial muscle activity, Response Entropy, and State Entropy indices during noxious stimuli in propofol-nitrous oxide or propofol-nitrous oxide-remifentanil anaesthesia without neuromuscular block. Br J Anaesth. 2009;102(2):227–33. doi: 10.1093/bja/aen356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sung PS, Zurcher U, Kaufman M. Reliability difference between spectral and entropic measures of erector spinae muscle fatigability. J Electromyogr Kinesiol. 2008;20:25–30. doi: 10.1016/j.jelekin.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 9.McGill SM. Low back exercises: evidence for improving exercise regimens. Phys Ther. 1998;78(7):754–65. doi: 10.1093/ptj/78.7.754. [DOI] [PubMed] [Google Scholar]
- 10.Mayer TG, et al. Lumbar myoelectric spectral analysis for endurance assessment. A comparison of normals with deconditioned patients. Spine. 1989;14(9):986–91. doi: 10.1097/00007632-198909000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Klein AB, et al. Comparison of spinal mobility and isometric trunk extensor forces with electromyographic spectral analysis in identifying low back pain. Physical Therapy. 1991;71(6):445–54. doi: 10.1093/ptj/71.6.445. [DOI] [PubMed] [Google Scholar]
- 12.Richardson CA, et al. The relation between the transversus abdominis muscles, sacroiliac joint mechanics, and low back pain. Spine. 2002;27(4):399–405. doi: 10.1097/00007632-200202150-00015. [DOI] [PubMed] [Google Scholar]
- 13.Richardson CA, et al. Therapeutic exercise for spinal segmental stabilization in low back pain. London: Harcourt Publishers; 1999. [Google Scholar]
- 14.Stewart M, Latimer J, Jamieson M. Back extensor muscle endurance test scores in coal miners in Australia. J Occup Rehabil. 2003;13(2):79–89. doi: 10.1023/a:1022547714552. [DOI] [PubMed] [Google Scholar]
- 15.Dvir Z, Keating JL. Trunk extension effort in patients with chronic low back dysfunction. Spine (Phila Pa 1976) 2003;28(7):685–92. doi: 10.1097/01.BRS.0000051917.04731.A4. [DOI] [PubMed] [Google Scholar]
- 16.Hunt A. Musculoskeletal fitness: the keystone in overall well-being and injury prevention. Clin Orthop Relat Res. 2003;(409):96–105. doi: 10.1097/01.blo.0000057787.10364.4e. [DOI] [PubMed] [Google Scholar]
- 17.Mannion AF, et al. A new skin-surface device for measuring the curvature and global and segmental ranges of motion of the spine: reliability of measurements and comparison with data reviewed from the literature. Eur Spine J. 2004;13(2):122–36. doi: 10.1007/s00586-003-0618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannion AF, et al. Comparison of three active therapies for chronic low back pain: results of a randomized clinical trial with one-year follow-up. Rheumatology (Oxford) 2001;40(7):772–78. doi: 10.1093/rheumatology/40.7.772. [DOI] [PubMed] [Google Scholar]
- 19.Sung PS. Multifidi muscles median frequency before and after spinal stabilization exercises. Arch Phys Med Rehabil. 2003;84(9):1313–18. doi: 10.1016/s0003-9993(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 20.Roy SH, et al. Classification of back muscle impairment based on the surface electromyographic signal. J Rehabil Res Dev. 1997;34(4):405–14. [PubMed] [Google Scholar]
- 21.Mannion AF, et al. The use of surface EMG power spectral analysis in the evaluation of back muscle function. J Rehabil Res Dev. 1997;34(4):427–39. [PubMed] [Google Scholar]
- 22.Lindstrom L, Magnusson R, Petersen I. Muscle load influence on myo-electric signal characteristics. Scand J Rehabil Med. 1974;0(Suppl):127–48. [PubMed] [Google Scholar]
- 23.Klenerman L, et al. The prediction of chronicity in patients with an acute attack of low back pain in a general practice setting. Spine. 1995;20(4):478–84. doi: 10.1097/00007632-199502001-00012. [DOI] [PubMed] [Google Scholar]
- 24.Zipp P. Recommendations for the standardization of lead positions in surface electromyography. Eur J Appl Physiol. 1982;50:41–54. [Google Scholar]
- 25.Lee TR, Kim YH, Sung PS. Spectral and entropy changes for back muscle fatigability following spinal stabilization exercises. J Rehabil Res Dev. 2010;47(2):133–42. doi: 10.1682/jrrd.2009.07.0088. [DOI] [PubMed] [Google Scholar]
- 26.O’Sullivan P, et al. Altered patterns of abdominal muscle activation in patients with chronic low back pain. Aust J Physiother. 1997;43(2):91–98. doi: 10.1016/s0004-9514(14)60403-7. [DOI] [PubMed] [Google Scholar]
- 27.Portney LGW. Foundations of clinical research applications to practice. 2000. [Google Scholar]
- 28.Fitzmaurice G. The meaning and interpretation of interaction. Nutrition. 2000;16(4):313–14. doi: 10.1016/s0899-9007(99)00293-2. [DOI] [PubMed] [Google Scholar]
- 29.Jackson CP, Brown MD. Is there a role for exercise in the treatment of patients with low back pain? Clin Orthop. 1983;(179):39–45. [PubMed] [Google Scholar]
- 30.McGill SM. Low back exercises: evidence for improving exercise regimens. Physical Therapy. 1998;78(7):754–65. doi: 10.1093/ptj/78.7.754. [DOI] [PubMed] [Google Scholar]
- 31.Roy SH, et al. Spectral electromyographic assessment of back muscles in patients with low back pain undergoing rehabilitation. Spine. 1995;20(1):38–48. doi: 10.1097/00007632-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Biedermann HJ, et al. Power spectrum analyses of electromyographic activity. Discriminators in the differential assessment of patients with chronic low-back pain. Spine. 1991;16(10):1179–84. [PubMed] [Google Scholar]
- 33.Sung PS, Lammers AR, Danial P. Different parts of erector spinae muscle fatigability in subjects with and without low back pain. Spine J. 2009;9(2):115–20. doi: 10.1016/j.spinee.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Richardson CA, Jull GA. Muscle control-pain control. What exercises would you prescribe? Manual Therapy. 1995;1(1):2–10. doi: 10.1054/math.1995.0243. [DOI] [PubMed] [Google Scholar]
- 35.Janda V. Muscles, central nervous motor regulation and back problems. In: Korr IM, editor. The Neurologic Mechanisms in Manulative Therapy. New York: Plenum Press; 1978. [Google Scholar]
- 36.Moffroid MT, et al. Endurance training of trunk extensor muscles. Phys Ther. 1993;73(1):10–17. [PubMed] [Google Scholar]
- 37.Roy SH, De Luca CJ, Casavant DA. Lumbar muscle fatigue and chronic lower back pain. Spine. 1989;14(9):–992–1001. doi: 10.1097/00007632-198909000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Jorgensen K, Nicolaisen T. Two methods for determining trunk extensor endurance. A comparative study. Eur J Appl Physiol Occup Physiol. 1986;55(6):639–44. doi: 10.1007/BF00423210. [DOI] [PubMed] [Google Scholar]
- 39.Jorgensen K, Nicolaisen T. Trunk extensor endurance: determination and relation to low-back trouble. Ergonomics. 1987;30(2):259–67. doi: 10.1080/00140138708969704. [DOI] [PubMed] [Google Scholar]
- 40.MacIntosh JE, Bogduk N. The biomechanics of the lumbar multifidus. Clinical Biomechanics. 1986;1:205–213. doi: 10.1016/0268-0033(86)90147-6. [DOI] [PubMed] [Google Scholar]
- 41.Flicker PL, et al. Lumbar muscle usage in chronic low back pain. Magnetic resonance image evaluation. Spine. 1993;18(5):582–86. doi: 10.1097/00007632-199304000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis: A new measure of complexity loss in heart failure. J Electrocardiol. 2003;36(Suppl):39–40. [Google Scholar]