Summary
Background
Peroxisome proliferator-activated Receptor-γ (PPAR-γ) and its nuclear partners, the Retinoid X Receptors (RXRs), have been recognized as crucial players in the pathogenesis of atherosclerosis. The present study aimed to assess the clinical significance of PPAR-γ and RXR-α expression in different cellular populations localized within advanced carotid atherosclerosis lesions.
Material/Methods
PPAR-γ and RXR-α expression was assessed by immunohistochemistry ïn 134 carotid atherosclerotic plaques obtained from an equal number of patients that underwent endarterectomy procedure for vascular repair, and was correlated with patients’ medical history, risk factors and medication intake.
Results
Increased incidence of low PPAR-γ expression in both macrophages and smooth muscle cells was noted in patients presenting coronary artery disease (p=0.032 and p=0.046, respectively). PPAR-γ expression in smooth muscle cells was borderline down-regulated in symptomatic compared to asymptomatic patients (p=0.061), reaching statistical significance when analyzing groups of patients with specific cerebrovascular events; amaurosis fugax (p=0.008), amaurosis fugax/stroke (p=0.020) or amaurosis fugax/transient ischemic attack patients (p=0.028) compared to asymptomatic patients. Low RXR-α expression in macrophages was more frequently observed in hypertensive (p=0.048) and hyperlipidemic patients (p=0.049). Increased incidence of low RXR-α expression in smooth muscle cells was also noted in patients presenting advanced carotid stenosis grade (p=0.015).
Conclusions
PPAR-γ and RXR-α expression down-regulation in macrophages and smooth muscle cells was associated with a more pronounced disease progression in patients with advanced carotid atherosclerotic lesions.
Keywords: carotid atherosclerosis, immunohistochemistry, macrophages, PPAR-γ, RXR-α, smooth muscle cells
Background
Atherosclerosis and its complications, such as myocardial infarction and stroke, are the leading cause of morbidity and mortality in the developed world [1]. Stress, tobacco smoking, alcohol consumption, hypertension, diabetes mellitus, obesity, insulin resistance and dyslipidemia have been identified as important risk factors predisposing to atherosclerosis [2,3]. At an early stage of disease, endothelial cells dysfunction causes the release of vasoactive molecules, which stimulate inflammatory responses and recruitment/migration of leukocytes into the arterial wall [4]. Macrophage stimulation leads to the secretion of cytokines, growth factors and other mediators, which promote smooth muscle cell proliferation and migration [5]. In later stages, smooth muscle cells activation stimulates the release of pro-inflammatory cytokines, which, combined with the secretion of matrix metalloproteinases (MMPs) and procoagulant factors, results in chronic inflammatory state and plaque instability [5,6]. The resulting chronic inflammatory state and the enrichment of lipid-laden macrophages (foam cells) contribute to the development of advanced atherosclerotic lesions, which ultimately causes luminal obstruction and plaque rupture, leading to thrombus formation and, eventually, ischemic events [6]. The multi-factorial nature of atherosclerosis involves chronic inflammation at every step from initiation to progression, suggesting that certain clinical risk factors may contribute to the pathogenesis of disease by aggravating the underlying inflammatory process [7].
PPAR-γ is a ligand-activated transcription factor, which belongs to the nuclear receptor superfamily [8]. Upon ligand activation, PPAR-γ forms heterodimers with its nuclear receptor partners, Retinoid X Receptors (RXRs) and binds to specific PPAR response elements (PPREs) in the promoter region of the target genes, regulating gene function through dissociation of co-repressors and recruitment of co-activators [9,10]. RXRs are common heterodimerization partners for several nuclear receptors beyond PPARs, including thyroid receptors (TRs), vitamin D receptor (VDR), and liver, farnesoid and pregnane X receptors (LXR, FXR and PXR, respectively) [11]. The RXR family includes 3 distinct subtypes, termed as RXR-α, -β and -γ; however, it still remains unclear whether their nuclear partners, such as PPAR-γ, exhibit a marked preference for 1 of them [12,13]. Moreover, it has not yet been elucidated whether their role is restricted to acting as heterodimerization partners for nuclear receptors, or if there is actually a separate RXR-mediated signalling pathway [12,13].
Currently, PPAR-γ constitutes a key regulator of glucose homeostasis and adipogenesis and is a promising therapeutic target for the treatment of patients with type 2 diabetes mellitus [14,15]. Two synthetic PPAR-γ activators, pioglitazone and rosiglitazone, which belong to the thiazolidinedione (TZD) class, have successfully been introduced in the market as oral antidiabetic agents to attenuate insulin resistance associated with obesity, hypertension and impaired glucose homeostasis [16,17]. Pleiotropic functions beyond this limit, such as anti-proliferative and anti-inflammatory effects against several pathophysiological states, including neoplasia, ischemia/reperfusion injury, gestational diseases and arthritis, are currently being explored in clinical studies [18–23]. An increasing body of evidence from preclinical and clinical studies has further supported that PPAR-γ ligands, and especially TZDs, exert a broad spectrum of anti-inflammatory and anti-proliferative effects on all cell types participating in the development of cardiovascular diseases [24–26]. Importantly, PPAR-γ was shown to be expressed in atherosclerotic lesions and in all vascular wall apparent cell types [27–30]. PPAR-γ ligand treatment was also reported to attenuate the development and progression of atherosclerotic lesions in several animal models [24,31–34]. Moreover, TZDs treatment was shown to reduce carotid artery intima/media thickness in patients with type 2 diabetes [24,30,35].
Taking into consideration the extensive use of PPAR-γ agonists in patients at high risk for cardiovascular diseases, the understanding of PPAR-γ function in the vasculature is not only of basic interest, but also carries important clinical implications. However, there is no comprehensive research so far concerning the clinical significance of PPAR-γ and its nuclear partner, RXR-α, in patients with advanced carotid atherosclerotic lesions. In view of the above considerations, our study aimed to assess the immunohistochemical expression of PPAR-γ and RXR-α in different cellular populations (macrophages, smooth muscle cells and endothelial cells), localized within advanced atherosclerotic lesions in patients that underwent carotid endarterectomy for vascular repair. Correlations of this differential cellular expression of PPAR-γ and RXR-α with patients’ medical history, risk factors and medication intake were examined.
Material and Methods
Clinical material
One hundred thirty-four (134) consecutive patients of Greek ethnicity that underwent carotid endarterectomy in Laikon Hospital between January 2006 and December 2008 were eligible for this study. The study was approved by the Hospital Ethics Committee, and informed consent was obtained from all participants. Indication for surgery was a symptomatic carotid stenosis ≥50% or an asymptomatic carotid stenosis ≥70% according to ESVS guidelines [36]. Preoperatively, patients had either carotid duplex ultrasound scans or digital subtraction angiograms, or both of these. Patients or plaques were defined as symptomatic when focal symptoms of cerebral ischemia were present, ipsilateral to the carotid lesions, such as transient ischemic attack (TIA), amaurosis fugax, or stroke, in the last 6 months. All patients were preoperatively on antiplatelet treatment that was interrupted 1 week before surgery.
A complete medical history, risk factors and medication intake were recorded, including: age, sex, coronary artery disease-CAD (angina pectoris, myocardial infarction and coronary artery by-pass grafting/percutaneous transluminal coronary angioplasty-CABG/PTCA), diabetes mellitus (controlled with diet, oral hypoglycemic agents or insulin; fasting glucose level ≥126mg/dL), hypercholesterolemia (total cholesterol ≥200mg/dL), hypertension (systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or self-report of high blood pressure) [37], peripheral artery disease (PAD), smoking history, and therapy with statins and angiotensin-converting enzyme (ACE) inhibitors. The demographic characteristics of the study population are summarized in Tables 1 and 2.
Table 1.
Associations of PPAR- γ expression in macrophages and smooth muscle cells with medical history, risk factors and medication intake in patients with advanced carotid atherosclerosis lesions.
| Clinical variables | N=134 | PPAR-γ expression in macrophages | PPAR-γ expression in smooth muscle cells | ||||
|---|---|---|---|---|---|---|---|
| Low (%) | High (%) | p-value | Low (%) | High (%) | p-value | ||
| 69 (51) | 65 (49) | 70 (52) | 64 (48) | ||||
| Age (mean ±SD) | 71.02±7.98 | 71.11±7.94 | 70.75±8.07 | 0.683 | 71.80±7.75 | 70.00±8.05 | 0.103 |
| Gender | 0.617 | 0.617 | |||||
| Male | 109 (81) | 55 (41) | 54 (40) | 58 (43) | 51 (38) | ||
| Female | 25 (19) | 14 (10) | 11 (8) | 12 (9) | 13 (10) | ||
| Carotid | 0.630 | 0.638 | |||||
| Right | 75 (56) | 40 (30) | 35 (26) | 37 (28) | 38 (28) | ||
| Left | 59 (44) | 29 (22) | 30 (22) | 33 (25) | 26 (19) | ||
| Stenosis grade | 0.862 | 0.999 | |||||
| <90% | 67 (50) | 34 (25) | 33 (25) | 35 (26) | 32 (24) | ||
| ≥90% | 67 (50) | 35 (26) | 32 (24) | 35 (26) | 32 (24) | ||
| Diabetes | 0.684 | 0.898 | |||||
| No | 97 (72) | 51 (38) | 46 (34) | 51 (38) | 46 (34) | ||
| Yes | 37 (28) | 18 (13) | 19 (14) | 19 (14) | 18 (13) | ||
| Hyperlipidemia | 0.328 | 0.464 | |||||
| No | 44 (33) | 20 (15) | 24 (18) | 21 (16) | 23 (17) | ||
| Yes | 90 (67) | 49 (37) | 41 (31) | 49 (37) | 41 (31) | ||
| Hypertension | 0.549 | 0.082 | |||||
| No | 32 (24) | 15 (11) | 17 (13) | 21 (16) | 11 (8) | ||
| Yes | 102 (76) | 54 (40) | 48 (36) | 49 (37) | 53 (40) | ||
| Smoking status | 0.178 | 0.716 | |||||
| No | 44 (33) | 19 (14) | 25 (19) | 22 (16) | 22 (16) | ||
| Yes | 90 (67) | 50 (37) | 40 (30) | 48 (36) | 42 (31) | ||
| Statins | 0.056 | 0.075 | |||||
| No | 86 (64) | 39 (29) | 47 (35) | 40 (30) | 46 (34) | ||
| Yes | 48 (36) | 30 (22) | 18 (13) | 30 (22) | 18 (13) | ||
| Antiplateles | 0.821 | 0.273 | |||||
| No | 94 (70) | 49 (37) | 45 (34) | 52 (39) | 42 (31) | ||
| Yes | 40 (30) | 20 (15) | 20 (15) | 18 (13) | 22 (16) | ||
| ACES | 0.173 | 0.630 | |||||
| No | 72 (54) | 41 (31) | 31 (23) | 39 (29) | 33 (25) | ||
| Yes | 62 (46) | 28 (21) | 34 (25) | 31 (23) | 31 (23) | ||
| CAD | 0.032 | 0.046 | |||||
| No | 76 (57) | 33 (25) | 43 (32) | 34 (25) | 42 (31) | ||
| Yes | 58 (43) | 36 (27) | 22 (16) | 36 (27) | 22 (16) | ||
| PAD | 0.254 | 0.517 | |||||
| No | 97 (72) | 47 (35) | 50 (37) | 49 (37) | 48 (36) | ||
| Yes | 37 (28) | 22 (16) | 15 (11) | 21 (16) | 16 (12) | ||
| CABG/PTCA | 0.007 | 0.028 | |||||
| No | 97 (72) | 43 (32) | 54 (40) | 45 (34) | 52 (39) | ||
| Yes | 37 (28) | 26 (19) | 11 (8) | 25 (19) | 12 (9) | ||
| Symptoms | 0.504 | 0.061 | |||||
| No | 62 (46) | 30 (22) | 32 (24) | 27 (20) | 35 (26) | ||
| Yes | 72 (54) | 39 (29) | 33 (25) | 43 (32) | 29 (22) | ||
Table 2.
Associations of RXR-α expression in macrophages and smooth muscle cells with medical history, risk factors and medication intake in patients with advanced carotid atherosclerosis lesions.
| Clinical variables | N=134 | RXR-α expression in macrophages | RXR-α expression in smooth muscle cells | ||||
|---|---|---|---|---|---|---|---|
| Low (%) | High (%) | p-value | Low (%) | High (%) | p-value | ||
| 62 (46) | 72 (54) | 70 (52) | 64 (48) | ||||
| Age (mean ±SD) | 71.02±7.98 | 70.98±7.67 | 70.90±8.28 | 0.966 | 70.61±8.51 | 71.29±7.38 | 0.846 |
| Gender | 0.800 | 0.071 | |||||
| Male | 109 (81) | 51 (38) | 58 (43) | 61 (46) | 48 (36) | ||
| Female | 25 (19) | 11 (8) | 14 (11) | 9 (7) | 16 (12) | ||
| Carotid | 0.806 | 0.774 | |||||
| Right | 75 (56) | 34 (25) | 41 (31) | 40 (30) | 35 (26) | ||
| Left | 59 (44) | 28 (21) | 31 (23) | 30 (22) | 29 (22) | ||
| Stenosis grade | 0.165 | 0.015 | |||||
| <90% | 67 (50) | 27 (20) | 40 (30) | 28 (21) | 39 (29) | ||
| ≥90% | 67 (50) | 35 (26) | 32 (24) | 42 (31) | 25 (19) | ||
| Diabetes | 0.466 | 0.898 | |||||
| No | 97 (72) | 43 (32) | 54 (40) | 51 (38) | 46 (34) | ||
| Yes | 37 (28) | 19 (14) | 18 (13) | 19 (14) | 18 (13) | ||
| Hyperlipidemia | 0.048 | 0.142 | |||||
| No | 44 (33) | 15 (11) | 29 (22) | 19 (14) | 25 (19) | ||
| Yes | 90 (67) | 47 (35) | 43 (32) | 51 (38) | 39 (29) | ||
| Hypertension | 0.049 | 0.771 | |||||
| No | 32 (24) | 10 (7) | 22 (16) | 16 (12) | 16 (12) | ||
| Yes | 102 (76) | 52 (39) | 50 (37) | 54 (40) | 48 (36) | ||
| Smoking status | 0.894 | 0.995 | |||||
| No | 44 (33) | 20 (15) | 24 (18) | 23 (17) | 21 (16) | ||
| Yes | 90 (67) | 42 (31) | 48 (36) | 47 (35) | 43 (32) | ||
| Statins | 0.662 | 0.698 | |||||
| No | 86 (64) | 41 (31) | 45 (34) | 46 (34) | 40 (30) | ||
| Yes | 48 (36) | 21 (16) | 27 (20) | 24 (18) | 24 (18) | ||
| Antiplateles | 0.184 | 0.735 | |||||
| No | 94 (70) | 47 (35) | 47 (35) | 50 (37) | 44 (33) | ||
| Yes | 40 (30) | 15 (11) | 25 (19) | 20 (15) | 20 (15) | ||
| ACEs | 0.557 | 0.892 | |||||
| No | 72 (54) | 35 (26) | 37 (28) | 38 (28) | 34 (25) | ||
| Yes | 62 (46) | 27 (20) | 35 (26) | 32 (24) | 30 (22) | ||
| CAD | 0.449 | 0.552 | |||||
| No | 76 (57) | 33 (25) | 43 (32) | 38 (28) | 38 (28) | ||
| Yes | 58 (43) | 29 (22) | 29 (22) | 32 (24) | 26 (19) | ||
| PAD | 0.732 | 0.795 | |||||
| No | 97 (72) | 44 (33) | 53 (40) | 50 (37) | 47 (35) | ||
| Yes | 37 (28) | 18 (13) | 19 (14) | 20 (15) | 17 (13) | ||
| CABG/PTCA | 0.963 | 0.517 | |||||
| No | 97 (72) | 45 (34) | 52 (39) | 49 (37) | 48 (36) | ||
| Yes | 37 (28) | 17 (13) | 20 (15) | 21 (16) | 16 (12) | ||
| Symptoms | 0.200 | 0.239 | |||||
| No | 62 (46) | 25 (19) | 37 (28) | 29 (22) | 33 (25) | ||
| Yes | 72 (54) | 37 (28) | 35 (26) | 41 (31) | 31 (23) | ||
Carotid specimens’ histopathology
The carotid plaque surgical specimens were collected intra-operatively immediately after endarterectomy and fixed in 10% buffered formalin for 24 hours. After decalcification, if necessary, specimens were embedded in paraffin wax using conventional techniques, cut transversely at 4ìm and stained with hematoxylin-eosin (H-E). After staining with H-E, 3 to 4 sections per specimen were examined. The section with plaque ulceration or thrombus, the most stenotic segment of the plaques, or both sections, were chosen for further analysis. Plaques were classified into 2 groups: (1) thrombotic plaques including (1a) plaque rupture, (1b) plaque erosion, and (1c) calcified nodule, and (2) plaques without acute thrombosis divided into: (2a) vulnerable and (2b) stable plaques [38].
Immunohistochemistry
Immunostainings for PPAR-γ and RXR-α were performed on paraffin-embedded carotid plaque surgical specimens using a commercially available mouse (IgG1) monoclonal antibody (E-8) that recognizes the carboxy terminus of human PPAR-γ (Santa Cruz Biochemicals, Santa Cruz, CA, USA), reacting with PPAR-γ1 and -γ2, and another rabbit polyclonal antibody that recognizes human RXR-α (D-20) (Santa Cruz Biochemicals). Briefly, 4μm thick tissue sections were dewaxed in xylene and were brought to water through graded alcohols. To remove the endogenous peroxidase activity, sections were then treated with freshly prepared 0.3% hydrogen peroxide in methanol in the dark, for 30 min (minutes), at room temperature. Antigen retrieval was performed for PPAR-γ antigen detection, by microwaving slides in 10mM citrate buffer (pH 6.0) for 15 min [39]. Non-specific antibody binding was then blocked using a specific blocking reagent for 5 min (Sniper, Biocare Medical, Walnut Creek, CA, USA). The sections were then incubated for 1 hour at room temperature, with the primary antibodies PPAR-γ and RXR-α diluted 1:100 in phosphate buffered saline (PBS). After washing 3 times with PBS, sections were incubated at room temperature with biotinylated linking reagent (Biocare Medical) for 10 min, followed by incubation with peroxidase-conjugated streptavidin label (Biocare Medical) for 10 min. The resultant immune peroxidase activity was developed in 0.5% 3,3′-diaminobenzidine hydrochloride (Vector Laboratories, Peterborough, United Kingdom) for 10 min. Sections were counterstained with Harris’ hematoxylin and mounted in Entellan (Merck, Darmstadt, Germany). Appropriate negative controls were performed by omitting the primary antibody and/or substituting it with an irrelevant anti-serum. As positive controls, pancreatic cancer tissue sections with known increased expression of PPAR-γ and RXR-α were used [39].
Immunohistochemical evaluation
The expression of PPAR-γ and RXR-α was assessed on macrophages, smooth muscle and endothelial cells of the carotid plaque specimens by 2 different observers (ST and GK) blinded to the clinical data with very good inter-observer agreement (κ=0.961, SE: 0.022). Carotid specimens were considered positive for PPAR-γ and RXR-α when more than 5% of macrophages, smooth muscle and endothelial cells were positively stained [39–42]. The median value of percentage expression for PPAR-γ and RXR-α in macrophages, smooth muscle and endothelial cells was used as a threshold to discriminate the carotid specimens into 2 categories: low and high expression [39–42]. In positively stained carotid specimens, the immunoreactivity of PPAR-γ and RXR-α was further classified according to staining intensity as mild (+), intermediate (++), and intense (+++) [39–42]. Specimens that exhibited percentage expression values equal to the median percentage value were considered to present low expression if their staining intensity was classified as mild (+), whereas they were considered to present high expression if their staining intensity was classified as intermediate (++) or intense (+++). In this way, we ensured that each group had a sufficient and more homogeneous number of cases in the cross-tables in order to be comparable with the other groups [39–42].
Plasma homocysteine and C-Reactive Protein (CRP) determination
Preoperative fasting blood samples were available for 123 patients. The blood samples were collected in Vacutainer tubes under sterile conditions. Plasma was separated from whole blood by centrifugation at 1500×g at 4°C for 10 min and the separated plasma was kept at −80°C until analysis. Plasma homocysteine levels were determined by reversed-phase High Performance Liquid Chromatography (HPLC) coupled to a fluorescence detector (ImmuChrom GmbH, Heppenheim, Germany). The albumin-bound and oxidized homocysteine was reduced and converted into a fluorescence probe in 1 step. The quantification was performed using a delivered plasma calibrator and the concentration was calculated via the internal standard method. The sensitivity of the homocysteine assay was 0.3 μmol/l. Inter- and intra-assay coefficients of variation were 1.7% to 3.5% and 0.9% to 1.0%, respectively. The manufacture’s normal values are <15 μmol/L. A concentration range from 15 to 30 μmol/L indicates lack of vitamin, while concentrations >30 μmol/L indicate a heterozygote homocysteinemia.
Plasma C-Reactive Protein (CRP) levels were measured on the BN ProSpec nephelometer (Dade Behring, Siemens Healthcare Diagnostics) with fully automated latex particle-enhanced immunonephelometric assay, according to the manufacturer’s instructions. The intra- and inter-assay CVs were less than 6% and less than 7%, respectively.
Statistical analysis
The associations of PPAR-γ and RXR-α expression with clinical variables of medical history, risk factors and medication intake were assessed by chi-square test. Spearman’s rank correlation coefficient (Rs) was used to evaluate the linear relationships PPAR-γ and RXR-α expression. The inter-observer agreement was determined with the use of κ statistics. The k value is indicated including the standard error (SE). A two-tailed p<0.05 was considered statistically significant. Statistical analysis was performed using the software package SPSS for Windows (version 11.0; SPSS Inc., Chicago, IL, USA).
Results
One hundred and thirty-four patients were evaluated. Main characteristics of the patient population are depicted in Tables 1 and 2. The mean age was 71.02±7.98 years, and the vast majority (81%) were male. Seventy-two patients (54%) suffered from carotid atherosclerosis-related neurological event (amaurosis fugax or stroke or TIA). A thrombotic plaque was observed in 49 (37%) cases, 44 (33%) of which were ruptured. Of the remaining non-thrombotic plaques, 30 (22%) were classified as vulnerable. Thrombotic plaques were observed more frequently in patients affected by stroke, TIA or amaurosis fugax as compared to asymptomatic patients (p<0.001).
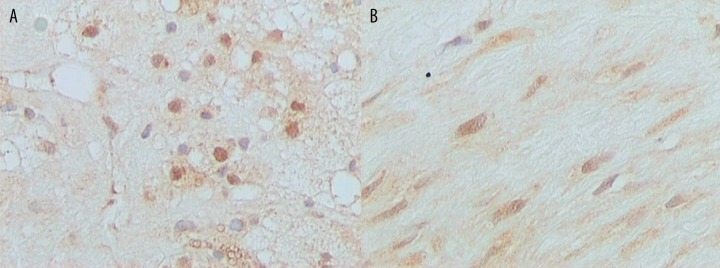
PPAR-γ positivity in macrophages and smooth muscle cells was noted in 84 (63%) and 95 (71%) out of 134 carotid specimens, respectively. RXR-α positivity in macrophages and smooth muscle cells was noted in 129 (96%) and 133 (99%) out of 134 carotid specimens, respectively. Representative immunostainings for PPAR-γ and RXR-α protein expression in macrophages and smooth muscle cells are depicted in Figures 1 and 2, respectively. The vast majority of carotid specimens did not show positive immunoreactivity for PPAR-γ and RXR-α in endothelial cells, as only 11 (8%) cases were PPAR-γ positive and only 13 (10%) cases were RXR-α positive (data not shown). The low incidence of PPAR-γ and RXR-α immunopositivity in endothelial cells did not permit any statistical analysis with clinical variables in this cellular population (data not shown).
Figure 1.
Representative immunostainings for PPAR-γ in (A). Macrophages and (B). Smooth muscle cells (original magnification ×400).
Figure 2.
Representative immunostainings for RXR-α in (A). Macrophages and (B). Smooth muscle cell (original magnification ×400).
Low PPAR-γ expression in macrophages was significantly more frequently observed in patients with history of CAD and CABG/PTCA (Table 1, p=0.032 and p=0.007, respectively). Accordingly, a significantly increased incidence of low PPAR-γ expression in smooth muscle cells of patients presenting history of CAD or CABG/PTCA was noted (Table 1, p=0.046 and p=0.028, respectively). Low PPAR-γ expression in macrophages and smooth muscle cells was also more frequently observed in patients receiving therapy with statins, without reaching statistical significance (Table 1, p=0.056 and p=0.075, respectively). An increased incidence of low PPAR-γ expression in smooth muscle cells was also noted in patients with no evidence of hypertension, without reaching statistical significance (Table 1, p=0.082).
Symptomatic patients more frequently showed low PPAR-γ expression in smooth muscle cells compared to asymptomatic patients, without reaching statistical significance (Table 1, p=0.061). When looking within specific cerebrovascular events, patients with amaurosis fugax more frequently presented low PPAR-γ expression in smooth muscle cells compared to asymptomatic patients, at a statistically significant level (p=0.008). PPAR-γ expression in smooth muscle cells was also significantly different between asymptomatic patients and those with combined amaurosis fugax/stroke (p=0.020), as well as amaurosis fugax/TIA (p=0.028).
A significantly increased frequency of low RXR-α expression in macrophages localized within carotid atherosclerotic lesions obtained from patients presenting history of hyperlipidemia was noted (Table 2, p=0.048). Hypertensive patients more frequently showed low RXR-α expression in macrophages compared to normotensive ones (Table 2, p=0.049). Patients presenting advanced carotid stenosis grade (≥90%) exhibited significantly increased incidence of low RXR-α expression in smooth muscle cells compared to those with carotid stenosis grade <90% (Table 2, p=0.015). Female patients more frequently presented high RXR-α expression in smooth muscle cells compared to males, without reaching statistical significance (Table 2, p=0.071).
We further statistically analyzed PPAR-γ and RXR-α expression in relation with patients’ plasma homocysteine and CRP levels. Patients presenting elevated plasma CRP levels were characterized by a significantly increased incidence of low PPAR-γ expression in macrophages (Table 3, p=0.038). PPAR-γ expression down-regulation in macrophages was also more frequently observed in patients with enhanced homocysteine levels, without reaching statistical significance (Table 3, p>0.005). PPAR-γ expression in smooth muscle cells, as well as RXR-α expression in both macrophages and smooth muscle cells did not show significant association with plasma homocysteine and CRP levels (Table 3, p>0.005).
Table 3.
Associations of PPAR-γ and RXR-α expression in macrophages and smooth muscle cells with plasma homocysteine and CRP levels of patients with advanced carotid atherosclerosis lesions.
| Clinical variables | N=123 | Homocysteine levels | CRP levels | ||||
|---|---|---|---|---|---|---|---|
| <24.18 μmol/L (%) | ≥24.18 μmol/L (%) | p-value | <2.68 mg/L (%) | ≥2.68 mg/L (%) | p-value | ||
| 60 (49) | 63 (51) | 61 (50) | 62 (50) | ||||
| PPAR-γ expression macrophages | 0.245 | 0.038 | |||||
| Low (%) | 64 (52) | 28 (23) | 36 (29) | 26 (21) | 38 (31) | ||
| High (%) | 59 (44) | 32 (26) | 27 (22) | 35 (28) | 24 (20) | ||
| PPAR-γ expression smooth muscle cells | 0.943 | 0.242 | |||||
| Low (%) | 66 (54) | 32 (26) | 34 (28) | 29 (24) | 36 (29) | ||
| High (%) | 57 (46) | 28 (23) | 29 (24) | 32 (26) | 26 (21) | ||
| RXR-α expression macrophages | 0.537 | 0.419 | |||||
| Low (%) | 58 (47) | 30 (24) | 28 (23) | 31 (25) | 27 (22) | ||
| High (%) | 65 (53) | 30 (24) | 35 (28) | 30 (24) | 35 (28) | ||
| RXR-α expression smooth muscle cells | 0.310 | 0.791 | |||||
| Low (%) | 66 (54) | 35 (28) | 31 (25) | 32 (26) | 34 (28) | ||
| High (%) | 57 (46) | 25 (20) | 32 (26) | 29 (24) | 28 (23) | ||
Spearman’s correlation analysis was used to evaluate the linear relationship between PPAR-γ and RXR-α expression. PPAR-γ expression in macrophages was positively associated with RXR-α expression (Rs=0.213, p=0.017). Accordingly, a positive association between PPAR-γ and RXR-α expression in smooth muscle cells was also noted (Rs=0.225, p=0.011). No significant association between PPAR-γ and RXR-α expression in endothelial cells was obtained (Rs=0.032, p=0.698).
Discussion
Carotid and cerebrovascular disease have major public health implications given the associated morbidity and mortality; however, the best treatment for this disease remains uncertain. Currently, carotid endarterectomy has proven useful in primary and secondary prevention of stroke episodes in respect to patients with advanced internal carotid artery stenosis [43,44]. However, a significant number of patients are considered at high risk for such surgical procedures and they therefore have relatively few treatment options [45,46].
In the last few years, PPAR-γ has been recognized as a crucial player in the pathogenesis of atherosclerosis and a promising therapeutic target for the treatment of cardiovascular complications [47]. In fact, TZDs treatment was shown to attenuate the progression of carotid artery intima/media thickness, a well-described surrogate marker for cardiovascular risk [24,48]. TZD therapy in patients undergoing coronary stent implantation was also associated with less in-stent restenosis and repeated revascularization. Importantly, cardiovascular outcome studies further suggested that pioglitazone reduced all-cause mortality, myocardial infarction, and stroke in patients with type 2 diabetes [24,48]. Moreover, PPAR-γ expression was enhanced in neointima formed after balloon injury of rat endothelium, suggesting that PPAR-γ expression may be up-regulated in vascular cells when the vasculature is damaged [49]. At a cellular level, it has certainly been well-established that PPAR-γ is expressed in monocytes/macrophages, smooth muscle and endothelial cells localized within injured vascular wall [27,29,49,50]. Notably, PPAR-γ ligands were shown to exert beneficial effects on the control of macrophage lipid metabolism and inflammatory status, which play crucial roles in atherosclerosis development and progression [24–26]. Emerging evidence has also implicated PPAR-γ as an essential transcriptional modulator of smooth muscle cell proliferation [51–53].
In this aspect, the present study aimed to assess the immunohistochemical expression of PPAR-γ and RXR-α in different cellular populations localized within advanced atherosclerotic lesions obtained from patients that underwent carotid endarterectomy for vascular repair. We showed that the incidence of low PPAR-γ expression in macrophages and smooth muscle cells was significantly increased in patients with history of CAD and CABG/PTCA. Symptomatic patients (with amaurosis fugax alone or combined amaurosis fugax/stroke or amaurosis fugax/TIA) also more frequently presented low PPAR-γ expression in smooth muscle cells compared to asymptomatic patients. The reduced levels of PPAR-γ expression may be indicative of a more pronounced disease progression, raising the possibility that PPAR-γ may be involved in carotid atherosclerotic plaque stabilization. These findings may also suggest that PPAR-γ activation could be proved to be a more effective therapeutic intervention in patients at an early stage of disease. In this context, recent studies showed that PPAR-γ mRNA levels were significantly reduced in occlusive and ectatic atherosclerotic tissues compared to arterial control ones, which was ascribed to the increased amount of cytokines in the plaque microenvironment [54]. Moreover, PPAR-γ1 expression in carotid atheromas was not down-regulated in any diabetic symptomatic patients compared to asymptomatic ones [55]. Such association was not obtained in diabetic patients, raising the possibility that certain clinical factors may affect the impact of PPAR-γ in the pathogenesis of atherosclerosis [55]. Notably, PPAR-γ 12Ala allele carriers were shown to have less widespread CAD, being also considerably protected against 10-year cardiovascular morbidity and mortality [56]. These long-term findings in patients with manifest CAD further supported an important role for PPAR-γ in determining vascular risk. We further found a significant association between PPAR-γ expression in macrophages and plasma CRP levels, which may suggest a possible interlink between carotid atherosclerosis, PPAR-γ and inflammatory infiltration associated with carotid plaque destabilization and subsequent neurologic events. In support of this view, several convincing pieces of evidence have implied a crucial role for PPAR-γ in certain inflammatory pathways related with atherosclerosis progression [25,26].
Accordingly, we showed that RXR-α expression down-regulation was associated with pronounced disease progression in patients with advanced atherosclerotic lesions. In fact, the incidence of low RXR-α expression in macrophages was significantly increased in patients with evidence of hyperlipidemia and hypertension. Patients with advanced carotid stenosis grade also presented significant increased frequency of low RXR-α expression in smooth muscle cells. In this context, several substantial studies have revealed that RXR may be involved in the pathogenesis of atherosclerosis. More to the point, RXRs have been identified as important regulators of glucose, fatty acid and cholesterol metabolism, which are associated with common metabolic disorders such as diabetes type 2, hyperlipidaemia and atherosclerosis [57]. RXR activation by bexarotene was shown to modulate essential metabolic pathways governing atherosclerosis in mice by improving, at least in part, the circulating cholesterol distribution profile [58]. RXR activation also considerably reduced the development of atherosclerosis in apolipoprotein E−/− mice [59]. Moreover, RXR agonists inhibited phorbol-12-myristate-13-acetate (PMA)-induced monocytic THP-1 cell differentiation into macrophage-like cells [60]. Notably, simultaneous PPAR-γ and RXR activation suppressed foam cell formation through enhanced cholesterol efflux despite the increased oxidized low density lipoprotein (oxLDL) uptake [61].
We further revealed that PPAR-γ expression in macrophages and smooth muscle cells was significantly associated with RXR-α expression. In this context, it should be noted that PPAR-γ forms heterodimers with RXRs in order to exert its function by regulating gene transcription or transrepression. Remarkably, RXR-α activation by 9-cis-retinoic acid (RA) synergistically enhanced the inhibition of human coronary artery vascular smooth muscle cell growth induced by PPAR-γ activation [62]. Thus, dimerization of PPAR-γ with RXR-α was required to achieve maximal suppression of coronary artery vascular smooth muscle cell growth [62]. An increased incidence of low PPAR-γ expression in macrophages and smooth muscle cells was also noted in patients receiving lipid-lowering therapy with statins. This could be ascribe to the fact that this patients’ subgroup had already experienced more pronounced disease progression, such as hyperlipidemia, evidence of CABG/PTCA and elevated plasma homocysteine levels in order to receive therapy with statins.
Conclusions
PPAR-γ and RXR-α immunohistochemical expression was assessed for the first time in patients with advanced carotid atherosclerotic lesions who underwent carotid endarterectomy for vascular repair and was associated with important clinicopathological parameters, such as patients’ medical history, risk factors and medication intake. Low levels of PPAR-γ and RXR-α expression in both macrophages and smooth muscle cells were associated with a more pronounced disease progression in patients with advanced carotid atherosclerotic lesions. However, further research conducted on distinct patients’ groups (e.g., only diabetic or non-diabetic patients, only symptomatic or asymptomatic patients, only patients with or without evidence of CAD) is required to delineate the clinical implications of PPAR-γ and RXR-α in advanced stages of carotid atherosclerosis.
Footnotes
Conflict of interest
No conflict of interest.
Source of support: Constantinos Giaginis was financially supported by a post-doctorate scholarship from the State Scholarship Foundation (I.K.Y.) of the Ministry of Education of Greece
References
- 1.Hansson GK. Inflammatory mechanisms in atherosclerosis. J Thromb Haemost. 2006;1:328–31. doi: 10.1111/j.1538-7836.2009.03416.x. [DOI] [PubMed] [Google Scholar]
- 2.Bonora E. The metabolic syndrome and cardiovascular disease. Ann Med. 2006;38:64–80. doi: 10.1080/07853890500401234. [DOI] [PubMed] [Google Scholar]
- 3.Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Established and emerging vascular risk factors and the development of aortic stenosis: an opportunity for prevention? Expert Opin Ther Targets. 2008;12:809–20. doi: 10.1517/14728222.12.7.809. [DOI] [PubMed] [Google Scholar]
- 4.Niessner A, Goronzy JJ, Weyand CM. Immune-mediated mechanisms in atherosclerosis: prevention and treatment of clinical manifestations. Curr Pharm Des. 2007;13:3701–10. doi: 10.2174/138161207783018626. [DOI] [PubMed] [Google Scholar]
- 5.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 6.Spagnoli LG, Bonnano E, Sangiorgi G, Mauriello A. Role of inflammation in atherosclerosis. J Nucl Med. 2007;48:1800–15. doi: 10.2967/jnumed.107.038661. [DOI] [PubMed] [Google Scholar]
- 7.Mallika V, Goswami B, Rajappa M. Atherosclerosis pathophysiology and the role of novel risk factors: a clinicobiochemical perspective. Angiology. 2007;58:513–22. doi: 10.1177/0003319707303443. [DOI] [PubMed] [Google Scholar]
- 8.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–88. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 9.Nolte RT, Wisely GB, Westin S, et al. Ligand binding and co-activator assembly of the peroxisome proliferators activated Receptor-γ. Nature. 1998;395:137–43. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- 10.Hamza MS, Pott S, Vega VB, et al. De-novo identification of PPARgamma/RXR binding sites and direct targets during adipogenesis. PLoS Onem. 2009;4:e4907. doi: 10.1371/journal.pone.0004907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Germain P, Chambon P, Eichele G, et al. International Union of Pharmacology. LXIII Retinoid X Receptors. Pharmacol Rev. 2006;58:760–72. doi: 10.1124/pr.58.4.7. [DOI] [PubMed] [Google Scholar]
- 12.Szanto A, Narkar V, Shen Q, et al. Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ. 2004;2:S126–43. doi: 10.1038/sj.cdd.4401533. [DOI] [PubMed] [Google Scholar]
- 13.Zoete V, Grosdidier A, Michielin O. Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators. Biochim Biophys Acta. 2007;1771:915–25. doi: 10.1016/j.bbalip.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Feige JN, Gelman L, Michalik L, et al. From: molecular action to physiological outputs: Peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res. 2006;45:120–59. doi: 10.1016/j.plipres.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Sugden MC, Zariwala MG, Holness MJ. PPARs and the orchestration of metabolic fuel selection. Pharmacol Res. 2009;60:141–50. doi: 10.1016/j.phrs.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Pourcet B, Fruchart JC, Staels B, Glineur C. Selective PPAR modulators, dual and pan PPAR agonists: multimodal drugs for the treatment of type 2 diabetes and atherosclerosis. Expert Opin Emerg Drugs. 2006;11:379–401. doi: 10.1517/14728214.11.3.379. [DOI] [PubMed] [Google Scholar]
- 17.Wysowski DK, Armstrong G, Governale L. Rapid increase in the use of oral Antidiabetic drugs in the United States, 1990–2001. Diabetes Care. 2003;26:1852–55. doi: 10.2337/diacare.26.6.1852. [DOI] [PubMed] [Google Scholar]
- 18.Giaginis C, Tsourouflis G, Theocharis S. Peroxisome Proliferator-Activated Receptor-γ (PPAR-γ) ligands: novel pharmacological agents in the treatment of ischemia reperfusion injury. Curr Mol Med. 2008;8:562–79. doi: 10.2174/156652408785748022. [DOI] [PubMed] [Google Scholar]
- 19.Giaginis C, Tsantili-Kakoulidou A, Theocharis S. Peroxisome Proliferator-Activated Receptors (PPARs) in the control of bone metabolism. Fundam Clin Pharmacol. 2007;21:231–44. doi: 10.1111/j.1472-8206.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 20.Giaginis C, Tsantili Kakoulidou A, Theocharis S. Peroxisome Proliferator Activated Receptor-γ (PPAR-γ) ligands: potential pharmacological agents for targeting the angiogenic signaling cascade in cancer. PPAR Res. 2008;2008:431763. doi: 10.1155/2008/431763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ondrey F. Peroxisome proliferator-activated receptor gamma pathway targeting in carcinogenesis: implications for chemoprevention. Clin Cancer Res. 2009;15:2–8. doi: 10.1158/1078-0432.CCR-08-0326. [DOI] [PubMed] [Google Scholar]
- 22.Giaginis C, Spanopoulou E, Theocharis S. PPAR-γ signaling pathway in placental development and function: A potential therapeutic target in the treatment of gestational diseases. Expert Opin Ther Targets. 2008;12:1049–63. doi: 10.1517/14728222.12.8.1049. [DOI] [PubMed] [Google Scholar]
- 23.Giaginis C, Giagini A, Theocharis S. Peroxisome Proliferator-Activated Receptor-γ (PPAR-γ) ligands as potential therapeutic agents to treat arthritis. Pharmacol Res. 2009;60:160–69. doi: 10.1016/j.phrs.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Gizard F, Bruemmer D. Transcriptional control of vascular smooth muscle cell proliferation by peroxisome proliferator-activated Receptor-γ: therapeutic implications for cardiovascular diseases. PPAR Res. 2008;2008:429123. doi: 10.1155/2008/429123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duan SZ, Usher MG, Mortensen RM. PPARs: the vasculature, inflammation and hypertension. Curr Opin Nephrol Hypertens. 2009;18:128–33. doi: 10.1097/MNH.0b013e328325803b. [DOI] [PubMed] [Google Scholar]
- 26.Takano H, Komuro I. Peroxisome proliferator-activated receptor gamma and cardiovascular diseases. Circ J. 2009;73:214–20. doi: 10.1253/circj.cj-08-1071. [DOI] [PubMed] [Google Scholar]
- 27.Marx N, Sukhova G, Murphy C, et al. Macrophage in human atheroma contain PPAR-γ: Differentiation-dependent peroxisome proliferator-activated receptor γ (PPARγ) expression and reduction of MMP-9 activity through PPARγ activation in mononuclear phagocytes in vitro. Am J Pathol. 1998;153:17–23. doi: 10.1016/s0002-9440(10)65540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marx N, Duez H, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors and atherogenesis: regulators of gene expression in vascular cells. Circ Res. 2004;94:1168–78. doi: 10.1161/01.RES.0000127122.22685.0A. [DOI] [PubMed] [Google Scholar]
- 29.Ricote M, Huang J, Fajas L, et al. Expression of the peroxisome proliferator activated receptor gamma (PPAR-γ) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low-density lipoprotein. Proc Natl Acad Sci USA. 1998;23:7614–19. doi: 10.1073/pnas.95.13.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bishop-Bailey D, Hla T, Warner TD. Intimal smooth muscle cells as a target of peroxisome proliferator-activated Receptor-γ ligand therapy. Circulation Res. 2002;91:210–17. doi: 10.1161/01.res.0000029080.15742.85. [DOI] [PubMed] [Google Scholar]
- 31.Li AC, Brown KK, Silvestre MJ, et al. Peroxisome proliferator-activated receptor γ ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Investig. 2000;106:523–31. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson E, Grieve DJ. Significance of peroxisome proliferator-activated receptors in the cardiovascular system in health and disease. Pharmacol Ther. 2009;122:246–63. doi: 10.1016/j.pharmthera.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Collins AR, Meehan WP, Kintscher U, et al. Troglitazone inhibits formation of early atherosclerotic lesions in diabetic and nondiabetic low density lipoprotein receptor-deficient mice. Arteriosclerosis Thromb Vasc Biol. 2001;21:365–71. doi: 10.1161/01.atv.21.3.365. [DOI] [PubMed] [Google Scholar]
- 34.Chen Z, Ishibashi S, Perrey S, et al. Troglitazone inhibits atherosclerosis in apolipoprotein e-knockout mice: pleiotropic effects on CD36 expression and HDL. Arteriosclerosis Thromb Vasc Biol. 2001;21:372–77. doi: 10.1161/01.atv.21.3.372. [DOI] [PubMed] [Google Scholar]
- 35.Hodis HN, Mack WJ, Zheng L, et al. Effect of peroxisome proliferator-activated receptor gamma agonist treatment on subclinical atherosclerosis in patients with insulin-requiring type 2 diabetes. Diabetes Care. 2006;29:1545–53. doi: 10.2337/dc05-2462. [DOI] [PubMed] [Google Scholar]
- 36.Liapis CD, Bell PR, Mikhailidis D, et al. ESVS guidelines. Invasive treatment for carotid stenosis: indication, techniques. Eur J Vasc Endovasc Surg. 2009;37:1–19. doi: 10.1016/j.ejvs.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Howard G, Prineas R, Moy C, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the reasons for geographic and racial differences in stroke study. Stroke. 2006;37:1171–78. doi: 10.1161/01.STR.0000217222.09978.ce. [DOI] [PubMed] [Google Scholar]
- 38.Virmani R, Kolodgie FD, Burke AP, et al. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–75. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 39.Giaginis C, Katsamangou E, Tsourouflis G, et al. Peroxisome Proliferator-Activated Receptor-gamma and RXR-alpha expression in pancreatic ductal adenocarcinoma: association with clinicopathological parameters, tumor proliferative capacity and patients’ survival. Med Sci Monit. 2009;15(5):BR148–56. [PubMed] [Google Scholar]
- 40.Katsargyris A, Theocharis SE, Tsiodras S, et al. Enhanced TLR4 endothelial cell immunohistochemical expression in symptomatic carotid atherosclerotic plaques. Expert Opin Ther Targets. 2010;14:1–10. doi: 10.1517/14728220903401294. [DOI] [PubMed] [Google Scholar]
- 41.Giaginis C, Vgenopoulou S, Tsourouflis G, et al. Expression and clinical significance of focal adhesion kinase in the two distinct histological types, intestinal and diffuse, of human gastric adenocarcinoma. Pathol Oncol Res. 2009;15:173–81. doi: 10.1007/s12253-008-9120-2. [DOI] [PubMed] [Google Scholar]
- 42.Giaginis C, Daskalopoulou S, Vgenopoulou S, et al. Heat shock protein-27, -60 and -90 expression in gastric cancer: association with clinicopathological variables and patient survival. BMC Gastroenterol. 2009;9:14. doi: 10.1186/1471-230X-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothwell PM, Eliasziw M, Gutnikov SA, et al. Carotid Endarterectomy Trialists Collaboration. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–24. doi: 10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 44.Rerkasem K, Rothwell PM. Systematic review of the operative risks of carotid endarterectomy for recently symptomatic stenosis in relation to the timing of surgery. Stroke. 2009;40:e564–72. doi: 10.1161/STROKEAHA.109.558528. [DOI] [PubMed] [Google Scholar]
- 45.Helton TJ, Bavry AA, Rajagopal V, et al. The optimal treatment of carotid atherosclerosis: a 2008 update and literature review. Postgrad Med. 2008;120:103–12. doi: 10.3810/pgm.2008.09.1911. [DOI] [PubMed] [Google Scholar]
- 46.Lal BK, Brott TG. The Carotid Revascularization Endarterectomy vs. Stenting Trial completes randomization: lessons learned and anticipated results. J Vasc Surg. 2009;50:1224–31. doi: 10.1016/j.jvs.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pascual G, Ricote M, Hevener AL. Macrophage peroxisome proliferator activated receptor γ as a therapeutic target to combat Type 2 diabetes. Expert Opin Ther Targets. 2007;11:1503–20. doi: 10.1517/14728222.11.11.1503. [DOI] [PubMed] [Google Scholar]
- 48.Zinn A, Felson S, Fisher E, Schwartzbard A. Reassessing the cardiovascular risks and benefits of thiazolidinediones. Clin Cardiol. 2008;31:397–403. doi: 10.1002/clc.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Law RE, Goetze S, Xi XP, et al. Expression and function of PPARγ in rat and human smooth muscle cells. Circulation. 2000;101(11):1311–18. doi: 10.1161/01.cir.101.11.1311. [DOI] [PubMed] [Google Scholar]
- 50.Marx N, Schonbeck U, Lazar MA, et al. Peroxisome proliferator-activated receptor gamma activators inhibit gene expression and migration in human vascular smooth muscle cells. Circ Res. 1998;83:1097–103. doi: 10.1161/01.res.83.11.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wakino S, Kintscher U, Kim S, et al. Peroxisome proliferator-activated receptor γ ligands inhibit retinoblastoma phosphorylation and G1→S transition in vascular smooth muscle cells. J Biol Chem. 2000;275:22435–41. doi: 10.1074/jbc.M910452199. [DOI] [PubMed] [Google Scholar]
- 52.De Dios ST, Bruemmer D, Dilley RJ, et al. Inhibitory activity of clinical thiazolidinedione peroxisome proliferator activating Receptor-γ ligands towards internal mammary artery, radial artery, and saphenous vein smooth muscle cell proliferation. Circulation. 2003;107:2548–50. doi: 10.1161/01.CIR.0000074040.31731.96. [DOI] [PubMed] [Google Scholar]
- 53.Neve BP, Fruchart JC, Staels B. Role of peroxisome proliferator-activated receptors (PPAR) in atherosclerosis. Biochem Pharmacol. 2000;60:1245–50. doi: 10.1016/s0006-2952(00)00430-5. [DOI] [PubMed] [Google Scholar]
- 54.Soumian S, Gibbs R, Davies A, Albrecht C. mRNA expression of genes involved in lipid efflux and matrix degradation in occlusive and ectatic atherosclerosis. J Clin Pathol. 2005;58:1255–60. doi: 10.1136/jcp.2005.026161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Golledge J, Mangan S, Clancy P. Effects of peroxisome proliferator-activated receptor ligands in modulating tissue factor and tissue factor pathway inhibitor in acutely symptomatic carotid atheromas. Stroke. 2007;38:1501–8. doi: 10.1161/STROKEAHA.106.474791. [DOI] [PubMed] [Google Scholar]
- 56.Regieli JJ, Jukema JW, Doevendans PA, et al. PPAR gamma variant influences angiographic outcome and 10-year cardiovascular risk in male symptomatic coronary artery disease patients. Diabetes Care. 2009;32:839–44. doi: 10.2337/dc08-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahuja HS, Szanto A, Nagy L, Davies PJ. The retinoid X receptor and its ligands: versatile regulators of metabolic function, cell differentiation and cell death. J Biol Regul Homeost Agents. 2003;17:29–45. [PubMed] [Google Scholar]
- 58.Lalloyer F, Fiévet C, Lestavel S, et al. The RXR agonist bexarotene improves cholesterol homeostasis and inhibits atherosclerosis progression in a mouse model of mixed dyslipidemia. Arterioscler Thromb Vasc Biol. 2006;26:2731–37. doi: 10.1161/01.ATV.0000248101.93488.84. [DOI] [PubMed] [Google Scholar]
- 59.Claudel T, Leibowitz MD, Fiévet C, et al. Reduction of atherosclerosis in apolipoprotein E knockout mice by activation of the retinoid X receptor. Proc Natl Acad Sci USA. 2001;98:2610–15. doi: 10.1073/pnas.041609298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou L, Shen LH, Hu LH, et al. Retinoid X receptor agonists inhibit phorbol-12-myristate-13-acetate (PMA)-induced differentiation of monocytic THP-1 cells into macrophages. Mol Cell Biochem. 2010;335(1–2):283–89. doi: 10.1007/s11010-009-0278-z. [DOI] [PubMed] [Google Scholar]
- 61.Argmann CA, Sawyez CG, McNeil CJ, et al. Activation of peroxisome proliferator-activated receptor gamma and retinoid X receptor results in net depletion of cellular cholesteryl esters in macrophages exposed to oxidized lipoproteins. Arterioscler Thromb Vasc Biol. 2003;23:475–82. doi: 10.1161/01.ATV.0000058860.62870.6E. [DOI] [PubMed] [Google Scholar]
- 62.Benson S, Padmanabhan S, Kurtz TW, Pershadsingh HA. Ligands for the peroxisome proliferator-activated Receptor-gamma and the retinoid X Receptor-alpha exert synergistic antiproliferative effects on human coronary artery smooth muscle cells. Mol Cell Biol Res Commun. 2000;3:159–64. doi: 10.1006/mcbr.2000.0209. [DOI] [PubMed] [Google Scholar]