Summary
Background
We performed a prospective observational cohort study to evaluate the causative bacteria and to identify risk factors for mortality in febrile neutropenic (FN) patients with blood stream infection (BSI).
Material/Methods
We conducted a prospective data collection on all patients with bacteremia or fungemia. The patients were assigned into low-risk and high-risk groups in accordance with the Multinational Association for Supportive Care in Cancer (MASCC) Risk Index.
Results
Throughout the study period, the patients developed 420 FN episodes. Out of 420 episodes, only 90 (21.4%) were found to have bloodstream infection. The mean age of the patients was 45.6±18.4 years and 55.6% of the patients were male. A total of 98 isolates were recovered from the cases of BSI. Coagulase-negative Staphylococcus spp (CoNS) were the most common isolates overall (33.7%). There was a significant increase in the rate of gram-negative bacteria throughout the study period (p=0.028). Overall mortality was 33%. Multivariate analyses showed that MASCC risk scores (p=0.0001, OR=15.1, CI%95 4.5–50.7), ICU wards (p=0.0002, OR= 8.6, Cl%95 1.101–68,157) and CoNS (p=0.004, OR=12.12, CI%95 2.3–64.7) were independent risk factors associated with mortality. BSI due to CoNS was associated with lower mortality; however, MASCC high risk score and ICU stay were associated with higher mortality.
Conclusions
The MASCC risk-index score and emergence of CoNS in positive blood cultures are valuable tools in the management of FN.
Keywords: bloodstream infection, febrile neutropenia, mortality
Background
Chemotherapy-induced neutropenia is a major adverse effect of cancer treatment. Bacteremia (BSI) was documented in blood cultures (BC) in 11–30% of the febrile neutropenia (FN) episodes [1–5]. The mortality from FN may be as high as 10%, depending on the population studied, and FN is still responsible for the majority of chemotherapy-associated deaths [6–8]. It includes a spectrum of clinical syndromes ranging from non-infectious fever to severe life-threatening infections. Patients with FN and bacteremia form a small subgroup within this spectrum, including severely infected patients. The epidemiology of bacteremic febrile neutropenia constitutes the basis for selection of empiric antibiotic therapy for febrile neutropenia [9]. There has been a shift from the predominance of gram-negative bacteria to predominance of gram-positive bacteria in many centers over the past 2 decades [10–13]; however, in recent years there has been a reverse of this trend in several centers. In fact, these centers reported the re-emergence of gram-negative bacteria in febrile neutropenic patients [14,15]. The significant variability between locations requires investigation of local trends to guide more appropriate antibiotic treatment.
Therefore, we performed a prospective cohort study to evaluate the causative bacteria and to identify risk factors for mortality in febrile neutropenic patients with BSI.
Material and Methods
This was a prospective observational cohort study. All patients with bacteremia or fungemia (thereafter referred to as bacteremia) and neutropenia (absolute neutrophil count of <500/mm3) consecutively hospitalized between December 2004 and December 2009 were included. Our hospital, Mersin University Faculty of Medicine, is a 402-bed tertiary-care, general medical ward of a general hospital, in Mersin, Turkey. Patients’ malignancies were treated with chemotherapy except in cases of stem cell transplantation. All patients with febrile episodes were evaluated and treated according to current guidelines by an infectious disease specialist. Prophylactic antibiotics was not used, but growth factor was used when necessary [16].
The patients were assigned into low-risk and high-risk groups in accordance with the Multinational Association for Supportive Care in Cancer (MASCC) Risk Index [17]. Those with a score of ≥21 were classified as low risk and those with a score of <21 classified as high risk [17]. All patients had a solid or hematologic malignancy. Mortality was defined as all-cause in-hospital mortality up to 30 days after BSI. BSI developing after 48 hours in hospital was considered as hospital-acquired.
Blood cultures were performed using the BACTEC 9240 (Becton Dickinson, Franklin Lakes, NJ, USA) automated system. Sensitivity to antibiotics was tested by the disk diffusion method on Mueller-Hinton agar according to Clinical and Laboratory Standards Institute (CCLS) procedures. Biological isolates were identified with the help of API (Bio Merieux SA Marcy I’Etoile, France). At least 2 positive cultures were required to define a bacteremia due to coagulase-negative staphylococci as a BSI.
Methicillin-resistant staphylococcus aureus (MRSA), vancomycin-resistant enterococcus spp. (VRE), pseudomonas aeruginosa, acinetobacter baumannii and stenotrophomonas maltophilia resistant to at least 3 different groups of antibiotics and extended spectrum beta-lactamase (ESBL) producing gram-negative bacilli were considered as multiple drug resistant (MDR) bacteria.
Statistical analysis
The SPSS 11.5 (Chicago, IL) package program was used for statistical analysis. Univariate statistical analysis including Student’s t test was used for continuous data. Chi-square and Fisher’s exact test were used for categorical data. P values of <0.05 were considered significant. Multivariate logistic regression analysis was performed to determine risk factors independently associated with mortality.
Results
Throughout the study period, the patients developed 420 febrile neutropenic episodes. Out of 420 episodes, only 90 (21.4%) were found to have BSI. The mean age of the patients was 45.6±18.4 years and 55.6% of the patients were male. The median time to development of BSI was 9.4±10.7 days (range 1–63). A total of 98 isolates were recovered from the cases of BSI. Polymicrobial bacteremia was detected in 6 episodes (6.7%). Forty-nine patients (54.4%) were in the low-risk group, 78 (86.7%) had hematogenous malignity, and 12 patients (13.3%) had solid malignancies. Thirty patients died. Of total bloodstream infections, 59.1% were nosocomial, and the most frequent underlying type of malignancy was AML (n=41). Table 1 shows the distribution of the patients by their diagnoses.
Table 1.
Distribution of the patients according to diagnosis.
| Patients diagnosis (n=90) | |
|---|---|
| Solid malignancies (n=12) | Gastrointestinal system (5) |
| Lung (3) | |
| Skin soft tissue(2) | |
| Mammary glands(1) | |
| Brain (1) | |
| Hematologic malignancies (n=78) | Acute myeloid leukemia (41) |
| Acute Lymphoblastic Leukemia (15) | |
| Hodgkin Diseases(5) | |
| Non-Hodgkin Lymphoma(5) | |
| Chronic Lymphoblastic Leukemia (7) | |
| Multiple myeloma(3) | |
| Chronic Myeloblastic Leukemia(2) |
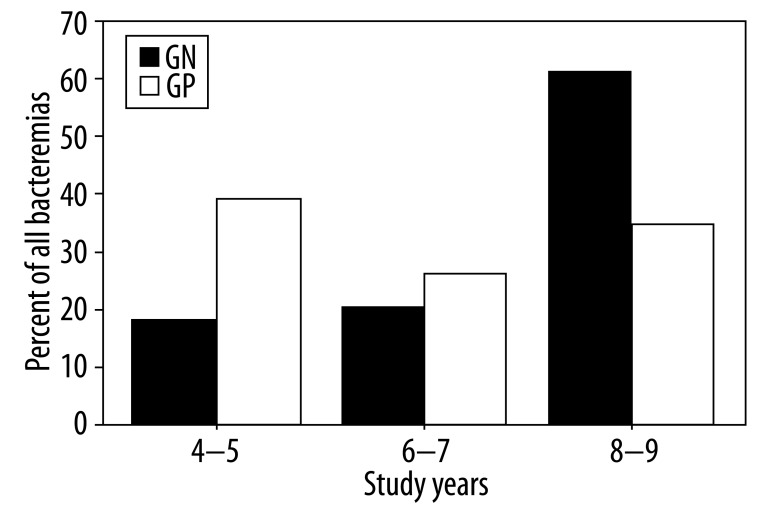
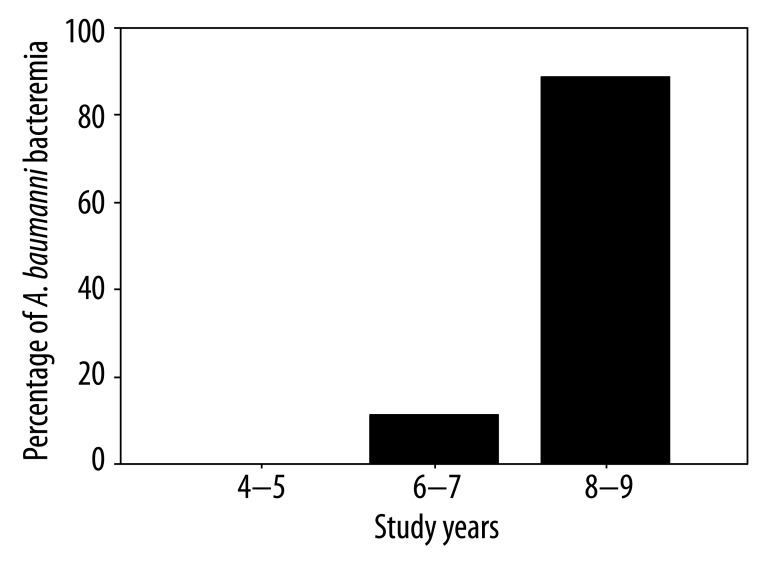
Gram-positive bacteria were more frequently isolated than gram-negative bacteria throughout the study period. Table 2 shows the distribution of causative bacteria. Coagulase-negative Staphylococcus (CoNS) spp. was the most common bloodstream isolate overall (33.7%), followed by E. coli (21.4%). Gram-negative bacteria were found in 30.7% of the isolates in 2004 and 2005, 42.8% of the isolates in 2006 and 2007, and 62.8% of the isolates in 2008 and 2009. The distribution of BSI episodes (n=90) according to study years were 26 episodes (28.9%) in 2004–5; 21 (23.3%) episodes in 2006–7 and 43 (47.8%) episodes in 2008–9. Percent of gram-positive bacteremia (n=42) according to study years were 18 (42.9%) episodes in 2004–5; 10 (23.8%) episodes in 2006–7, and 14 (33.3%) episodes in 2008–9. Occurrence of gram-negative bacteremia (n=44) according to study years was 8 (18.2%) episodes in 2004–5, 9 (20.5%) episodes in 2006–7, and 27 (61.4%) episodes in 2008–9 (Figure 1). There was a significant increase in the rate of gram-negative bacteria throughout the study period (p=0.028); in fact, there was a striking increase, especially in the rate of acinetobacteria (p=0.029). Acinetobacteria were not found (0%) in the first 2 years of the study, comprised 4.7% of the isolates in the second 2 years of the study, and 18.6% of the isolates in the third 2 years of the study (Figure 2). Thirty-five microorganisms isolated (35.7%) were MDR.
Table 2.
Microorganism (n=98) causing positive blood cultures in febrile neutropenic patients, 2004–2009.
| Microorganisms | No. (n MDR) |
|---|---|
| Monomicrobial | |
| Gram negative, total | 44 |
| E. coli | 21 (8) |
| P. aeruginosa | 6 |
| K. pneumoniae | 4 |
| A. baumannii | 9 (9) |
| Enterobacter spp. | 2 (2) |
| Serratia spp. | 2 |
| Gram positive, total | 47 |
| Coagulase-negative Staphylococcus spp. | 33 (18) |
| S. aureus | 7 (3) |
| Enterococcus spp. | 4 |
| α-Hemolytic streptococci | 1 |
| Streptococcus pneumoniae | 2 |
| Candida spp. total | 7 |
| C. albicans | 2 |
| C. non-albicans | 5 |
| Polymicrobial | |
| Only Gram-positive organisms* | 3 |
| Both Gram positive and Gram negative organisms** | 3 |
Infection with the following: (1) S. aureus and coagulase-negative Staphylococcus spp.; (2). S. aureus and coagulase-negative Staphylococcus spp.; (3) S. aureus, Metisilin-sensitive coagulase-negative Staphylococcus spp. and Metisilin-resistant coagulase-negative Staphylococcus spp.;
Infection with the following: (1) E. coli and, S. aureus; (2) E. coli, Metisilin-sensitive coagulase-negative Staphylococcus spp. and Metisilin-resistant coagulase-negative Staphylococcus spp.; (3) Enterococcus spp. and P. aeruginosa.
Figure 1.
Percent of gram positive and gram negative bacteremia throughout the study period.
Figure 2.
Increase in A. baumanni bacteremia throughout the study period.
Univariate analyses (Table 3) showed a significant relation between mortality and MASSC scores (p=0.000) and several characteristics of causative agents. The relation between mortality and gram-negative bacteria, acinetobacteria, nonfermentative bacteria, stay in intensive care unit (ICU) wards and coagulase-negative staphylococci (CoNS) was significant (p=0.002, 0.005, 0.009,0.002,0.0001, 0.0001, respectively).
Table 3.
Risk factors for mortality in febrile neutropenic cancer patients with BSI by univariate and multivariate analysis.
| Univariate analysis | Nonsurvivors (n=30) | Survivors (n=60) | p value |
|---|---|---|---|
| Age (years) | 45.2±18.1 | 45.8±18.6 | 0.897 |
|
| |||
| Male/Female gender | 18/12 | 32/28 | 0.549 |
|
| |||
| Hospital stay(day) | 27.4±17.5 | 34.8±27 | 0.308 |
|
| |||
| MASSC score | |||
| Low-risk group (≥21) (n=49) | 5 | 44 | 0.0001 |
| High-risk group (<21) (n=41) | 25 | 16 | 0.0001 |
| High-risk group <15 (n=13) | 10 | 3 | 0.0001 |
|
| |||
| Etiologic agents | |||
| Gram positive bacteremia (n=42) | 7 | 34 | 0.002 |
| Gram negative bacteremia (n=44) | 21 | 23 | 0.005 |
|
| |||
| Nonfermentative bacteremia (n=13) | 9 | 4 | 0.002 |
|
| |||
| CoNS bacteremia (n=29) | 2 | 27 | 0.0001 |
|
| |||
| ICU wards (n=11) | 9 | 2 | 0.0001 |
|
| |||
| Nosocomial BSI (n=26) | 13 | 13 | 0.717 |
|
| |||
| Multivariate analysis | OR (95% CI) | p value | |
|
| |||
| MASCC score index | 15.1 (4.5–50.7) | 0.0001 | |
|
| |||
| CoNS bacteremia | 12.12 (2.3–64.7) | 0.004 | |
|
| |||
| ICU wards | 8.6 (1.101–68,157) | 0.002 | |
|
| |||
| Gram negative bacteremia | 1.2 (0.449–68.9) | 0.181 | |
|
| |||
| Nonfermentative bacteremia | 1.1(0.064–22.43) | 0.903 | |
Multivariate analyses showed that MASSC scores (p=0.0001, OR=15.1, CI%95 4.5–50.7), stay in ICU wards (p=0.0002, OR= 8.6, Cl%95 1.101–68,157) and CoNS were independent risk factors associated with mortality (p=0.004, OR=12.12, CI%95 2.3–64.7) (Table 3). BSI due to CoNS was associated with lower mortality; however, MASCC high risk score and ICU stay were associated with higher mortality.
Discussion
Our study found that 21.4% of the febrile neutropenic patients with cancer had BSI. This is consistent with other reports showing that up to 30% of the episodes of FN are associated with confirmed bacteremia [5,6–18]. The most commonly isolated bacteria in the present series were CoNS, which accounted for 33 of 98 (33.7%) blood culture isolates, similar to other studies [5,19].
Overall, gram-positive organisms accounted for nearly 50% (n=47, 47.9%) of all blood culture isolates. These data support the documented high rates of gram-positive infections in cancer patients with FN [6–19]. In fact, prior studies revealed that 50%–71% of the etiological agents found in microbiological analyses in FN patients with BSI were gram-positive bacteria [5,21,22,23]; however, the rate of gram-negative infections is on the rise in some centers [24,25,26]. Recently, non-fermentative gram-negative rods such as Acinetobacter species have appeared as pathogens in FN patients [20,21].
Although the most frequent causative agents were gram-positive bacteria throughout the study period, there was a significant increase in gram-negative bacteria, especially acinetobacteria, in the last 2 years of the study; 30.7% and 62.8% of the isolates had gram-negative bacteria in the first and the last 2 years of the study, respectively. Recently, Chen et al reported that gram-negative bacteria were the predominant pathogens (60%) and that fungi were relatively uncommon (6%) in bloodstream infections in patients with neutropenia. In addition, the number of Acinetobacter and Stenotrophomonas infections increased from 2002 to 2006, and were the third (7%) and fourth (6%) most frequent after E. coli and Klebsiella[27].
A. baumannii and S. maltophilia were found in 1–3% of the bloodstream isolates from neutropenic patients in the USA and Europe [22,28]. However, in some areas the frequency of A. baumannii complex in neutropenic patients is reportedly higher, at 6–9% [21,24,29]. According to data from the infection control committee of our hospital, there has been an increase in acinetobacteria resistant to multiple antibiotics in the past few years. This increased rate of acinetobacteria in FN patients can be attributed to nosocomial transmission.
Our multivariate analysis revealed that isolation of a CoNs strain and MASSC index-score are independent predictors of mortality in patients with FN and BSI. Uys et al showed that the MASCC risk-index score correctly identifies low- and high-risk patients at presentation with febrile neutropenia [30]. This study showed that the MASCC risk-index score had a positive predictive value of 98.3% and a negative predictive value of 86.4%, with both a sensitivity and specificity of 95%.
We found that mortality was 10.2% in the low-risk patients with the MASSC score of 21 and above and 60.8% in the high-risk patients (p=0.000). Consistent with the results of the present study, Klastersky et al. [22] reported a relatively low rate of overall complications (18%) and death (3%) in low-risk patients with bacteremia and MASCC scores of >21, but that when the MASCC score was <21, the corresponding figures were 49% and 19% (P<0.001). When the score was <15, overall complications (79%) and mortality (36%) were even higher. In fact, when the score was 15–20, the rates of complications and mortality were 40% and 14%, respectively. In our study, 13 patients had a MASCC score index <15. Mortality rate was higher (76.9%) in this group. This results was statistically significant (p=0.0001) and similar to the results of Klastersky’s study. Based on MASSC scores, low-risk and high-risk patients with BSI can be identified easily, and, accordingly, an appropriate therapy can be given to the high-risk patients in order to decrease the very high overall complication and mortality rates seen in these patients [22]. We also found that CoNS bacteremia was a predictor of a very low mortality (6.9%). Klastersky et al. found a mortality of 6% in patients with CoNS and a higher mortality of 18% in patients with gram-negative bacteria (p<0.001). In the present study, the mortality was 16% in patients with gram-positive bacteremia, but 47% in patients with gram-negative bacteremia.
Prior studies have revealed that gram-negative bacteremia is usually associated with higher case fatality rates than gram-positive infections, and that the risk is further increased due to antimicrobial resistance if effective treatment is delayed [23,31–34].
In the present study, 43.2% of gram-negative bacteria were multi-drug resistant, and 20.5% of these bacteria were highly resistant bacteria such as acinetobacteria. This might have contributed to the increase in the mortality due to gram-negative bacteria. In addition, gram-positive bacteria are isolated easily and quickly; therefore, they are easily identified, which allows treatment. This may explain the low mortality from gram-positive bacteria.
Conclusions
There has been an increase in the rate of bloodstream infections due to antimicrobial-resistant gram-negative bacteria in FN patients. Bacterial epidemiology and antimicrobial resistance in these patients should be regularly monitored, which will provide guidance for local policies for the use of antimicrobial agents and the assist in the choice of agents for empirical antibiotic therapy and prophylaxis in FN patients. The MASCC risk-index score and emerging of CoNS in positive blood cultures are valuable tools in the management of patients with febrile neutropenia, and can be used to accurately predict mortality in patients with BSI.
Footnotes
Source of support: Departmnetal sources
References
- 1.Petrilli AS, Melaragno R, Barros KV, et al. Fever and neutropenia in children with cancer: a therapeutic approach related to the underlying disease. Pediatr Infect Dis J. 1993;12(11):916–21. doi: 10.1097/00006454-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Oude Nijhuis C, Kamps WA, Daenen SM, et al. Feasibility of withholding antibiotics in selected febrile neutropenic cancer patients. J Clin Oncol. 2005;23(30):7437–44. doi: 10.1200/JCO.2004.00.5264. [DOI] [PubMed] [Google Scholar]
- 3.Hodgson-Viden H, Grundy PE, Robinson JL. Early discontinuation of intravenous antimicrobial therapy in pediatric oncology patients with febrile neutropenia. BMC Pediatr. 2005;5(1):10. doi: 10.1186/1471-2431-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santolaya ME, Villarroel M, Avendano LF, Cofre J. Discontinuation of antimicrobial therapy for febrile neutropenic children with cancer: a prospective study. Clin Infect Dis. 1997;25(1):92–97. doi: 10.1086/514500. [DOI] [PubMed] [Google Scholar]
- 5.Morris PG, Hassan T, McNamara M, et al. Emergence of MRSA in positive blood cultures from patients with febrile neutropenia a cause for concern. Support Care Cancer. 2008;16(9):1085–88. doi: 10.1007/s00520-007-0398-5. [DOI] [PubMed] [Google Scholar]
- 6.Sipsas NV, Bodey GP, Kontoyiannis DP. Perspectives for the management of febrile neutropenic patients with cancer in the 21st century. Cancer. 2005;103:1103–13. doi: 10.1002/cncr.20890. [DOI] [PubMed] [Google Scholar]
- 7.Smith PF, Birmingham MC, Noskin GA, et al. Safety, efficacy and pharmacokinetics of linezolid for treatment of resistant Gram-positive infections in cancer patients with neutropenia. Ann Oncol. 2003;14:795–801. doi: 10.1093/annonc/mdg211. [DOI] [PubMed] [Google Scholar]
- 8.Elting LS, Rubenstein EB, Rolston K, et al. Time to clinical response: an outcome of antibiotic therapy of febrile neutropenia with implications for quality and cost of care. J Clin Oncol. 2000;18:3699–706. doi: 10.1200/JCO.2000.18.21.3699. [DOI] [PubMed] [Google Scholar]
- 9.Paul M, Gafter-Gvili A, Leibovici L, et al. The epidemiology of bacteremia with febrile neutropenia: experience from a single center, 1988–2004. Isr Med Assoc J. 2007;9(6):424–29. [PubMed] [Google Scholar]
- 10.Viscoli C, Varnier O, Machetti M. Infections in patients with febrile neutropenia: epidemiology, microbiology, and risk stratification. Clin Infect Dis. 2005;40(Suppl 4):S240–45. doi: 10.1086/427329. [DOI] [PubMed] [Google Scholar]
- 11.Ramphal R. Changes in the etiology of bacteremia in febrile neutropenic patients and the susceptibilities of the currently isolated pathogens. Clin Infect Dis. 2004;39(Suppl 1):S25–31. doi: 10.1086/383048. [DOI] [PubMed] [Google Scholar]
- 12.Wisplinghoff H, Seifert H, Wenzel RP, Edmond MB. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin Infect Dis. 2003;36:1103–10. doi: 10.1086/374339. [DOI] [PubMed] [Google Scholar]
- 13.Zinner SH. Changing epidemiology of infections in patients with neutropenia and cancer: emphasis on gram-positive and resistant bacteria. Clin Infect Dis. 1999;29:490–94. doi: 10.1086/598620. [DOI] [PubMed] [Google Scholar]
- 14.Haupt R, Romanengo M, Fears T, et al. Incidence of septicaemias and invasive mycoses in children undergoing treatment for solid tumours: a 12-year experience at a single Italian institution. Eur J Cancer. 2001;37:2413–19. doi: 10.1016/s0959-8049(01)00274-x. [DOI] [PubMed] [Google Scholar]
- 15.Wang FD, Lin ML, Liu CY. Bacteremia in patients with hematological malignancies. Chemotherapy. 2005;51:147–53. doi: 10.1159/000085623. [DOI] [PubMed] [Google Scholar]
- 16.Meunier F, Lukan C. The First European Conference on Infections in Leukaemia – ECIL1: a current perspective. Eur J Cancer. 2008;44(15):2112–17. doi: 10.1016/j.ejca.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Klastersky J, Paesmans M, Rubenstein EB, et al. The Multinational Association for Supportive Care in Cancer risk index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol. 2000;18:3038–51. doi: 10.1200/JCO.2000.18.16.3038. [DOI] [PubMed] [Google Scholar]
- 18.Rolston KV. Challenges in the treatment of infections caused by Gram-positive and Gram-negative bacteria in patients with cancer and neutropenia. Clin Infect Dis. 2005;40:S246–52. doi: 10.1086/427331. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Barca E, Fernandez-Sevilla A, Carratala J, Grañena A, Gudiol F. Prospective study of 288 episodes of bacteremia in neutropenic cancer patients in a single institution. Eur J Clin Microbiol Infect Dis. 1996;15:291–96. doi: 10.1007/BF01695660. [DOI] [PubMed] [Google Scholar]
- 20.Wojak I, Gospodarek E. Analysis of microorganisms isolated from febrile neutropenic children with neoplastic disease. Med Dosw Mikrobiol. 2004;56:411–19. [PubMed] [Google Scholar]
- 21.Irfan S, Idrees F, Mehraj V, et al. Emergence of Carbapenem resistant Gram negative and vancomycin resistant Gram positive organisms in bacteremic isolates of febrile neutropenic patients: a descriptive study. BMC Infect Dis. 2008;8:80. doi: 10.1186/1471-2334-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klastersky J, Ameye L, Maertens J, et al. Bacteraemia in febrile neutropenic cancer patients. Int J Antimicrob Agents. 2007;30(Suppl 1):S51–59. doi: 10.1016/j.ijantimicag.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Tumbarello M, Spanu T, Caira M, et al. Factors associated with mortality in bacteremic patients with hematologic malignancies. Diagn Microbiol Infect Dis. 2009;64(3):320–26. doi: 10.1016/j.diagmicrobio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Chen CY, Tang JL, Hsueh PR, et al. Trends and antimicrobial resistance of pathogens causing bloodstream infections among febrile neutropenic adults with hematological malignancy. J Formos Med Assoc. 2004;103:526–32. [PubMed] [Google Scholar]
- 25.Guven GS, Uzun O, Cakir B, et al. Infectious complications in patients with hematological malignancies consulted by the Infectious Diseases team: a retrospective cohort study (1997–2001) Support Care Cancer. 2006;14:52–55. doi: 10.1007/s00520-005-0836-1. [DOI] [PubMed] [Google Scholar]
- 26.Velasco E, Byington R, Martins CS, et al. Bloodstream infection surveillance in a cancer centre: a prospective look at clinical microbiology aspects. Clin Microbiol Infect. 2004;10:542–49. doi: 10.1111/j.1469-0691.2004.00874.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen CY, Tsay W, Tang JL, et al. Epidemiology of bloodstream infections in patients with haematological malignancies with and without neutropenia. Epidemiol Infect. 2009;27:1–8. doi: 10.1017/S0950268809991208. [DOI] [PubMed] [Google Scholar]
- 28.Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 29.Velasco E, Byington R, Martins CA, et al. Comparative study of clinical characteristics of neutropenic and non-neutropenic adult cancer patients with bloodstream infections. Eur J Clin Microbiol and Infect Dis. 2006;25:1–7. doi: 10.1007/s10096-005-0077-8. [DOI] [PubMed] [Google Scholar]
- 30.Uys A, Rapoport BL, Anderson R. Febrile neutropenia: a prospective study to validate the Multinational Association of Supportive Care of Cancer (MASCC) risk-index score. Support Care Cancer. 2004;12:555–60. doi: 10.1007/s00520-004-0614-5. [DOI] [PubMed] [Google Scholar]
- 31.Anderson DJ, Engemann JJ, Harrell LJ, et al. Predictors of mortality in patients with bloodstream infection due to ceftazidime-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2006;50:1715–20. doi: 10.1128/AAC.50.5.1715-1720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blot S, Vandewoude K, De Bacquer D, Colardyn F. Nosocomial bacteremia caused by antibiotic-resistant Gram-negative bacteria in critically ill patients: clinical outcome and length of hospitalization. Clin Infect Dis. 2002;34:1600–6. doi: 10.1086/340616. [DOI] [PubMed] [Google Scholar]
- 33.Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother. 2007;60:913–20. doi: 10.1093/jac/dkm318. [DOI] [PubMed] [Google Scholar]
- 34.Tumbarello M, Sanguinetti M, Montuori E, et al. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase – producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother. 2007;51:1987–94. doi: 10.1128/AAC.01509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]