Summary
Background
Enterobacteriaceae producing ESBL and AmpC enzymes can be associated with failure of antibiotic therapy and related morbidity and mortality. Their routine detection in microbiology laboratories is still a problem. The aim of this study was to compare the sensitivity of selected phenotypic methods.
Material/Methods
A total of 106 strains of the Enterobacteriaceae family were tested, in which molecular biology methods confirmed the presence of genes encoding ESBL or AmpC. In ESBL-positive strains, the sensitivity of the ESBL Etest (AB Biodisk) and a modified double-disk synergy test (DDST) were evaluated. AmpC strains were tested by a modified AmpC disk method using 3-aminophenylboronic acid. For simultaneous detection of ESBL and AmpC, the microdilution method with a modified set of antimicrobial agents was used.
Results
The sensitivity of the ESBL Etest was 95%; the modified DDST yielded 100% sensitivity for ESBL producers and the AmpC test correctly detected 95% of AmpC-positive strains. The sensitivity of the modified microdilution method was 87% and 95% for ESBL and AmpC beta lactamases, respectively.
Conclusions
The detection of ESBL and AmpC beta lactamases should be based on specific phenotypic methods such as the modified DDST, ESBL Etest, AmpC disk test and the modified microdilution method.
Keywords: ESBL, AmpC, detection, phenotypic methods
Background
At present, Enterobacteriaceae producing broad-spectrum beta-lactamases belong to the most feared group of bacterial pathogens. Of particular clinical importance are ESBL and AmpC enzymes capable of hydrolyzing broad-spectrum penicillins, monobactams and cephalosporins. The prevalence of broad-spectrum beta-lactamases in pathogenic bacteria is clinically very important. It increases the risk of failure of antibiotic therapy, resulting in higher morbidity, mortality and, last but not least, treatment costs. For example, Kang et al reported initial antibiotic therapy fails significantly more often (73%) in patients with spontaneous bacterial peritonitis due to ESBL-positive strains as compared with peritonitis of ESBL-negative etiology (17%) [1]. Moreover, they showed 30-day mortality to be higher in patients with ESBL-positive pathogens (60%) than in those with ESBL-negative Enterobacteriaceae (23%) [1]. Pai et al documented high rates of failure of cefotaxime or ceftazidime therapy in patients with bloodstream infections caused by Klebsiella pneumoniae strains producing AmpC beta-lactamases [2]. Tumbarello et al also reported mortality associated with bloodstream infections due to ESBL-positive Enterobacteriaceae of as much as 60% in cases of inadequate antibiotic therapy as compared with 19% when therapy was adequately targeted to the above multiresistant bacteria [3]. Schwaber et al documented a 30% mortality rate in patients with bacteremia due to ESBL-producing strains of Escherichia coli, Klebsiella spp. and Proteus spp. as compared with 16% mortality in patients with non-ESBL-associated bacteremia [4].
The frequency and number of different types of ESBL and AmpC enzymes continue to increase. The EARSS data on the resistance of invasive isolates of Escherichia coli and Klebsiella pneumoniae suggest that the frequency of third-generation cephalosporin resistance has increased in the recent years. This negative trend is assumed to be determined by the spread of strains producing broad-spectrum beta-lactamases, in particular ESBL [5]. In their multicentre study, Paterson et al found that 31% of Klebsiella pneumoniae strains isolated from blood produced ESBL, with ESBL production being detected in 44% of isolates from intensive care units (ICUs) [6]. In their 2004 multicentre study comprising 16 health care facilities, Kolar et al detected ESBL production in 26% of Klebsiella pneumoniae strains, with the prevalence reaching 39% in ICUs [7].
The laboratory detection of broad-spectrum beta-lactamases remains difficult. A study of 220 ESBL-positive isolates of Klebsiella spp. from 35 European ICUs showed that the collaborating laboratories incorrectly identified 29% of ESBL-positive isolates as susceptible to ceftriaxone and 37% as susceptible to cefotaxime [8]. According to Tenover et al, the proportion of laboratories that did not detect production of ESBL and AmpC enzymes in Enterobacteriaceae ranged from 24% to 32%, depending on the types of enzymes present [9]. A 2006 Italian study showed that all tested ESBL producers were correctly detected by only 42% of laboratories, while a 2008 Spanish study reported that all tested ESBL producers were correctly detected by 91% of laboratories, but only 47% of laboratories correctly identified all AmpC strains [10, 11].
For detection, phenotypic and genotypic methods may be used, with phenotyping being preferred in routine microbiology practice. In the case of ESBL, the double-disk synergy test (DDST), Etest or CLSI test are used, which are based on inhibiting the activity of these enzymes by their inhibitors (e.g., clavulanic acid) and decreasing the minimum inhibitory concentration (MIC) value of a particular cephalosporin, enlarging the zone of growth inhibition around the disk or its extension and formation of a characteristic pattern between the substrate disk and the disk containing the inhibitor [12–14]. AmpC beta-lactamases may be detected using a modified AmpC disk test with disks containing ceftazidime, ceftazidime with clavulanic acid and their combination with 3-aminophenylboronic acid, an inhibitor of AmpC beta-lactamases [15].
Our study aimed to compare the sensitivity of phenotypic methods for detecting ESBL and AmpC enzymes in a group of strains with their production detected by molecular biology methods.
Material and Methods
Bacterial strains
A total of 106 strains of the Enterobacteriaceae family were tested. The strains were identified by standard microbiology methods and the Phoenix automated system (Becton Dickinson).
PCR
Molecular biology methods confirmed the presence of genes encoding ESBL or AmpC enzymes. ESBL detection was based on PCR amplification of blaTEM, blaSHV and blaCTX-M genes encoding the most frequent types of these beta-lactamases, with subsequent determination of a particular variation by direct sequencing [16–18]. AmpC beta-lactamases were determined by multiplex PCR capable of differentiation of various types of these enzymes [19].
ESBL and AmpC detection
For the isolates, the results of the disk diffusion and microdilution methods and the accuracy of the Phoenix automated system were assessed [13,14]. In ESBL-positive strains, the sensitivity of a modified microdilution method with determination of cefoxitin MIC and the ratio of MIC of cefoperazone with the cefoperazone/sulbactam combination (Chart 1), as well as of the ESBL Etest (AB Biodisk) and a modified DDST (Chart 2) were assessed. AmpC strains were tested by a modified AmpC disk test using 3-aminophenylboronic acid (Chart 3) and the modified microdilution method with the determination of cefoxitin MIC and the ratio of MIC of cefotaxime and ceftazidime with the cefotaxime/3-aminophenylboronic acid and ceftazidime/3-aminophenylboronic acid combinations (Chart 1). The criteria of positivity for the individual phenotypic tests are summarized in Table 1.
Chart 1.
A modified set of antimicrobial agents for the standard microdilution method for simultaneous detection of ESBL and AmpC production (concentrations in mg/L).
| AMS | AZT | CXT | CTX | CTX/3APB | CTZ | CTZ/3APB | CPR | CPS | CPM | PIP | PPT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 64 | 64 | 64 | 64 | 64/300 | 64 | 64/300 | 64 | 64 | 64 | 256 | 256 |
| 32 | 32 | 32 | 32 | 32/300 | 32 | 32/300 | 32 | 32 | 32 | 128 | 128 |
| 16 | 16 | 16 | 16 | 16/300 | 16 | 16/300 | 16 | 16 | 16 | 64 | 64 |
| 8 | 8 | 8 | 8 | 8/300 | 8 | 8/300 | 8 | 8 | 8 | 32 | 32 |
| 4 | 4 | 4 | 4 | 4/300 | 4 | 4/300 | 4 | 4 | 4 | 16 | 16 |
| 2 | 2 | 2 | 2 | 2/300 | 2 | 2/300 | 2 | 2 | 2 | 8 | 8 |
| 1 | 1 | 1 | 1 | 1/300 | 1 | 1/300 | 1 | 1 | 1 | 4 | 4 |
| 0.5 | 0.5 | 0.5 | 0.5 | 0.5/300 | 0.5 | 0.5/300 | 0.5 | 0.5 | 0.5 | 2 | 2 |
AMS – ampicillin/sulbactam; AZT – aztreonam; CXT – cefoxitin; CTX – cefotaxime; CTZ – ceftazidime; CPR – cefoperazone; CPS – cefoperazone/sulbactam; CPM – cefepime; PIP – piperacillin; PPT – piperacillin/tazobactam; 3APB – 3-aminophenylboronic acid.
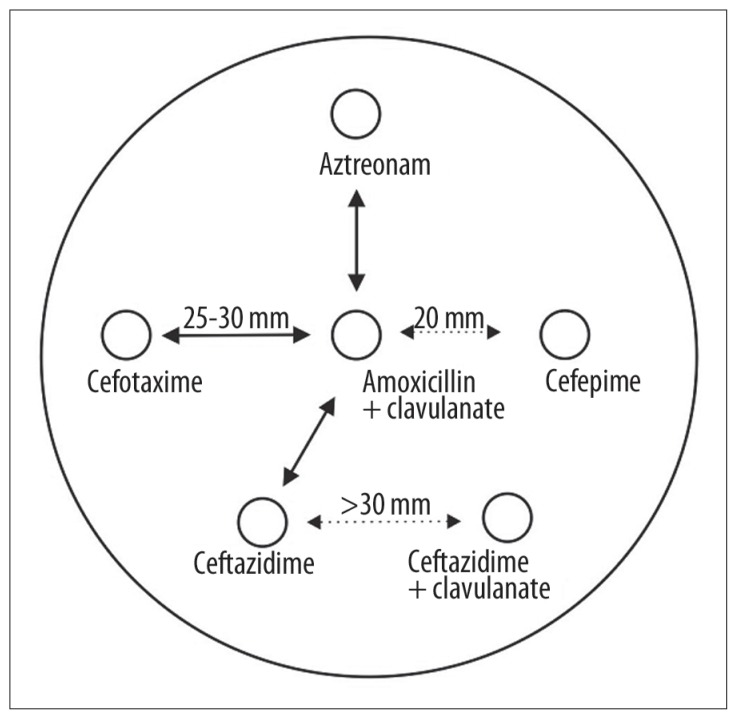
Chart 2.
Modified DDST for ESBL detection.
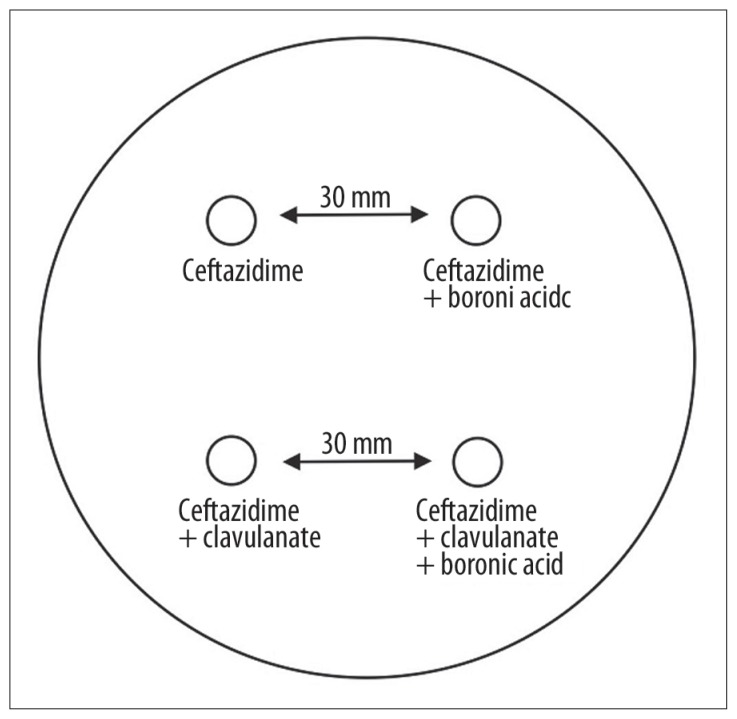
Chart 3.
AmpC test for AmpC detection.
Table 1.
Criteria of positivity of the phenotypic tests used to detect ESBL and AmpC enzymes.
| Test | ESBL detection | AmpC detection |
|---|---|---|
| mDDST* | Extension of the inhibition zone towards the disk with the inhibitor and/or enlargement of the zone by more than 5 mm around the disk with the inhibitor (Figure 1) | |
| mAmpC test** | Enlargement of the inhibition zone by more than 5 mm as compared with the disk without 3-aminophenylboronic acid (Figure 2) | |
| mMIC*** | Cefoxitin MIC ≤16 mg/L and a ratio of MIC of cefoperazone and cefoperazone/sulbactam >2:1 | Cefoxitin MIC ≥16 mg/L and a ratio of MIC of cefotaxime and cefotaxime/3-aminophenylboronic acid or MIC of ceftazidime and ceftazidime/3-aminophenylboronic acid >2:1 |
| ESBL Etest | At least 4-fold decrease of MIC of the particular third- or fourth-generation cephalosporin (cefotaxime and ceftazidime in case of E. coli and K. pneumoniae, and in addition cefepime in S. marcescens) in the presence of the inhibitor (cefotaxime/clavulanic acid and ceftazidime/clavulanic acid, and additionally cefepime/clavulanic acid in S. marcescens) (Figure 3) |
Modified double-disk synergy test;
modified AmpC disk method;
modified microdilution method.
Results
A total of 106 Enterobacteriaceae strains were tested. PCR and direct sequencing were used to detect ESBL production in 85 strains and AmpC production in 21 strains (Table 2).
Table 2.
A group of ESBL- and AmpC-positive isolates for determination of sensitivity of phenotypic methods.
| Species | Number of strains | Broad-spectrum beta-lactamase type |
|---|---|---|
| Escherichia coli | 50 | ESBL (CTX-M-1, CTX-M-9, CTX-M-14, CTX-M-15, CTX-M-27, SHV-12) |
| 4 | AmpC (CMY-2) | |
| Klebsiella pneumoniae | 33 | ESBL (SHV-2, SHV-2a, SHV-12, CTX-M-1-like, CTX-M-9-like) |
| 11 | AmpC (DHA type) | |
| Serratia marcescens | 2 | ESBL (SHV-12) |
| Enterobacter cloacae | 3 | AmpC (EBC type) |
| Citrobacter freundii | 2 | AmpC (CIT type) |
| Providencia rettgeri | 1 | AmpC (DHA type) |
When using the microdilution method with the CLSI criteria, the tested strains were shown to be highly false-susceptible to cefoperazone (21% for ESBL and 76% for AmpC), cefotaxime (29% and 67%), ceftazidime (32% and 29%) and cefepime (60% for ESBL). Also with the disk method, a large percentage of strains were identified as false-susceptible to third- and fourth-generation cephalosporins according to the CLSI criteria (cefoperazone 9% for ESBL and 43% for AmpC; cefotaxime 8% and 14%; ceftazidime 45% and 14%; cefepime 52%). The results are summarized in Tables 3 and 4.
Table 3.
Percentage of false susceptibility to third- and fourth-generation cephalosporins in the groups of ESBL and AmpC producers when using the microdilution method.
| Antibiotic | False susceptibility | |||||
|---|---|---|---|---|---|---|
| CLSI criteria | EUCAST criteria | |||||
| Breakpoint (mg/L) | ESBL strains (%) | AmpC strains (%) | Breakpoint (mg/L) | ESBL strains (%) | AmpC strains (%) | |
| Cefoperazone | ≤16 | 21 | 76 | not stated | – | – |
| Cefotaxime | ≤8 | 29 | 67 | ≤1 | 7 | 43 |
| Ceftazidime | ≤8 | 32 | 29 | ≤1 | 5 | 5 |
| Cefepime | ≤8 | 60 | – | ≤1 | 8 | – |
Table 4.
Percentage of false susceptibility to third- and fourth-generation cephalosporins in the groups of ESBL and AmpC producers when using the disk diffusion method.
| Antibiotic | False susceptibility (%) | |||||
|---|---|---|---|---|---|---|
| CLSI criteria | EUCAST criteria | |||||
| Breakpoint (mm) | ESBL strains (%) | AmpC strains (%) | Breakpoint (mm) | ESBL strains (%) | AmpC strains (%) | |
| Cefoperazone | ≥21 | 9 | 43 | not stated | – | – |
| Cefotaxime | ≥23 | 8 | 14 | ≥21 | 11 | 29 |
| Ceftazidime | ≥18 | 45 | 14 | ≥20 | 38 | 10 |
| Cefepime | ≥18 | 52 | – | ≥24 | 19 | – |
After the breakpoints for cefepime, cefotaxime and ceftazidime were decreased to 1 mg/L, as recommended by the EUCAST, the percentage of false susceptibility in the tested strains of Enterobacteriaceae producing ESBL and AmpC enzymes decreased significantly.
Although the sensitivity of ESBL detection by the Phoenix automated system was high (99%), it only reached 38% in the group of AmpC-positive strains. High sensitivity was achieved with tests specific for a given type of a broad-spectrum beta-lactamase. The ESBL Etest detected 95% of ESBL-positive strains. The modified DDST showed 100% sensitivity for ESBL producers, whereas the modified AmpC test with 3-aminophenylboronic acid proved correct in detecting 95% of AmpC-positive strains. In the group of ESBL-positive strains, 87% of strains were shown to have cefoxitin MIC of 16 mg/L or less and a ratio of MIC of cefoperazone and cefoperazone/sulbactam >2:1. The sensitivity of the modified microdilution method for AmpC beta-lactamases was 95% (Table 5).
Table 5.
Sensitivity of the tested phenotypic methods in the groups of ESBL and AmpC producers.
| Phenotypic method | Sensitivity (%) | |
|---|---|---|
| ESBL | AmpC | |
| Phoenix | 99 | 38 |
| mDDST | 100 | – |
| mAmpC test | – | 95 |
| mMIC | 87 | 95 |
| ESBL Etest | 95 | – |
Discussion
From a microbiological and clinical point of view, early and correct detection of ESBL and AmpC beta-lactamase production is essential. In routine microbiology practice, this remains complicated by false-negative results, especially if traditional methods for determination of bacterial resistance to antimicrobial agents are used. In severe bacterial infections, particularly in intensive care patients, antibiotic therapy should be initiated as soon as possible, preferably within an hour of diagnosis. However, microbiological results of phenotypic tests (including correct determination of susceptibility/resistance of bacterial pathogens to antimicrobial agents) remain an important primary source for selecting adequate antibiotic therapy and, if needed, de-escalation antibiotic therapy.
Results of our study show a high percentage of false susceptibility in the standard microdilution method (21–76%) and disk diffusion method (8–52%) when using the 2009 CLSI criteria [13]. When decreasing the breakpoints for cefepime, ceftazidime and cefotaxime to 1 mg/L, in accordance with the EUCAST clinical breakpoints, false susceptibility drops to 5–43% [14]. The use of the EUCAST criteria for the disk diffusion method results in a clear decrease of false susceptibility to 10–38% [14].
Detection of broad-spectrum beta-lactamases should be based on specific phenotypic methods, especially the modified DDST and AmpC disk method. Sensitivity of the mDDST used for ESBL detection was 100%, and 95% in the mAmpC test. Although very good results were also obtained with the ESBL Etest (a sensitivity of 95%), this might not be the most suitable method for common use in microbiology due to higher operating costs associated with this test. The standard microdilution method with a modified set of antibiotics seems to be an effective method since it meets the criteria of high sensitivity (87% for ESBL and 95% for AmpC) and simultaneous detection of the 2 enzyme types. Wiegand et al tested 3 semi-automated and 3 manual phenotypic methods for ESBL detection. In the semi-automated systems, the sensitivity rates were 99% for Phoenix, 86% for VITEK 2 and 84% for MicroScan. The compared manual methods included agar diffusion assays, the DDST, ESBL Etest and CLSI disk method. Sensitivity of these tests for ESBL detection ranged from 93% to 94%. Thus, the manual diffuse tests were more precise than the automated systems, which is in accordance with our results documenting 100% sensitivity of the mDDST [20]. Linscott and Brown compared 4 phenotypic methods for ESBL detection; however, their production was not confirmed by PCR. Unlike the study by Wiegand et al., they reported higher sensitivity of semi-automated methods (MicroScan ESBL plus ESBL confirmation panel 100%; VITEK 1 99%) as compared with agar diffusion assays (ESBL Etest 97%; CLSI ESBL disk method 96%) [21].
Conclusions
It is obvious that 100% sensitivity in the detection of ESBL production is difficult to achieve. Our results, especially those of the modified DDST, suggest that the best solution seems to be a combination of 2 phenotypic methods. In case of a high prevalence of AmpC-positive Enterobacteriaceae, routine testing should include a phenotypic method to detect potential AmpC production. As a suitable highly sensitive method, the AmpC disk test with 3-aminophenylboronic acid could be recommended. For basic screening, it would be advisable to use the microdilution method with a modified set of antibiotics able to detect both ESBL and AmpC enzyme production in a single assay.
Figure 1.
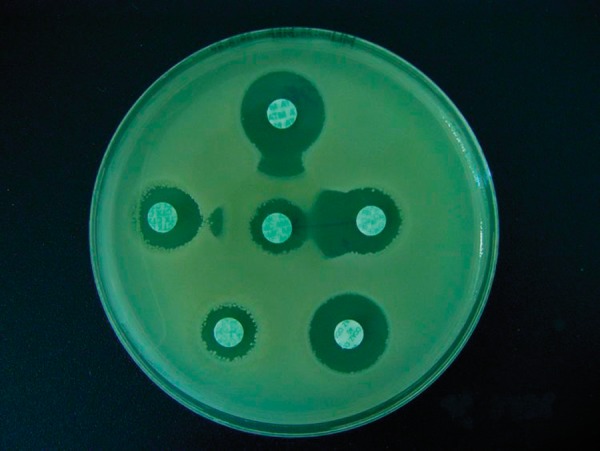
Positive result of the modified DDST in an ESBL-positive strain of Klebsiella pneumoniae.
Figure 2.
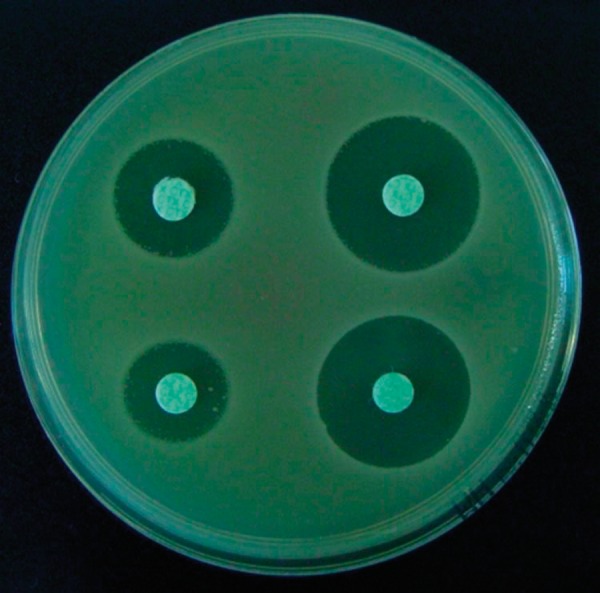
Positive results of the modified AmpC test in an AmpC-positive strain of Klebsiella pneumoniae.
Figure 3.
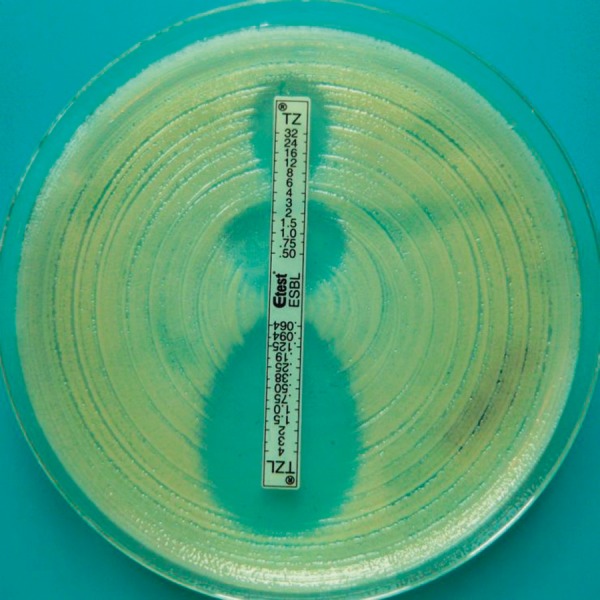
Positive result of the ESBL Etest in an ESBL-positive strain of Klebsiella pneumoniae.
Footnotes
The reported results were partially presented at the 20th European Congress of Clinical Microbiology and Infection Diseases, Vienna, Austria, 2010
Conflict of interest
The authors declare that they have no conflict of interest.
The manuscript has been read and approved by all the authors and each author believes the manuscript represents honest work.
Source of support: The study was supported by the Czech Ministry of Health grant no. IGA MZ CR 9950-3 and LF_2010_002
References
- 1.Kang CI, Sung HK, Park WB, et al. Clinical outcome of bacteremic spontaneous bacterial peritonitis due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumonia. Korean J Intern Med. 2004;19:160–65. doi: 10.3904/kjim.2004.19.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pai H, Kang CI, Byeon JH, et al. Epidemiology and clinical features of bloodstream infections caused by AmpC-type-beta-lactamase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2004;48:3720–28. doi: 10.1128/AAC.48.10.3720-3728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tumbarello M, Sanguinetti M, Montuori E, et al. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother. 2007;51:1987–94. doi: 10.1128/AAC.01509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwaber MJ, Navon-Venezia S, Kaye KS, et al. Clinical and economic impact of bacteremia with extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2006;50:257–62. doi: 10.1128/AAC.50.4.1257-1262.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Antimicrobial Resistance Surveillance System. [Accessed 10. 1. 2010]. Available from http://www.rivm.nl/earss.
- 6.Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum-beta-lactamase production in nosocomial infections. Ann Intern Med. 2004;140:26–32. doi: 10.7326/0003-4819-140-1-200401060-00008. [DOI] [PubMed] [Google Scholar]
- 7.Kolar M, Latal T, Cermak P, et al. Prevalence of extended-spectrum-lactamase-positive Klebsiella pneumoniae isolates in the Czech Republic. Int J Antimicrob Ag. 2006;28:49–53. doi: 10.1016/j.ijantimicag.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Livermore DM, Yuan M. Antibiotic resistance and production of extended-spectrum beta-lactamases amongst Klebsiella spp. from intensive care units in Europe. J Antimicrob Chemother. 1996;38:409–24. doi: 10.1093/jac/38.3.409. [DOI] [PubMed] [Google Scholar]
- 9.Tenover FC, Mohammed MJ, Gorton TS, Dembek ZF. Detection and reporting of organisms producing extended-spectrum β-lactamases: survey of laboratories in Connecticut. J Clin Microbiol. 1999;37:4065–70. doi: 10.1128/jcm.37.12.4065-4070.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luzzaro F, Gesu G, Endimiani A, et al. Performance in detection and reporting beta-lactam resistance phenotypes in Enterobacteriaceae: a nationwide proficiency study in Italian laboratories. Diagn Microbiol Infect Dis. 2006;55:311–18. doi: 10.1016/j.diagmicrobio.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Conejo MC, Mata C, Navarro F, Pascual A the GEMARA collaborative group. Detection and reporting beta-lactam resistance phenotypes in Escherichia coli and Klebsiella pneumoniae: a multicenter proficiency study in Spain. Diagn Microbiol Infect Dis. 2008;62:317–25. doi: 10.1016/j.diagmicrobio.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Jarlier V, Nicolas MH, Fournier G, Philippon A. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer b-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–78. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, nineteenth informational supplement. 2009;29(3):M100–S19. [Google Scholar]
- 14.The European Committee on Antimicrobial Susceptibility Testing. 2009. [Accessed 15. 4. 2010]. Available from http://www.eucast.org/clinical_breakpoints.
- 15.Yagi T, Wachino J, Kurokawa H, et al. Practical methods using boronic acid compounds for identification of class C beta-lactamase-producing Klebsiella pneumoniae and Escherichia coli. J Clin Microbiol. 2005;43:2551–58. doi: 10.1128/JCM.43.6.2551-2558.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arlet G, Brami G, Decre D, et al. Molecular characterisation by PCR-restriction fragment length polymorphism of TEM beta-lactamases. FEMS Microbiol Lett. 1995;134:203–8. doi: 10.1111/j.1574-6968.1995.tb07938.x. [DOI] [PubMed] [Google Scholar]
- 17.Chanawong A, M’Zali FH, Heritage J, et al. Characterisation of extended-spectrum beta-lactamases of the SHV family using a combination of PCR-single strand conformational polymorphism (PCR-SSCP) and PCR-restriction fragment length polymorphism (PCR-RFLP) FEMS Microbiol Lett. 2000;184:85–89. doi: 10.1111/j.1574-6968.2000.tb08995.x. [DOI] [PubMed] [Google Scholar]
- 18.Pagani L, Dell’Amico E, Migliavacca R, et al. Multiple CTX-M-type extended-spectrum beta-lactamases in nosocomial isolates of Enterobacteriaceae from a hospital in Northern Italy. J Clin Microbiol. 2003;41:4264–69. doi: 10.1128/JCM.41.9.4264-4269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pérez-Pérez FJ, Hanson ND. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol. 2002;40:2153–62. doi: 10.1128/JCM.40.6.2153-2162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiegand I, Geiss HK, Mack D, et al. Detection of extended-spectrum beta-lactamases among Enterobacteriaceae by use of semiautomated microbiology systems and manual detection procedures. J Clin Microbiol. 2007;45:1167–74. doi: 10.1128/JCM.01988-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linscott AJ, Brown WJ. Evaluation of four commercially available extended-spectrum beta-lactamase phenotypic confirmation tests. J Clin Microbiol. 2005;43:1081–85. doi: 10.1128/JCM.43.3.1081-1085.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]