Summary
Background
It is widely accepted that T helper 2 (Th2) cells, Th17 cells and their cytokines orchestrate the feature of asthma. However, most of studies on asthma mechanisms use a single allergen challenge model. Actually, humans are concurrently exposed to various allergens, and the mechanism of asthma with complex allergen exposure is less well defined. To explore whether the mechanism would be altered if asthma patients are re-exposed to another allergen, we exposed the chicken egg albumin (OVA) induced-asthmatic mice to house dust mite (HDM).
Material/Methods
HE staining was used to analyze pathologic variation in lung tissue of mice in each sub-group: control group, HDM alone group, OVA alone group and OVA+HDM group. Th1, Th2 and Th17 associated gene mRNA expressions were detected by quantitative PCR; associated cytokines were determined by ELISA or immunohistochemistry.
Results
The severe of inflammatory cell infiltration, the augmentation of Th17 and Th2 related gene mRNA expressions and the increase of Th17 associated cytokines expression were shown in OVA+HDM group in comparison with OVA alone group. However, Th2 related cytokines were increased with no significant difference in OVA+HDM group compared with OVA alone group.
Conclusions
We have found that Th17 response is connected with inflammation in the OVA-induced asthmatic mice exposed to HDM. When OVA-induced asthmatic mice are re-exposed to HDM, the pathomechanism is different from OVA alone exposure. HDM, indoor allergen, may be an important interferential factor for asthma therapy. It will give an important direction in the development of future asthma therapy.
Keywords: house dust mite, T helper 17, asthma
Background
Asthma is a complex syndrome, characterized by intermittent reversible obstruction, airway hyper-responsiveness (AHR) and pulmonary inflammation in which many cells and cellular elements play a vital role, such as eosinophils, mast cells, T lymphocytes, macrophages, neutrophils and epithelial cells. Asthma is usually divided into allergic asthma and non-allergic asthma, and approximately two-thirds of asthma cases are allergic [1,2]. It is widely accepted that antigen-specific T helper cell type 2 (Th2) and their cytokines such as IL-5, IL-4, and IL-13 orchestrate the feature of asthma [3,4]. Recently, the classical theory has been expanded to include Th17 cells and their associated cytokines [5,7]. Many therapies have been adopted basis on pathogenesis, but there is still a definite increase in prevalence of asthma. Perhaps the complexity of the disease and allergen exposure is beyond our imaginations.
CD4+T cells are differentiated into Th1 or Th2 cells depending on the distinct involved cytokines [8]. Recently, a new phenotype of Th cell, Th17 cell, has been identified. Th17 cells, with its secretion IL-17A, IL-17F and IL-22, play a vital role in host defense and in induction and propagation of autoimmunity in animal models [9]. IL-17A and IL-17F, augmentative in the inflammation of bronchial submucosa in mild to moderate asthma, contribute to excessive mucus and airway smooth muscle proliferation [6,10]. Both the maturation of Th17 cells and secretion of IL-17 are related with IL-23 [11].
House dust mite (HDM), a major source of allergen in house dust, is closely associated with development of asthma. It can lead to prominent and sustained airway eosinophilic inflammation, along with elevated serum levels of Th2-associated immunoglobulins and cytokines [12,13]. HDM directly induces the release of pro-inflammation cytokines and chemokines from bronchial epithelial cells and airway epithelial cells and evokes the direct, non-allergic inflammation [14]. Many researchers merely investigate the mechanism of asthmatic mice challenged by HDM alone, the complex HDM exposure is less well defined. So the morbidity of HDM related asthma is still increasing sharply [15,16]. The role of Th17 response in asthma remains a controversial problem, especially it in HDM-alone-induced asthmatic mice [17,18]. To know more about the asthma patients who re-expose to HDM allergen, we pay attention to Th2 and Th17 responses in our complex allergen challenge model, OVA-induced asthmatic mice exposed to HDM.
More inflammatory cell infiltrations were observed in HDM alone groups than that in control group, which demonstrates that HDM can directly evoke non- allergic inflammatory response. The expression of Th17 related cytokines were augmented in OVA+HDM group compared with OVA alone group. Th2 related cytokines were increased in OVA+HDM group in comparison with OVA alone group at gene level, while at protein level, they were increased with no significant difference. We confirm that the augmented Th17 response is linked with the inflammation in OVA-induced asthmatic mice exposed to HDM. When the OVA-induced asthmatic mice are re-exposed to HDM, the pathomechanism is different from OVA alone exposure. Thus, HDM is an important interferential factor for asthma therapy in the indoor living environment of asthma patients. It will give an important direction in the development of future asthma therapy.
Material and Methods
Mice and reagents
The 6-week-old male Balb/c mice were purchased from the Animal Center of Si Chuan University, and were cared for and experimented on under specific, pathogen-free conditions at our animal facilities in the Laboratory Animal Center of West China Medical School, Sichuan University. HDM (50000 BU/ml) was obtained from Allergopharma (Reinbeck, Germany).
OVA-induced asthmatic mice exposed to HDM
The mice were divided into 4 groups: Control group, HDM alone group, OVA alone group, and OVA+HDM group (Table 1) with 10 mice in each group. OVA (100 μg/ml, Sigma, Bornem, Belgium) was mixed with an equal volume of 10% (w/v) alum (Sigma, USA) in distilled water. To establish the OVA asthmatic model, the mice were sensitized by intra-peritoneal injections of OVA/alum (100 μg/ml) for 13 days. Then the mice were aerosol inhalation challenged with 10 ml OVA/PBS (2 mg/ml) during day 33–37. In OVA+HDM group, OVA-induced asthmatic mice received nasal drops 100 μl HDM 10 BU/ml (total 1 BU) 1 hour after aerosol inhalation of OVA/PBS, while in control group, mice were sensitized by intra-peritoneal injection of PBS, were aerosol inhalation challenged with PBS solution, and received nasal drops with PBS. The number of sneezing and nasal rubbing movement of mice were counted in an observation cage for 20 min. The observer assessed movements by the double-blinded method. Mice were sacrificed by ether anesthesia at 24 h after the last administration. Serum, bronchial alveolus lavage fluid, spleen, thymus and lung tissues were obtained for further analysis.
Table 1.
Four groups of mice sensitized by allergen.
| Group | Intra-peritoneal injection | Aerosol inhalation | Nosing dropping | The relevant clinical meanings |
|---|---|---|---|---|
| Control group | PBS | PBS | PBS | Healthy people |
| HDM alone group | PBS | PBS | HDM | Susceptible population |
| OVA alone group | OVA | OVA | PBS | Asthma patients |
| OVA+HDM group | OVA | OVA | HDM | Asthma patients inhaled again another allergen |
Histopathology and immunohistochemistry
The original pathologic specimen of lung tissues were fixed in 10% neutral buffered formalin and processed to paraffin, and 4–5 μm sections were prepared. For histopathology, routine HE staining was carried out. For immunohistochemistry, sections were incubated with 1:200 diluted anti-IL-17 antibody (Boaosen, Beijing, China) overnight at 4°C. Then the sections were washed with PBS and incubated with 1:1000 diluted biotinylated secondary anti-goat immunoglobulin (Zhongshan, Beijing, China). Diaminobenzidine was used as a substrate chromogen. Slides were counterstained with hematoxylin. The positively stained cells were assessed in 20 randomly selected fields of view on each image at a final magnification of ×400.
Quantitative PCR
mRNA level of RORc (Retinoic acid-related orphan receptor C) IL-17A, IL-23, T-bet (T-box transcription factor), GATA-3 (GATA binding protein) were estimated by quantitative PCR. Total RNA was purified using TAKARA kit (Takara, Dalian, China) following manufacture’s instructions. cDNA was synthesized by the Prime Script RT Reagent Kit (Takara, Dalian, China). cDNA, equivalent to 40 ng total RNA, was examined by an iCycler iQ quantitative PCR Detection System (Bio-Rad, CA, USA) by their specific primer sequences: (1) RORc forward: TC TGG GAT CCA CTA CGG G and reverse: AAA CTT GAC AGC ATC TCG GG; (2) IL-17A forward: CTG TGT CTC TGA TGC TGT TGC and reverse: CGT GGA ACG GTT GAG GTA GT; (3) T-bet forward: GCC AGG GAA CCG CTT ATA T and reverse: GAG TGA TCT CTG CGT TCT GGT; (4) GATA-3 forward: TGA CGG AAG AGG TGG ACG TA and reverse: TGG ATG GAC GTC TTG GAG AA; (5) IL-23 forward: CCA GTT CTG CTT GCA AAG G and reverse: GGT GAT CCT CTG GCT GGA; (6) β-actin forward: AGG GTG TGA TGG TGG GAA T and reverse: CTC GGT GAG CAG CAC AGG. PCR amplification consisted of 40 cycles of denaturation at 95°C for 15 s and annealing and extension at 60°C for 45 s. The target product was detected by SYBR Premix Ex Taq II kit (Takara, Dalian, China) and β-actin was used to normalize for transcription and amplification variations among samples. The relative expression quantity was assessed by the Gene Expression Macro Version 1.1 software (Bio-Rad, CA, USA).
Enzyme-linked Immunosorbent Assay (ELISA)
The Serum, bronchial alveolus lavage fluid, spleen, thymus and lung tissues were gathered from mice at room temperature and stored at −80°C. The levels of INF-γ, IL-5, IL-23 and IL-17 cytokines were analyzed by commercial sandwich ELISA kits (R&D Systems, Minneapolis, USA). Color reaction was measured as OD450 units on Bio-Rad microplate reader. The minimum detectable dose of cytokines was 1.0 pg/ml.
Statistical analysis
Statistical analysis was performed with SPSS 13.0 (SPSS, Chicago, USA). Two-way ANOVA test and post-hoc analysis were used for intra-group comparisons. P value less than 0.05 was considered as statistically significant.
Results
The manifestations of OVA-induced asthmatic model
The classical OVA-induced asthmatic mice were generated referencing the method of other investigators [19,20]. After the final administration, the mice in the HDM alone group, OVA alone group and OVA+HDM group were slow in movement and showed various degrees of respiratory failure. The increase frequency of nasal rubbing and sneezing were found in OVA alone group and OVA+HDM group compared with control group. The dull fur of the mice was also observed in these two groups.
HDM directly induced non-allergic asthma pathology
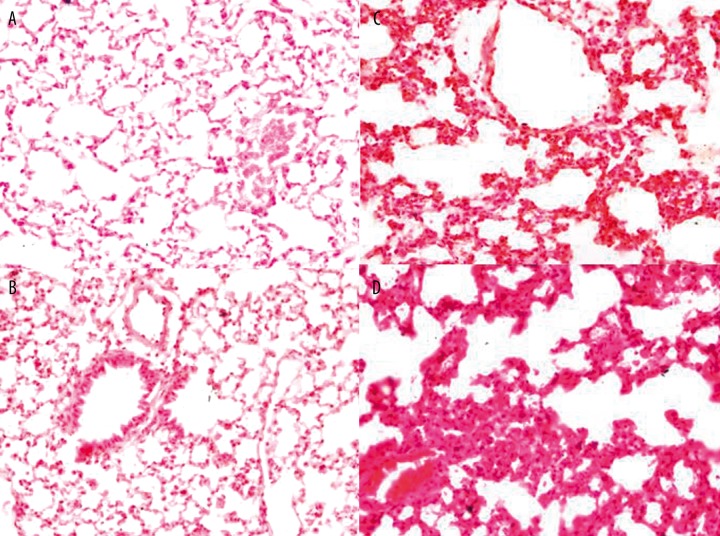
Eosinophil infiltrations, epithelial cell hyperplasia and airway obstruction were evident in each experimental group in comparison with control group. More inflammatory cell infiltration was observed in HDM alone group than that in control group (Figure 1A, B). It indicated that HDM can directly evoke non-allergic inflammatory response, in accordance with others’ conclusions [14]. Compared with OVA alone group, the inflammatory response in OVA+HDM group was more evident (Figure 1 C, D). HDM can evoke non-allergic inflammatory, we assume that the non-allergic inflammatory is present in OVA+HDM group.
Figure 1.
HE staining of paraffin-embedded lung tissue in control group (A), HDM alone group (B), OVA alone group (C) and OVA+HDM group (D). Magnification was all 400×.
Th2 and Th17 response relative gene mRNA expressions were augmented in OVA-induced asthmatic mice exposed to HDM
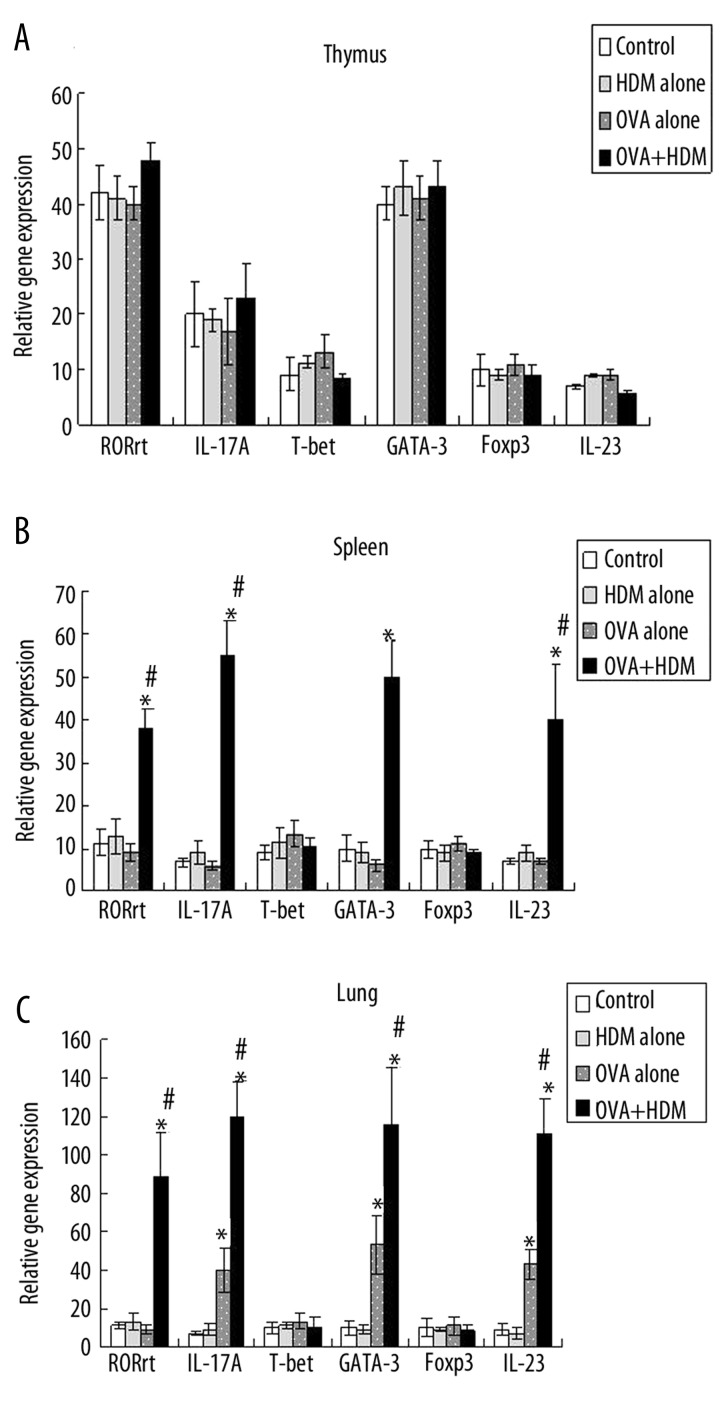
In spleen tissue, RORc, IL-17, IL-23 and GATA3 mRNA expressions were increased in OVA+HDM group, while they were elevated with no significant difference in HDM alone and OVA alone group in comparison with control group (Figure 2A). In thymus tissue, there was no significant difference in gene mRNA expressions among all the groups. (Figure 2B). Th2 and Th17 response in spleen tissue was more obvious than that in thymus tissue. We deduced that spleen tissue was easy respone to the stimulation of allergen. In lung tissue, Th2 and Th17 related gene mRNA expressions were not significant increased in HDM alone group as we supposed. They were significant elevated in OVA alone group and OVA+HDM group compared with control group. In comparison with OVA alone group, Th2 and Th17 responses were still significant augmented in OVA+HDM group. (Figure 2C). Th2 and Th17 response related gene mRNA expressions were augmented when OVA-induced asthmatic mice were exposed to HDM.
Figure 2.
The expressions of RORc, IL-17A, IL-23 and GATA-3 gene mRNA were detected in each group in spleen, thymus and lung tissue.spleen tissue (A), thymus tissue (B) and in lung tissue (C). * P<0.05, Statistical differences were compared to control group by two-way ANOVA test and post-hoc pairwise analysis, # P<0.05, Statistical differences were compared to OVA alone group by two-way ANOVA test and post-hoc pairwise analysi).
Th17 response related cytokines were increased in OVA-induced asthmatic mice exposed to HDM
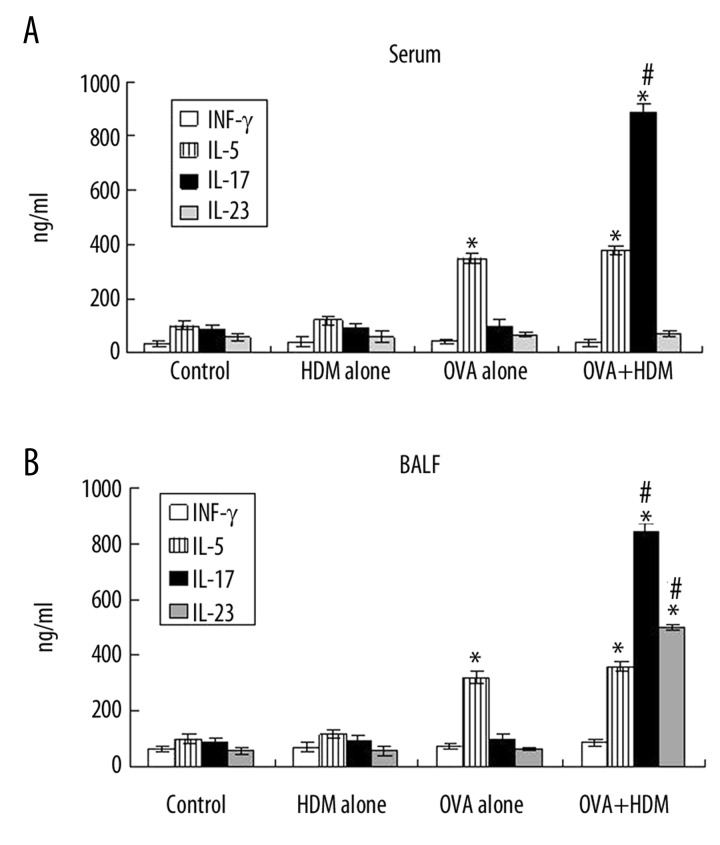
Th2 and Th17 response related cytokines in serum and bronchoalveolar lavage fluid were detected by ELISA. In serum, IL-17 was sharply up-regulated only in OVA+HDM group compared with other groups, while INF-γ and IL-23 showed no evident increase (Figure 3A). Both IL-17 and IL-23 were apparently increased in bronchoalveolar lavage fluid in OVA+HDM group, but not in HDM alone group and OVA alone group (Figure 3B). IL-5 was augmented in both OVA alone group and OVA+HDM group in serum and bronchoalveolar lavage fluid compared to control group (Figure 3A, B). In comparison with OVA alone group, IL-5 was increased with no significant difference in OVA+HDM group in bronchoalveolar lavage fluid (Figure 3A). In other words, Th2 relative response increased in OVA alone group not in OVA+HDM group.
Figure 3.
The levels of Th1, Th2 and Th17 related cytokines were detected in serum (A) and bronchoalveolar lavage fluid (B). * P<0.05, Statistical differences were compared to control group by two-way ANOVA test and post-hoc pairwise analysis, # P<0.05, Statistical differences were compared to OVA alone group by two-way ANOVA test and post-hoc pairwise analysis.
IL-17 was up-regulated in OVA-induced asthmatic mice exposed to HDM
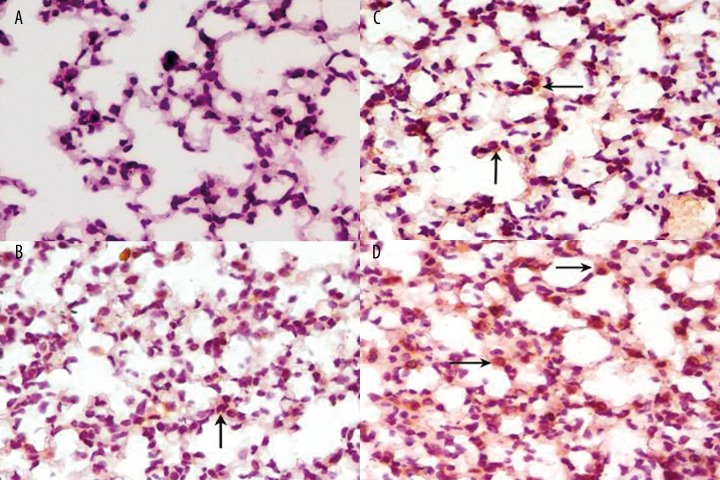
IL-17 staining was low to moderate intensity in inflammatory cells in HDM alone group (Figure 4B). Abundant infiltrating inflammatory cells were moderate positive for IL-17 in OVA alone group (Figure 4C). In accordance with ELISA and gene mRNA expressions results, IL-17 was strongly positive in inflammatory cells in OVA+HDM group compared with other groups by immunohistochemistry (Figure 4A, D and Table 2)).
Figure 4.
The expression of IL-17 was detected by immunochemistry in lung tissue in control group (A), HDM alone group (B), OVA alone group (C) and OVA+HDM group (D). 400×. Arrow, the positive cells for IL-17.
Table 2.
The statistic results of IL-17 positive cells in four groups in lung tissue.
| Group | Positive cells/field |
|---|---|
| Control group | 15.43±6.52 |
| HDM alone group | 18.29±4.38 |
| OVA alone group | 36.91±5.73* |
| OVA+HDM group | 92.62±11.35** |
P<0.05, Two-way ANOVA test and post-hoc pairwise analysis for OVA+HDM group compared with control group and HDM alone group.
P<0.05, Two-way ANOVA test and post-hoc pairwise analysis for OVA+HDM group compared with control group, HDM alone group, and OVA alone groups
Discussion
In a study performed in United States, 80% of allergic asthmatic adults were challenged by more than one aeroallergen [21]. However, when investigating the pathomechanism of asthma, many researchers merely focus on a single allergen challenge model [22,23]. In recent years, the morbidity of asthma induced by HDM has increased sharply. HDM, a main allergen in asthma patient living environment, is strongly associated with the development of asthma. The mechanism of asthma patients who re-exposed to HDM is less well defined. To know more about the asthma patient who re-expose to HDM allergen, we establish our experimental model.
RORc, IL-17 and IL-23 were chosen as Th17 response related genes referencing previous studies [25,26]. As for Th2 response, GATA3 and IL-5 were selected, while T-beta and INF-γ were selected for Th1 response. In our study, Th1, Th2 and Th17 response related genes were detected in lung tissue and central immune organs of our model. Unexpectedly, Th17 response associated genes were significant elevated in spleen tissue in OVA+HDM group compared with OVA alone group, while the tendency was not observed in thymus tissue. We deduced that spleen tissue was easy response to stimulation of allergen. Interestingly, Th2 and Th17 response related gene mRNA expressions, not Th1, were significant augmented in OVA+HDM group compared with OVA alone group in lung tissue. Given that the gene mRNA expression results were not as we expected, Th1, Th2 and Th17 responses related cytokines were detected in serum, bronchoalveolar lavage fluid and lung tissue. IL-17 and IL-23 were sharply augmented in OVA+HDM group compared with OVA alone group, while IL-5 was increased with not significant difference. As the result of transcription regulation, the mRNA expressions of Th2 response related gene was inconsistent with protein expression in OVA+HDM group. We confirm that the augmentation of Th17 response is linked to the inflammation in the OVA-induced asthmatic mice exposed to HDM. We also conclude that Th17 and Th2 responses are definitely augmented in the OVA-asthmatic model, but when it is re-exposed to HDM, Th17 response is dominant. That is to say, if the OVA-induced asthmatic mice is re-exposed to HDM, the pathogenesis would be different from OVA alone exposure. Therefore, HDM is an important interferential factor for asthma therapy, which also has important implications in the development of future asthma therapy. IL-17 increased in BALF and serum, while IL-23 was only elevated in BALF. High level of IL-23 in lung tissue induces Th17 cell to participate in the inflammation of asthma [27], so that we can widely find IL-17 cytokine in BALF and serum. The augmented Th17 cells also have a particular ability to recruit granulocytes to participate in [28]. Hence, the inflammation in OVA +HDM group was more serious than that in OVA alone group. To our knowledge, this is the first study to investigate the pathogenesis of asthma with complex HDM exposure.
Recent study has revealed that HDM can evoke the non-allergic asthma, which is similar with the allergic asthma [14]. We observed inflammatory cell infiltrations in lung tissue in HDM alone group, which was consistent with previous findings. Thus, we assume that non-allergic inflammatory response is present in OVA+HDM group. However, single HDM challenge may not be enough to achieve reliable lung function changes, and it appears that multiple challenges may be necessary in the model [13,29,30]. This is the reason we have difficulty in inducing the asthmatic model by a single HDM challenge. From the inflammation in HDM alone group, we infer that the longer people are exposed to HDM, the more risk of developing to asthma they will undertake.
Conclusions
The non-allergic inflammatory response may be present in the inflammation in OVA- induced asthmatic mice exposed to HDM. The augmented Th17 response is linked with the inflammation in OVA-induced asthmatic mice exposed to HDM. When the OVA-induced asthmatic mice are re-exposed to HDM, the pathomechanism is different from OVA alone exposure. Thus, HDM is an important interferential factor for asthma therapy in the indoor living environment of asthma patients. It will give an important direction in the development of future asthma therapy.
Abbreviations
- HDM
house dust mite
- OVA
chicken egg albumin
- Th
T helper cell type
- C RORc
retinoic acid-related orphan receptor
- T-bet
T-box transcription factor
- GATA3
GATA binding protein 3
Footnotes
Source of support: This study was supported by grant 136050132 from Start up Foundation of West China Hospital of Sichuan University
References
- 1.Bollag U, Grize L, Braun-Fahrländer C. Is the ebb of asthma due to the decline of allergic asthma? A prospective consultation-based study by the Swiss Sentinel Surveillance Network, 1999–2005. Fam Pract. 2009;6:96–101. doi: 10.1093/fampra/cmn104. [DOI] [PubMed] [Google Scholar]
- 2.Hamelmann E. The rationale for treating allergic asthma with anti-IgE. European Respiratory Review. 2007;16:61–66. [Google Scholar]
- 3.Kay AB. Allergy and allergic diseases First of two parts. N Engl J Med. 2001;344:30–37. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 4.Hellings PW, Kasran A, Liu Z, et al. Interleukin-17 Orchestrates the Granulocyte Influx into Airways after Allergen Inhalation in a Mouse Model of Allergic Asthma. Am J Respir Cell Mol Biol. 2003;28:42–50. doi: 10.1165/rcmb.4832. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Yang J, Gao YD, Guo W. Th17 immunity in patients with allergic asthma. Int Arch Allergy Immunol. 2010;151:297–307. doi: 10.1159/000250438. [DOI] [PubMed] [Google Scholar]
- 6.Doe C, Bafadhel M, Siddiqui S, et al. Expression of the Th17-associated cytokines interleukin (IL)-17A and F in asthma and chronic obstructive pulmonary disease. Chest. 2010;10:1378–406. [Google Scholar]
- 7.McKinley L, Alcorn JF, Peterson A, et al. Th17 Cells Mediate Steroid-Resistant Airway Inflammation and Airway Hyper responsiveness in Mice. J Immunol. 2008;181:4089–97. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosmann TR, Cherwinski H, Bond MW, et al. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 9.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–57. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008;266:55–79. doi: 10.1111/j.1600-065X.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 11.Medeiros KC, Faustino L, Borduchi E, et al. Preventive and curative glycoside kaempferol treatments attenuate the TH2-driven allergic airway disease. Int Immuno Pharmacol. 2009;9:1540–48. doi: 10.1016/j.intimp.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Fukushima C, Matsuse H, Fukahori ST, et al. Aspergillus fumigatus synergistically enhances mite-induced allergic airway inflammation. Med Sci Monit. 2010;16(7):BR197–202. [PubMed] [Google Scholar]
- 13.Gregory LG, Causton B, Murdoch JR, et al. Inhaled house dust mite induces pulmonary T helper 2 cytokine production. Clin Exp Allergy. 2009;39:1597–610. doi: 10.1111/j.1365-2222.2009.03302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Alba J, Raemdonck K, Dekkak A, et al. House dust mite induces direct airway inflammation in vivo: implications for future disease therapy? Eur Respir J. 2010;35:1377–87. doi: 10.1183/09031936.00022908. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Gjesing B, Lai X, et al. The IgE cross-reactivity between house dust mites and storage mites in patients from Guangzhou city. Allergy. 2009;64:378–79. [Google Scholar]
- 16.Phua G, Koh M, Tan L, Chan P. Sensitisation to house dust mites in adults with asthma and allergic rhinitis in Singapore. Allergy. 2009;64:377–78. [Google Scholar]
- 17.Phipps S, Lam CE, Kaiko GE, et al. Toll/IL-1 signaling is critical for house dust mite-specific helper T cell type 2 and type 17. Am J Respir Crit Care Med. 2009;179:883–93. doi: 10.1164/rccm.200806-974OC. [DOI] [PubMed] [Google Scholar]
- 18.McGee HS, Stallworth AL, Agrawal T, et al. Fms-like tyrosine kinase 3 ligand decreases T helper type 17 cells and suppressors of cytokine signaling proteins in the lung of house dust mite-sensitized and -challenged mice. Am J Respir Cell Mol Biol. 2010;43:520–29. doi: 10.1165/rcmb.2009-0241OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yim Yk, Lee H, Hong KE, et al. Anti-inflammatory and Immune-regulatory Effects of Subcutaneous Perillae Fructus Extract Injections on OVA-induced Asthma in Mice. Evid Based Complement Altern Med. 2010;7:79–86. doi: 10.1093/ecam/nem118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Secor ER, Carson WF, Singh A, et al. Oral Bromelain Attenuates Inflammation in an Ovalbumin-induced Murine Model of Asthma. Evid Based Complement Altern Med. 2008;5:61–69. doi: 10.1093/ecam/nel110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hervas D, Rodriguez R, Garde J. Role of aeroallergen nasal challenge in asthmatic children. Allergol Immunopathol (Madr) 2011;39(1):17–22. doi: 10.1016/j.aller.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Cheung PF, Wong CK, Lam CW. Molecular Mechanisms of Cytokine and Chemokine Release from Eosinophils Activated by IL-17A, IL-17F, and IL-23: Implication for Th17 Lymphocytes-Mediated Allergic Inflammation. J Immunol. 2008;180:5625–35. doi: 10.4049/jimmunol.180.8.5625. [DOI] [PubMed] [Google Scholar]
- 23.Labonté I, Hassan M, Risse PA, et al. The effects of repeated allergen challenge on airway smooth muscle structural and molecular remodeling in a rat model of allergic asthma. Am J Physiol Lung Cell Mol Physiol. 2009;297:698–705. doi: 10.1152/ajplung.00142.2009. [DOI] [PubMed] [Google Scholar]
- 24.Phipps S, Lam CE, Kaiko GE, et al. Toll/IL-1 Signaling Is Critical for House Dust Mite–specific Th1 and Th2 Responses. Am J Respir Crit Care Med. 2009;179:883–93. doi: 10.1164/rccm.200806-974OC. [DOI] [PubMed] [Google Scholar]
- 25.Klotz L, Burgdorf S, Dani I, et al. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J Exp Med. 2009;206:2079–89. doi: 10.1084/jem.20082771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Tato CM, Muul L, et al. Distinct regulation of interleukin-17 in human T helper lymphocyte. Arthritis Rheum. 2007;56:2936–46. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakashin H, Hirose K, Maezawa Y, et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008;178:1023–32. doi: 10.1164/rccm.200801-086OC. [DOI] [PubMed] [Google Scholar]
- 28.Annunziato F, Cosmi L, Liotta F, et al. The phenotype of human Th17 cells and their precursors, the cytokines that mediate their differentiation and the role of Th17 cells in inflammation. Int Immunol. 2008;20:1361–68. doi: 10.1093/intimm/dxn106. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs B, Braun A. Improved mouse models of allergy and allergic asthmachances beyond ovalbumin. Curr Drug Targets. 2008;9:495–502. doi: 10.2174/138945008784533589. [DOI] [PubMed] [Google Scholar]
- 30.Hammad H, Chieppa M, Perros F, et al. House dust mite allergen induces astham via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–16. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]