Summary
Background
The aim of this retrospective study was to evaluate the efficacy and safety of weekly high-dose 5-fluorouracil (5-FU)/folinic acid (FA) as 24-h infusion (AIO regimen) plus irinotecan in patients with histologically proven metastatic gastroesophageal adenocarcinoma (UICC stage IV).
Material/Methods
From 08/1999 to 12/2008, 76 registered, previously untreated patients were evaluable. Treatment regimen: irinotecan (80 mg/m2) as 1-h infusion followed by 5-FU (2000 mg/m2) combined with FA (500 mg/m2) as 24-h infusion (d1, 8, 15, 22, 29, 36, qd 57).
Results
Median age: 59 years; male/female: 74%/26%; ECOG ≤1: 83%; response: CR: 1%, PR: 16%, SD: 61%, PD: 17%, not evaluable in terms of response: 5%; tumor control: 78%; median OS: 11.2 months; median time-to-progression: 5.3 months; 1-year survival rate: 49%; 2-year survival rate: 17%; no evidence of disease: 6.6%; higher grade toxicities (grade 3/4): anemia: 7%, leucopenia: 1%, ascites: 3%, nausea: 3%, infections: 12%, vomiting: 9%, GI bleeding of the primary tumor: 4%, diarrhea: 17%, thromboembolic events: 4%; secondary metastatic resection after downsizing: 16 patients (21%), R-classification of secondary resections: R0/R1/R2: 81%/6%/13%, median survival of the 16 patients with secondary resection: 23.7 months.
Conclusions
Combined 5-FU/FA as 24-h infusion plus irinotecan may be considered as an active palliative first-line treatment accompanied by tolerable toxicity; thus offering an alternative to cisplatin-based treatment regimens. Thanks to efficient interdisciplinary teamwork, secondary metastatic resections could be performed in 16 patients. In total, the patients who had undergone secondary resection had a median survival of 23.7 months, whereas the median survival of patients without secondary resection was 10.1 months (p≤0.001).
Keywords: gastroesophageal cancer, palliative chemotherapy, irinotecan, 5-fluorouracil
Background
Although the incidence of gastric cancer has continually decreased in Germany over the last 20 years, gastric cancer still remains a common cause of cancer mortality in Germany. To date, an estimated 18 800 new cases of gastric cancer will be diagnosed in Germany each year, mostly affecting male patients [1]. In Europe, an incidence of 159 900 cases of gastric cancer was registered in 2006, which is consistent with 5% of all causes of cancer. In Europe, gastric cancer is the fourth leading cause of cancer mortality [2]; moreover, it is the second most common malignity worldwide, with approximately 700 000 cancer-related deaths. The geographic distribution is characterized by enormous international differences; as gastric cancer is frequently diagnosed in particular in East Asia (China, Japan), in Eastern Europe and in parts of Central and South America [3].
In contrast to gastric cancer, the incidence of carcinomas of the esophagogastric junction has clearly increased over the past 20 years. It is mainly diagnosed in the white male population of Western industrial nations. In 2000, an annual increase in incidence ranging from 8.7% to 15.2% was observed in Central Europe [4]. This rate of increase outranges that of other entities of solid tumors [5].
Gastric cancer patients have a relatively poor prognosis [6]. More than 60% of the patients present with either advanced or metastatic disease (in accordance with UICC (International Union Against Cancer) stage III or IV) [7]; thus rendering the performance of curative resections impossible. As a result, even under the best supportive care, median overall survival (OS) in advanced non-resectable or metastatic gastric cancer only ranges from 3 to 5 months [8,9].
Over the last few years, the development of active combined chemotherapy regimens have improved the median OS, time-to-progression (TTP) and quality of life factors in advanced and metastatic gastric cancer [10]. The outcome of a trial conducted by Glimelius et al demonstrated that patients with metastatic gastric cancer showed a prolonged OS of 3 additional months, as well as an increase in median TTP ranging from 2 to 5 months, if they had received palliative treatment (either with 5-fluorouracil/folinic acid (5-FU/FA) and etoposide (ELF) or with 5-FU/FA alone) in contrast to best supportive care alone [8].
At present, combination chemotherapy, in particular cisplatin-based chemotherapy regimens combined with either 5-FU or 5-FU/FA, is a widely accepted standard treatment for metastatic gastric cancer. The so-called PLF regimen (5-FU/FA as 24h infusion plus cisplatin) or further infusional regimens (5-FU/FA) in combination with cisplatin, yielded response rates ranging from 27% to 46% as well as median overall survival times ranging from 9.2 to 9.7 months [11–13]. However, they produced relatively high rates of adverse effects in terms of NCI-CTC (National Cancer Institute Common Toxicity Criteria) grade 3 or 4 toxicities (71% of the patients revealed higher grade hematological toxicities, 25% presented gastrointestinal toxicities) [11]. In addition, the so-called ECF regimen (epirubicin/cisplatin/5-FU) has been established as a standard treatment schedule, particularly in the UK. In comparatively large phase III trials the ECF regimen achieved response rates from 41% to 42% accompanied by a median OS of between 9.4 and 9.9 months. In terms of toxic adverse effects, higher grade neutropenia (grade 3 or 4) was observed in 32–41% of the patients [14,15]. The objective of the subsequent studies was to reduce the toxic adverse effects of cisplatin-based regimens while maintaining their efficacy; it could be demonstrated in 2 phase III trials that a similar level of efficacy accompanied by a better toxicity profile could be achieved by replacing the relatively toxic cisplatin agent with oxaliplatin [14,16].
Adding docetaxel to 5-FU plus cisplatin (DCF) significantly improved median OS, which increased from 8.6 to 9.2 months in a large phase III trial related to the treatment of metastatic gastric cancer. In this trial on the approval of docetaxel (TAX 325), which was conducted by Van Cutsem et al, combined docetaxel presented a clear superiority of docetaxel over the reference regimen based on CF (cisplatin/5-FU). Overall response increased from 25% to 37%, and median TTP could be significantly prolonged from 3.7 to 5.6 months (primary endpoint). The secondary endpoints were also improved by use of DCF [17–19], although severe hematological toxicities (neutropenia grade 3 and 4) were observed in 82% of the patients. Moreover, treatment-related deaths occurred in 3% (n=6 patients) of patients due to febrile neutropenia after having applied the DCF schedule [19].
Early in 2010, trastuzumab, a monoclonal humanized antibody against HER2 (human epidermal growth factor receptor 2), was approved for the treatment of metastatic gastric cancer (together with combined 5-FU and cisplatin in first-line treatment) in Germany. In a randomized phase III trial (ToGA Trial), 3807 patients were checked for their HER-2 status; however, only 22.1% out of those 3807 patients revealed a positive expression. The subgroup, comprising 594 patients with a positive HER-2 status, was randomly assigned to 2 treatment arms, and subsequently received combined treatment (5-FU or capecitabine and cisplatin) or a combined cisplatin-based treatment plus trastuzumab. The patient group treated with trastuzumab had a significantly prolonged median OS (13.8 months vs. 11.1 months) and an improved overall response rate (47% vs. 35%). Both arms were comparable in terms of their toxicity profile. In total, trastuzumab combined with 5-FU and cisplatin may be considered as an efficient immunological agent accompanied by good tolerability for the treatment of metastatic gastric cancer of the subgroup with HER-2 positive tumors [20].
Irinotecan, which is used in the treatment of metastatic gastric cancer, is another active drug [21–23]. Single-agent irinotecan yielded response rates ranging from 12% to 23%, accompanied by a median survival rate of 6.4 to 7.0 months in the treatment of metastatic gastric cancer [21,24,25]. Grade 3 and 4 neutropenias were observed in 23% to 39% of the patients and diarrhea in 20–30% of the patients [21,25]. High-dose 5-FU/FA as 24-h infusion (AIO regimen, Arbeitsgemeinschaft Internistische Onkologie,) plus irinotecan yielded noteworthy results in terms of efficiency and tolerability in the first- and second-line treatment of metastatic colorectal cancer [22,26]; this combination was also used in trials on metastatic gastroesophageal adenocarcinoma [27–29]. Irinotecan plus 5-FU/FA proved to be an active drug combination in terms of median OS and response, it offered good tolerability and yielded positive results in several trials on advanced and metastatic gastroesophageal adenocarcinoma [11,27–29]. Accordingly, this combination has been used in our department for first-line treatment since 1999. Here, we present the evaluation of the documented patients treated in the Medical Department 1, Erlangen University, as a validation collective of a previous phase II trial published by our study group [29]. The objective was two-fold – we analyzed the specific chemotherapeutical data, and we evaluated whether secondary metastatic resection after downsizing offered an improved prognosis for the analyzed patient group.
Material and Methods
Patients
We evaluated the data collected by the prospective tumor registry of the Medical Department 1 of Erlangen University from August 1999 to December 2008 on patients with the following inclusion criteria: Either a histologically proven metastatic adenocarcinoma (UICC stage IV) of the stomach or esophagogastric junction (AEG type I, II, III) had to be present. Previously, the non-resectability of the distant metastases had been assessed by the interdisciplinary tumor board of Erlangen University. At least 1 bi-dimensional lesion had to be detectable by imaging procedures (CT scan of the abdomen or chest, respectively). All patients were chemonaive and received high-dose 5-FU/FA as 24-h infusion (AIO regimen) plus irinotecan as palliative first-line treatment on an outpatient basis. Further inclusion criteria were an ECOG (Eastern Cooperative Oncology Group) index ≤2 prior to initiating treatment, adequate bone marrow function (leucocytes ≥3500/μl, platelets ≥100.000/μl), sufficient liver function (serum bilirubin ≤2× the upper reference range), and adequate renal function (creatinine ≤1.5× the upper reference range). Exclusion criteria were hypersensitivity against 5-FU, FA or irinotecan, previous palliative chemotherapy, concurrent radiochemotherapy (exception: pain treatment), clinically relevant cardiac disease, cerebral metastases or other malignancy. Further exclusion criteria were chronic diarrhea, a chronic inflammatory bowel disease or a subtotal bowel obstruction. Before initiating treatment, a medical history, physical examination, laboratory tests (blood count, serum analysis, coagulation test) including tumor marker determination and a spiral CT scan of the abdomen or the chest were performed.
Treatment protocol
Prior to treatment, a Port-a-Cath was surgically implanted. For palliative treatment, the patients received in out-patient care 80 mg/m2 irinotecan as 1-h intravenous (i.v.) infusion followed by 2000 mg/m2 5-FU and 500 mg/m2 FA as a 24-h infusion (AIO regimen) via a miniature pump system on days 1, 8, 15, 22, 29, and 36. The procedure was repeated from day 57 onwards. One treatment cycle comprised 6 applications followed by 2 weeks of rest. As prophylactic antiemetic, 1 mg granisetron i.v. was applied prior to initiating treatment, and 0.25 mg atropine s.c. was given to avoid the occurrence of an acute cholinergic syndrome. If, during the course of treatment, nausea or vomiting of NCI-CTC toxicity grade ≥2 occurred, antiemetic treatment was intensified by 8 mg of dexamethason i.v. In the event of diarrhea, the patient was instructed to take loperamide immediately after every bowel movement according to a strictly defined schedule. Prior to each weekly application and after terminating each cycle, the NCI-CTC toxicity was determined by performing laboratory tests and taking medical history. Treatment was continued up to tumor progression. Treatment was discontinued if severe side effects, withdrawal of the patients request for chemotherapy treatment, or the necessity of other measures (eg, surgical intervention for secondary resection), presented.
Methods
After every cycle (every 8 weeks), a follow-up examination comprising laboratory tests and tumor marker control (CEA (Carcinoembryonic antigen), CA 19-9 (Carbohydrate Antigen 19-9) and CA 72-4 (Carbohydrate Antigen 72-4), a CT scan (of the abdomen or chest, depending on the localization of the metastases) or further imaging procedures (e.g., a PET-CT scan) were performed. Antitumor activity was evaluated in accordance with WHO (World Health Organization) criteria [30].
All toxic events were registered and categorized in accordance with the NCI-CTC criteria. Prior to each application (exception: alopecia), an NCI-CTC index of ≤1 was required. If an NCI-CTC toxicity ≥2 was present, treatment was delayed by 1 week or more until a toxicity grade ≤1 was achieved. If higher grade toxic adverse effects (NCI-CTC-Index ≥2) persisted, the chemotherapy dose was reduced by 25% at first.
After progress during first-line treatment with 5-FU/FA and irinotecan, 29 patients (38%) presenting in a good general state of health and a satisfactory state of nutrition and with the request of further treatment, received palliative second-line treatment consisting of either an oxaliplatin-based regimen (55%) or a cisplatin-based regimen (28%). In some patients (8%), palliative third-line treatment, mainly with docetaxel (83%), was performed. Only a few patients (1%) received palliative fourth-line treatment.
Statistical considerations and study endpoints
The primary endpoints of this evaluation were the response rate (CR+PR) and the achieved tumor control (CR+PR+SD). Further endpoints related to median OS (calculated from the first chemotherapy application up to the event of death or end of trial (31 December, 2008)); TTP according to radiological imaging procedures (calculated from the first chemotherapy application up to progression); the NCI-CTC toxicity grade (version 3.0) and the rate of secondary resections (related to median OS). In addition, the CEA, CA 19-9 and CA 72-4 tumor markers, and the respective ECOG index values were evaluated as prognostic factors related to median OS. The last date of evaluation was 31 December, 2008. At that time, 64 out of the 76 evaluated patients (84%) were dead. One patient still received first-line treatment, and 6 patients (8%) received further palliative treatment lines.
In total, toxicity data of 75 patients (99%) were collected and evaluated. Furthermore, response-to-treatment data of 72 patients (95%) were analyzed. In 4 patients (5%), response could not be evaluated for the following reasons: 2 out of the 76 evaluable patients (2.6%) died within 30 days after initiating palliative first-line treatment, each one after having received 2 applications of the first cycle. Most probably the causes of death were both a high tumor burden and a clinically suspected tumor progression. One patient refused treatment continuation after having received 5 applications of the first cycle, and another patient had to undergo an emergency intervention in the form of a Billroth I operation due to extreme bleeding out of the primary tumor after the first chemotherapy application.
Both median OS and TTP parameters were analyzed in accordance with the Kaplan-Meier method, beginning with the first chemotherapy application as the respective starting point. Differences between the survival times were analyzed for statistical significance using the log-rank test. The significance level α was defined as 0.05. All statistical tests were bilateral. The 95% confidence interval (95% CI) was calculated in accordance with the Greenwood method [31]. All analyses were performed using the statistics software SPSS for Windows version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics (Table 1)
Table 1.
Patient characteristics (n=76).
| Characteristics | Number | Percentage (%) |
|---|---|---|
| Total | 76 | (100) |
| Age (years) | ||
| Median | 59 | |
| Range | 26 – 78 | |
| Gender | ||
| Female | 20 | (26) |
| Male | 56 | (74) |
| Performance Status (ECOG) | ||
| 0 | 18 | (24) |
| 1 | 45 | (59) |
| 2 | 13 | (17) |
| Histology | ||
| Adenocarcinoma | 76 | (100) |
| Grading | ||
| G1 | 1 | (1) |
| G2 | 18 | (24) |
| G3 | 45 | (59) |
| G4 | 9 | (12) |
| Not evaluable | 3 | (4) |
| UICC Stage | ||
| IV | 76 | (100) |
| Localisation of the primary tumor | ||
| AEG I | 14 | (18) |
| AEG II | 19 | (25) |
| AEG III | 1 | (1) |
| Corpus | 28 | (37) |
| Antrum | 13 | (17) |
| Unknown | 1 | (1) |
| Localisation of the metastases | ||
| Liver (HEP) | 30 | (39) |
| Lymph nodes (M1LYM) | 45 | (59) |
| Peritoneum (PER) | 34 | (45) |
| Lungs (PUL) | 9 | (12) |
| Skeleton (OS) | 6 | (8) |
| Ovary | 3 | (4) |
| Liver only | 7 | (9) |
ECOG – Eastern Cooperative Oncology Group; UICC – International Union Against Cancer.
The median age was 59 years (range: 26–78 years), the majority of the patients were male (74%), and 20 out of the 76 treated patients (26%) were female. At treatment initiation, the following co-morbidities were observed: arterial hypertonia in 27 patients (36%), thromboembolic events in 14 patients (18%), diabetes mellitus in 11 patients (15%), coronary artery disease in 6 patients (8%), and cardiac arrhythmia in 4 patients (5%). Prior to initiating treatment, 63 patients (83%) had an ECOG index ≤1, and in 13 patients (17%) an ECOG index equal 2 was observed. In total, 47 patients (62%) suffered from pain symptoms during palliative treatment. Fourteen of those 47 patients were treated with non-steroid antirheumatic agents, and 23 patients received opiate analgesia.
All 76 patients had a histologically proven adenocarcinoma; 1% had a G1 classified tumor, 24% a G2 tumor, 59% a G3 and 12% a G4 tumor. For 3 patients (4%) no grading had been registered. Gastric adenocarcinoma was diagnosed in 41 out of 76 patients (54%), and adenocarcinoma of the gastroesophageal junction in 35 patients (46%). In 14 (18%) out of the 35 patients with adenocarcinoma of the gastroesophageal junction, the primary tumor was classified as AEG type I, in 19 out of those 35 patients (25%) as type II, and in 2 out of the 35 patients (3%) as AEG type III. In 28 cases of gastric cancer (37%), the tumor was located within the gastric corpus, and in 13 cases (17%) it was situated in the gastric antrum. Prior to palliative treatment, a surgical intervention with curative intent (gastrectomy, esophageal resection) (R0=75%, R1=15%, R2=10%) was performed in 20 out of the 76 evaluated patients (26%) as soon as the initial diagnosis had been histologically confirmed. Fifty-six out of 76 patients (74%) revealed synchronous distant metastases and were considered as eligible for palliative treatment.
Treatment and toxicity (Table 2)
Table 2.
Maximum toxicity per patient (n=75) – 1293 chemotherapy applications and 226 cycles.
| Toxicity | NCI-CTC Grad [n (%)] | ||||
|---|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Haematological | |||||
| Anaemia | 34 (45) | 14 (18) | 22 (29) | 5 (7) | – |
| Leucopenia | 44 (59) | 20 (27) | 10 (13) | 1 (1) | – |
| Thrombopenia | 71 (95) | 4 (5) | – | – | – |
| Nonhaematological | |||||
| Diarrhea | 17 (23) | 26 (35) | 19 (25) | 13 (17) | – |
| Nausea | 11 (15) | 46 (61) | 16 (21) | 2 (3) | – |
| Vomiting | 27 (36) | 33 (44) | 8 (11) | 7 (9) | – |
| Loss of appetite | 35 (46) | 28 (37) | 11 (15) | 1 (1) | – |
| Fatigue | 44 (59) | 28 (37) | 3 (4) | – | – |
| Hand-foot syndrome | 59 (79) | 15 (20) | 1 (1) | – | – |
| Mucositis | 61 (81) | 13 (17) | 1 (1) | – | – |
| Neurological failures | 70 (93) | 5 (7) | – | – | – |
| Thromboembolism | 65 (87) | 3 (4) | 4 (5) | 2 (3) | 1 (1) |
| Increase of creatinine | 57 (76) | 12 (16) | 5 (7) | 1 (1) | – |
| Infections | 52 (69) | 8 (11) | 6 (8) | 8 (11) | 1 (1) |
| Fever | 59 (79) | 5 (7) | 10 (13) | 1 (1) | – |
| Ascites | 53 (71) | 12 (16) | 8 (11) | 2 (3) | – |
| Oedema | 68 (91) | 3 (4) | 4 (5) | – | – |
| GI bleeding | 65 (87) | 5 (7) | 2 (3) | 2 (3) | 1 (1) |
| Obstipation | 61 (81) | 14 (19) | – | – | – |
| Dysphagia | 64 (85) | 7 (9) | 2 (3) | 1 (1) | 1 (1) |
| Alopecia | 63 (83) | 10 (13) | 2 (3) | – | – |
GI – Gastrointestinal.
Seventy-five out of 76 patients (98.7%) were evaluable for toxicity. In total, 1293 chemotherapy applications were administered, median 16 applications per patient (range: 1–59 applications). Altogether, 226 cycles were applied, consisting of a minimum of 1 up to a maximum of 10 cycles (median 3 cycles per patient). The following higher grade non-hematological toxicities (grade 3+4) were observed: the predominating toxicity was diarrhea occurring in 13 patients (17%), followed by infections in 9 patients (12%), vomiting in 7 patients (9%), and nausea in 2 patients (3%). Anemia was observed as the predominating hematological toxicity; it occurred in 5 patients (7%), in 3 cases most probably associated with gastrointestinal bleeding of the primary tumor, and in 2 cases it was associated with chemotherapy. Table 2 presents a complete overview of toxicities. Altogether, dose reductions due to higher grade toxicity (grade ≥ 2) had to be decided in 26 out of 76 patients (34%).
Response, TTP and median survival (Tables 3 and 4)
Table 3.
Response rate (n=76).
| Response | Number | Percentage (%) |
|---|---|---|
| Total | 76 | (100.0) |
| Complete remission (CR) | 1 | (1.3) |
| Partial remission (PR) | 12 | (15.8) |
| Stable Disease (SD) | 46 | (60.5) |
| Progressive disease (PD) | 13 | (17.1) |
| Not evaluable | 4 | (5.3) |
| Tumor control (CR, PR, SD) | 59 | (77.6) |
| No evidence of disease (NED) | 5 | (6.6) |
Table 4.
Survival (n=76).
| Median | 95% CI | |
|---|---|---|
| Time (months) | ||
| Overall survival (n=76) | 11.2 | 8.1–14.4 |
| With secondary resection (n=16) | 23.7 | 12.5–34.8 |
| Without secondary resection (n=60) | 10.1 | 7.8–12.5 |
| Normal CA 19-9 (n=39) | 15.5 | 10.1–21.0 |
| Elevated CA 19-9 (n=30) | 8.5 | 5.5–11.4 |
| Time-to-progression (TTP) (months) | 5.3 | (range 4.3–6.3) |
| Median Follow-up (months) | 11.4 | (range 0.36–86.9) |
Seventy-two out of 76 patients (95%) were evaluable for response to first-line treatment. Complete remission (CR), the best response-to-treatment, was observed in 1 patient (1%), partial remission (PR) in 12 patients (16%), and stable disease (SD) in 46 patients (61%). Thirteen patients (17%) had progressive disease (PD). In total, a tumor control of 78% (CR+PR+SD) was achieved. As soon as response-to-treatment became evident, the corresponding CT scans were regularly presented at the interdisciplinary tumor board of Erlangen University, discussing the feasibility of secondary metastatic resection. The median follow-up period (registered from the first application onwards) was 11.4 months (range 0.4–86.9 months). Median TTP was 5.3 months (95% confidence interval (CI): 4.2–6.5 months). During the total follow-up period, a NED (no evidence of disease) status was achieved in 5 patients (6.6%). The median OS of all patients (n=76) was 11.2 months (95% CI: 8.1–14.4 months). The 1-year survival rate was 48.7%, and the 2-year survival rate was 17.1%.
Prognostic factors (CEA, CA 72-4, CA 19-9 and ECOG-Index)
Prior to initiating first-line treatment, the tumor marker status of CEA, CA 72-4 and CA 19-9 was evaluated as a potential prognostic factor for median survival in a specific subgroup analysis. CEA was determined in 71 out of 76 patients (93%) before the first application was administered; in 25 patients (35%) it exceeded the normal range (≥5 ng/ml). No significant relationship between median survival and the CEA value was observed. The CA 72-4 tumor marker was analyzed for 53 out of 76 patients (70%); in 37 patients (70%) it was elevated (≥4 U/ml). Again, no significant relationship between median survival and the CA 72-4 value was observed. The CA 19-9 value was evaluable in 69 out of 76 patients (91%). Here, 30 patients (43%) presented with an elevated value (≥37 U/ml) at the first date of first-line treatment. Overall, it was evident that patients with an elevated CA 19-9 tumor marker value at treatment initiation had a significantly shorter median survival than the patients whose CA 19-9 values were within the normal range. Patients with an elevated CA 19-9 value (n=30 patients) had a median survival of 8.5 months, whereas the median survival time of the patient group (n=39 patients) with normal CA 19-9 values was 15.5 months (Log Rank [Mantel Cox]: Chi-Square: 8.810, d.f. =1, p≤0.003).
The analysis of patient groups who presented with differing ECOG performance status levels at the first day of first-line treatment revealed a trend (p=0.083) towards a prolonged survival accompanied by a better general condition from treatment initiation onwards. A median survival period of 13.1 months (95% CI 8.8–17.4) was observed in patients with an ECOG index value of 0 plus 1 (n=63 patients). In contrast, patients with an ECOG index =2 (n=13 patients) had a distinctly shorter survival period of only 5.2 months (95% CI 0.8–9.5).
Secondary metastatic resection
After having achieved a downsize of the tumor by first-line treatment, the patients were again presented in the interdisciplinary tumor board of Erlangen University, and 16 out of 76 patients (21.1%) in a good general state of health were subsequently evaluated as potentially resectable. In 10 out of 16 previously unresected patients (62.5%) a curative gastrectomy with extended lymphadenectomy (D2: 40%, D3: 60%) was performed. In 4 of those 10 patients a peritonectomy combined with HIPEC (hyperthermal intraperitoneal chemoperfusion) (25%) was performed in addition to the gastrectomy. A further primarily unresected patient (6.3%) underwent an esophageal resection with gastric interposition and radical lymph node dissection of the upper abdomen and mediastinum. Two primarily resected patients (12.6%) underwent liver segment resection. Furthermore, 1 peritoneal metastasis was resected from the small intestine in another 2 previously resected patients (12.6%). In 1 patient (6.3%) it was only possible to perform an explorative laparotomy due to an advanced peritoneal carcinosis. In 13 out of 16 secondarily resected patients (81.3%) a R0 status could be confirmed by means of a histological examination. After performance of the secondary resection, 1 patient (6.3%) had a histological R1 status and 2 patients (12.5%) had R2 status.
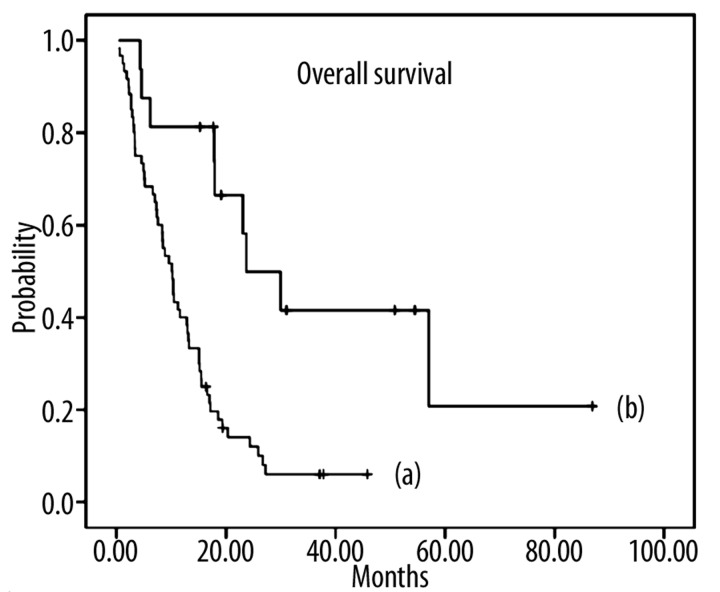
Patients (n=16) who had undergone a secondary resection in addition to palliative chemotherapy achieved a significantly longer median survival of 23.7 months (95% CI: 12.5–34.8) compared to patients (n=60) without secondary resection (10.1 months; 95% CI: 7.8–12.5. Log Rank [Mantel Cox]: X2=13.2, d.f.=1, p≤0.001). Median survival (counted from the date of surgery up to the event of death or end of trial) was 17.7 months (95% CI: 10.8–24.6) in the patient group which underwent secondary resection (n=16). Figure 1 presents the Kaplan-Meier curves for median survival of both subgroups and Figure 2 presents the case of 1 of the secondary resected patients.
Figure 1.
Kaplan-Meier curve: Median overall survival in patients with metastatic adenocarcinoma of the stomach or esophagogastric junction (UICC stage IV) after treatment with high-dose 5-FU/FA as 24h-infusion (AIO regimen) plus irinotecan. (a) Survival curve for patients without secondary resection (n=60) with a median survival of 10.1 months; (b) Survival curve for patients with secondary resection (n=16) with a median survival of 23.7 months.
Figure 2.
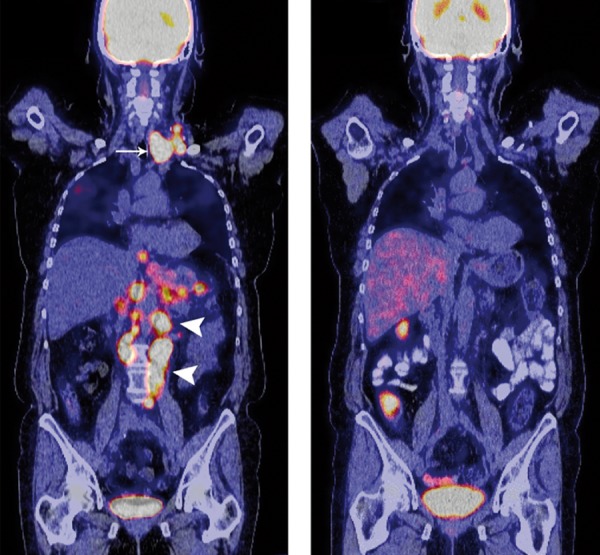
Multi-layer PET-CT scan with intravenous application of 18fluor-desoxyglucosis (FDG), coronary layers: 67-year-old female patient with multiple intra-thoracic and intra-abdominal lymph node metastases and histologically proven gastric adenocarcinoma. (A) Cervical (white arrow) and paraaortal (white arrowheads) lymph node metastases (M1 LYM) in a representative PET-CT scan prior to initiating chemotherapy treatment; (B) After 3 cycles (6 months) of high-dose 5-FU/FA as 24h-infusion (AIO regimen) plus irinotecan, the PET-CT scan reveals a complete remission (CR) of the cervical and paraaortal lymph node metastases without evidence of vital tumor tissue. Subsequently, an extended gastrectomy including a dissection of the cervical and paraaortal lymph nodes (D3) was performed after tumor downsizing (PR). The histopathological examination of the resected tumor sample revealed both an R0 situation (ypT3, ypN3 (18/38), L1, V0, M0, G3) and a tumor regression of 40%.
Discussion
Almost 40% of all gastric cancer patients present at a metastatic stage (UICC stage IV) [32]. In general, the surgical resection of a primary tumor without evidence of metastatic disease (UICC stage I–III) is considered as a potentially curative option, although more than 50% of all curatively resected patients will develop either distant metastases or a local recurrence [33]. For those patients, palliative chemotherapy treatment may offer a prolonged median survival and an improved quality of life. Gastric cancer patients frequently reveal clinical symptoms when presenting at a metastatic stage [34] since 50% are older than 70 years [32]. Thus, an efficient chemotherapy treatment with low toxicity plays a major role in palliative first-line therapy.
The efficacy of combined irinotecan plus 5-FU as an infusional regimen and folinic acid in the treatment of gastroesophageal adenocarcinomas has been investigated in various phase II and phase III trials [11,27,28,35,36]. An overview on these trials is offered in Table 5. The outcome of a multicenter phase II trial conducted by Moehler et al on 56 patients with metastatic or locally advanced gastric cancer, who were treated with 5-FU/FA as a 24-h infusion (modified AIO regimen) plus irinotecan, is promising, and achieved a 43% response rate. Median OS was 10.8 months, and TTP was 4.5 months. In terms of median OS and TTP, the efficacy is comparable with the outcome of our evaluation presented here. Nevertheless, the response rate of 43% documented by Moehler et al is distinctly higher than the response rate of 17% observed in our analysis. This may be due to the rate of locally advanced gastric carcinomas in the trial conducted by Moehler et al (because locally advanced gastric cancer generally yields higher response rates) compared with our patient group that exclusively comprised patients with metastatic gastroesophageal adenocarcinoma (UICC stage IV). In terms of higher grade toxicities (NCI-CTC grade 3+4), Moehler et al observed diarrhea as the predominating symptom of toxicity, occurring in 18% of the participating patients [28]. This is equivalent to the rate of higher grade diarrhea (occurring in 17% of the patients) observed in our own evaluation. A further phase II trial (n=75 patients) investigated the efficacy of irinotecan combined with 5-FU/FA (AIO regimen) at the same dosage but with a reduced application time (FA as 2-h infusion and 5-FU as 22-h infusion instead of 24-h infusion) compared with our analysis. The phase II trial achieved a response rate of 42.4% and a tumor control rate of 84.8%; TTP was 6.5 months, and median OS was 10.7 months [36]. The data on median OS and TTP are comparable with the outcome of our analysis; however, a clearly higher response rate was obtained in the phase II trial. Among higher grade toxicities (NCI-CTC grade 3+4), neutropenia was observed in 26% of the patients, and diarrhea in 27% of the patients [36]. Thus, the phase II trial described a higher grade of toxicity related to diarrhea than the trial by Moehler et al. [28] and our analysis.
Table 5.
Efficacy, toxicity and secondline treatment in studies on irinotecan, 5-FU and folinic acid as combination chemotherapy in gastroesophageal adenocarcinoma.
| Author | Phase | No. of patients | Treatment | UICC IV | Med. OS | ORR | TTP | Higher grade toxicity (3+4) | 2.line | Secondary metastatic resection |
|---|---|---|---|---|---|---|---|---|---|---|
| Dank et al. [27] | III | 333 | AIO/Iri n=170 | 96 % | 9.0 months | 31.8% | 5.0 months | Diarrhea (22%), neutropenia (25%) | n.i. | n.i. |
| Moehler et al. [28] | II | 120 | AIO/Iri n=56 | n.i. | 10.8 months | 43.0% | 4.5 months | Diarrhea (18%), nausea (16%) | n.i. | n.i. |
| Pozzo et al. [36] | II | 115 | AIO/Iri n=75 | 92 % | 10.7 months | 42.4% | 6.5 months | Diarrhea (27%), neutropenia (26%) | n.i. | n.i. |
| Bouché et al. [11] | II | 136 | FOLFIRI n=45 | 100 % | 11.3 months | 40.0% | 6.9 months | Diarrhea (22%), neutropenia (40%) | 51% | 7% |
| Yilmaz et al. [35] | n.i. | 25 | FOLFIRI n=25 | n.i. | 11.6 months | 36.0% | 8.6 months | Diarrhea (16%), neutropenia (20%) | n.i. | n.i. |
| Koucky et al. [53] | n.i. | 76 | AIO/Iri n=76 | 100 % | 11.2 months | 17.0% | 5.3 months | Diarrhea (17%), infection (12%) | 38% | 21 % |
no. – number; Iri: irinotecan; UICC – International Union Against Cancer; med. OS – median overall survival; ORR – overall response rate; TTP – time to progression; 2.line – second line therapy; n.i. – no information.
Other phase II trials employed the FOLFIRI schedule instead of the AIO regimen plus irinotecan and achieved response rates of 36–40% accompanied by a TTP of 6.9–8.6 months [11,35]. In terms of response, the FOLFIRI schedule seems to be comparable with the AIO regimen plus irinotecan, although the TTP was shorter for the AIO regimen plus irinotecan, amounting to 4.5–6.5 months [27,28,36].
A median OS of 11.3–11.6 months was achieved by the FOLFIRI regimen, which is equivalent to the outcome of our analysis, with a median OS of 11.2 months [11,35]. Likewise, the 1-year survival rate of 48.7% and 2-year survival rate of 17.1%, which could be verified by our evaluation for palliative first-line treatment, are both comparable with the results of a phase II trial based on the FOLFIRI regimen which demonstrates a 1-year survival rate of 48% and a 2-year survival rate of 17.8% [35]. Higher grade toxicities (NCI-CTC grade 3+4) in the form of neutropenia were observed in 20–40% of the patients, and in the form of diarrhea in 16–22% of the patients. As far as diarrhea is concerned, the toxicity profiles of the FOLFIRI regimen and the AIO regimen plus irinotecan are comparable. However, the FOLFIRI regimen produces a higher frequency of alopecia (NCI-CTC grade ≥2), which occurred in 12–13% of the patients [11,35]. In contrast, we observed alopecia (NCI-CTC grade ≥2) in only 2% of our patients, which is comparable to the outcome of the trial by Moehler et al with alopecia (grade ≥3) occurring in 5% of the patients [28].
The evaluation of our data demonstrates an overall response rate of 17%, consisting of 1 case of complete remission (1%) and 12 cases of partial remission (16%). This response rate is distinctly lower than the response rates of comparable phase II trials on the AIO regimen plus irinotecan (Table 5). The main reason for this difference might be seen in the variable percentage of UICC stage IV patients in the patient population of different studies. In contrast to various other trials, our analysis exclusively comprised patients with histologically proven metastatic disease (UICC stage IV=100%), which generally is a patient collective with a poor prognosis. Table 5 demonstrates that other trials on the AIO regimen plus irinotecan either included a patient collective which was inhomogeneous in terms of the UICC stage (UICC IV, valid for 92–96%), or that the authors completely refrained from publishing the UICC stage.
Despite achieving a relatively low response rate of 17%, a median OS of 11.2 months, accompanied by 1- and 2-year survival rates of 48.7% and 17.1%, respectively, was observed in our analysis. This might be caused by employing a sequential treatment, a therapy method which is increasingly gaining in importance in clinical daily routine [37–39]. According to the data of our evaluation collective, 38% of the patients received second-line treatment after having developed progressive disease during first-line treatment, 8% received third-line treatment, and 1% fourth-line treatment. Another reason might be seen in the performance of a secondary resection subsequent to first-line treatment. In total, this surgical intervention yielded a clear prolongation of median OS. The outcome of our subgroup analysis demonstrates that the secondarily resected patients (n=16) had a significantly prolonged median survival of 23.7 months, whereas the median survival of unresected patients was only 10.1 months (p<=0.001). In 6.6% of the patients a NED status was recorded during the observation period. The sequential treatment factor and the interdisciplinary concept of secondary metastatic resection factor are 2 aspects which remain unconsidered in most trials (Tables 5 and 6).
Table 6.
Efficacy and toxicity in phase II and III studies on chemotherapy treatment of gastroesophageal adenocarcinoma.
| Author | Phase | No. of patients | Treatment | UICC IV | Med. OS | ORR | TTP | Higher grade toxicity (3+4) | Secondary metastatic resection |
|---|---|---|---|---|---|---|---|---|---|
| Van Cutsem et al. [19] | III | 445 | DCF, n=221 | 96% | 9.2 months | 37% | 5.6 months | Neutropenia (82%), diarrhea (19%) | n.i. |
| Al-Batran et al. [43] | II | 59 | FLOT, n=59 | 93% | 11.1 months | 58% | 5.2 months | Neutropenia (48%), diarrhea (15%) | n.i. |
| Ross et al. [15] | III | 580 | ECF, n=289 | 53% | 9.4 months | 42% | 7.0 months | Neutropenia (32%), nausea (11%), | n.i. |
| Lutz et al. [12] | II | 145 | PLF, n=51 | 88% | 9.7 months | 46% | 6.1 months | Neutropenia (20%), alopecia (18%) | n.i. |
| Cunningham et al. [14] | III | 1002 | ECF, n=263 | 80% | 9.9 months | 41% | 6.2 months | Neutropenia (42%), alopecia (44%) | n.i. |
| Al-Batran et al. [16] | III | 220 | PLF, n=108 | 91% | 8.8 months | 25% | 3.9 months | Neutropenia (15%), nausea (9%) | n.i. |
| Roth et al. [54] | II | 121 | DCF, n=41 | 95% | 10.4 months | 37% | 4.6 months | Neutropenia (80%), alopecia (41%) | n.i. |
| Bang et al. [20] | III | 594 | H+CT, n=298 | 97% | 13.8 months | 47% | 6.7 months | Neutropenia (27%), anemia (12%) | n.i. |
no. – number; H+CT – trastuzumab plus 5-fluorouracil or capecitabine and cisplatin; UICC – International Union Against Cancer; med. OS – median overall survival; ORR – overall response rate; TTP – time to progression; n.i. – no information.
A comparison between the AIO regimen plus irinotecan and current cisplatin-based standard regimens for the treatment of metastatic gastroesophageal cancer demonstrated that irinotecan-based regimens achieved a similar level of efficacy accompanied by a better toxicity profile. Two phase II trials comprising a total of 159 patients, which investigated the PLF regimen (5-FU/FA as 24-h infusion plus cisplatin) in advanced or metastatic gastric adenocarcinomas, yielded response rates of 25–46% and 8.8–9.7 months median OS. This is comparable with the efficacy of irinotecan-based regimens in other trials (Table 5), accompanied by a more unfavorable toxicity profile (neutropenia of NCI-CTC grade 3+4 in 15–20%, and alopecia grade 3+4 in 18%) [12,16].
In a large phase III trial (n=221 patients), the DCF regimen (docetaxel, cisplatin, 5-FU) demonstrated a similar activity in terms of TTP (5.6 months) and median OS (9.2 months). The 1-year survival rate was 40% and the 2-year survival rate was 18%, which is comparable with our results. However, more higher grade toxicities (NCI-CTC grade 3+4) were observed under the DCF regimen than in our analysis – 82% of the participating patients suffered from neutropenia and 65% from leucopenia, 19% of the patients had diarrhea and 14% had either nausea or vomiting. There were 6 (2.7%) toxicity-related deaths under the DCF regimen [19], whereas no toxicity-related death was observed in our own evaluation. As the addition of taxanes to 5-FU and cisplatin, improved all primary and secondary endpoints of the V325 trial, taxanes were admitted to the market for palliative first-line treatment of gastroesophageal adenocarcinoma in 2006 [17–19]. Further phase II trials tried to improve the toxicity profile of the DCF regimen by either modifying the application schedules or including other combined agents, maintaining the same efficacy level. The Gastro-Tax-1-phase II trial applied a reduced dose of docetaxel (40 mg/m2 every 2 weeks) due to the occurrence of higher grade toxicities, which yielded a dose reduction of <80% in 80% of the participating patients [40]. In other trials, cisplatin was exchanged for the less toxic and equally active oxaliplatin [14,41,42]. This reduced the occurrence of febrile neutropenias to <5% and achieved an overall response of 44–53% [40–42].
Promising results were achieved with a taxane-based combination schedule of a phase II trial conducted by Al-Batran et al, in which 59 patients with locally advanced or metastatic gastric cancer were enrolled and subsequently treated with combined docetaxel, oxaliplatin and 5-FU/FA (FLOT). The outcome of this trial presented a very high response rate of 57.7% and a median OS of 11.1 months. As higher grade toxicities (NCI-CTC grade 3+4), neutropenia and diarrhea were observed in 48% and 15% of the patients, respectively. Second-line treatment was given to 50% of the enrolled patients [43], a fact that remains unmentioned in the study protocol, but seems to be a normal procedure in clinical routine.
Furthermore, the results of 2 large phase III trials (n=552 patients) that applied the ECF schedule (epirubicin, cisplatin, 5-FU) in patients with gastroesophageal adenocarcinomas are comparable with the outcome achieved by trials based on the AIO regimen plus irinotecan in terms of median OS (ranging from 9.4 to 9.9 months). The ECF regimen yielded response rates from 41–42%, accompanied by higher grade toxicities (NCI-CTC grade 3+4) in the form of neutropenia in 32–42%. The percentage of alopecia (≥grade 2), which was observed under ECF treatment and amounted to 44–59%, seems particularly important [14,15].
The high response rates of 37–58% that were achieved with the DCF, ECF, PLF and FLOT regimens, and which are contrary to our own evaluation (ORR: 17%), may be primarily due to the enrolment of inhomogeneous patient collectives that widely differed in terms of UICC stage assignment. In trials based on the DCF, ECF, PLF and FLOT regimens, the proportion of patients with proven metastatic disease (UICC stage IV) ranges from 53% to 96% (Table 6). In terms of efficacy (median OS), the DCF, ECF, PLF and FLOT schedules are comparable with irinotecan-based regimens. Nevertheless, DCF and ECF in particular yield distinctly more unfavorable toxicity profiles than the AIO regimen plus irinotecan. The FLOT schedule especially seems to offer a promising taxane-based alternative, as it yields higher efficacy (ORR: 57.7%) accompanied by a better toxicity profile than the DCF regimen.
Primary metastatic resection is considered as an established standard treatment in colorectal cancer [44–46]. Few trials have investigated metastatic resection in gastric cancer [47,48]. An overview by Lehnert et al demonstrated that the 5-year survival rate of 195 gastric cancer patients who underwent liver segment resections was 20%; thus, because of extremely low morbidity and mortality rates, surgical interventions seem to offer a potentially curative treatment option [33].
Likewise, the treatment procedure of performing a secondary resection after having achieved downsizing of the metastases through palliative chemotherapy has begun to be established in colorectal cancer [49–52]; however, this procedure has not yet been established as a standardized treatment procedure in metastatic gastric cancer. Due to the heterogeneous metastatic patterns (frequently including peritoneal metastases) of gastric cancer, the performance of a secondary metastatic resection with a curative intent appears to be clearly more difficult. According to our analysis, only 9% of the patients with metastatic adenocarcinoma of the stomach or esophagogastric junction revealed “liver-only metastases”. Nevertheless, the data of our analysis demonstrate that an interdisciplinary procedure comprising palliative chemotherapy treatment followed by secondary resection could significantly prolong the median survival from 10.1 months to 23.7 months (p≤0.001). Using this procedure, the 11.2 months median OS of our total population was achieved despite the relatively low response rate of 17%; this outcome was certainly positively influenced by the performance of secondary metastatic resections. In spite of generally satisfying response rates of the phase II and phase III trials (Tables 5 and 6), the performance of secondary metastatic resections is rarely mentioned nor analysed.
Conclusions
Due to a promising median OS of 11.2 months accompanied by a good tolerability, high dose 5-FU/FA as 24-h infusion (AIO regimen) plus irinotecan may be considered as active and well tolerated. It offers an efficient platin-free option for the first-line treatment of metastatic adenocarcinoma of the stomach or esophagogastric junction, in particular for a multimorbid patient collective. This analysis also demonstrates that secondary metastatic resection after downsizing by palliative chemotherapy yields a significant survival advantage for patients with metastatic gastroesophageal adenocarcinoma, due to interdisciplinary teamwork. The patient collective who underwent a secondary metastatic resection demonstrated a significantly prolonged median survival of 23.7 months compared with the unresected patient group whose median survival was only 10.1 months. After a short median follow-up of 11.4 months, 6.6% of the patients (n=5) were still disease-free and have a curative option. These aspects should be intensively evaluated in future prospective trials with a longer follow-up.
Acknowledgements
The authors are indebted to Mr. K. Mach, Cancer Registry of the Department of Internal Medicine 1, and the nurses of the outpatients Department of the Internal Medicine 1 of Erlangen University, Germany.
Footnotes
Source of support: This study was partially supported by a grant from Pfizer Pharma GmbH, Germany
References
- 1.Krebs in Deutschland 2003–2004. Häufigkeiten und Trends. 6. überarbeitete Auflage. Robert Koch-Institut (Hrsg) und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e. V. (Hrsg). Berlin, 2008
- 2.Ferlay J, Autier P, Boniol M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–92. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Bollschweiler E, Wolfgarten E, Gutschow C, Hölscher AH. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer. 2001;92:549–55. doi: 10.1002/1097-0142(20010801)92:3<549::aid-cncr1354>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Ilson DH. Phase II trial of weekly irinotecan/cisplatin in advanced esophageal cancer. Oncology (Williston Park) 2004;18(14 Suppl 14):22–25. [PubMed] [Google Scholar]
- 6.Alici S, Kaya S, Izmirli M, et al. Analysis of survival factors in patients with advanced-stage gastric adenocarcinoma. Med Sci Monit. 2006;12(5):CR221–29. [PubMed] [Google Scholar]
- 7.Heise K, Bertran E, Andia ME, Ferreccio C. Incidence and survival of stomach cancer in a high-risk population of Chile. World J Gastroenterol. 2009;15:1854–62. doi: 10.3748/wjg.15.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glimelius B, Ekström K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163–68. doi: 10.1023/a:1008243606668. [DOI] [PubMed] [Google Scholar]
- 9.Pyrhönen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995;71:587–91. doi: 10.1038/bjc.1995.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database of Systematic Reviews. 2010;3:CD004064. doi: 10.1002/14651858.CD004064.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Bouché O, Raoul JL, Bonnetain F, et al. Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus irinotecan in patients with previously untreated metastatic gastric cancer: a Federation Francophone de Cancerologie Digestive Group Study – FFCD 9803. J Clin Oncol. 2004;22:4319–28. doi: 10.1200/JCO.2004.01.140. [DOI] [PubMed] [Google Scholar]
- 12.Lutz MP, Wilke H, Wagener DJ, et al. Weekly infusional high-dose fluorouracil (HD-FU), HD-FU plus folinic acid (HD-FU/FA), or HD-FU/FA plus biweekly cisplatin in advanced gastric cancer: randomized phase II trial 40953 of the European Organisation for Research and Treatment of Cancer Gastrointestinal Group and the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2007;25:2580–85. doi: 10.1200/JCO.2007.11.1666. [DOI] [PubMed] [Google Scholar]
- 13.Kundel Y, Purim O, Figer A, et al. Weekly infusional high-dose 5-fluorouracil and leucovorin and biweekly cisplatin: a convenient treatment option in advanced gastric cancer. Med Sci Monit. 2008;14:190–95. [PubMed] [Google Scholar]
- 14.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 15.Ross P, Nicolson M, Cunningham D, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol. 2002;20:1996–2004. doi: 10.1200/JCO.2002.08.105. [DOI] [PubMed] [Google Scholar]
- 16.Al-Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–42. doi: 10.1200/JCO.2007.13.9378. [DOI] [PubMed] [Google Scholar]
- 17.Ajani JA, Moiseyenko VM, Tjulandin S, et al. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25:3210–16. doi: 10.1200/JCO.2006.08.3956. [DOI] [PubMed] [Google Scholar]
- 18.Ajani JA, Moiseyenko VM, Tjulandin S, et al. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25:3205–9. doi: 10.1200/JCO.2006.10.4968. [DOI] [PubMed] [Google Scholar]
- 19.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–97. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 20.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 21.Köhne CH, Catane R, Klein B, et al. Irinotecan is active in chemonaive patients with metastatic gastric cancer: a phase II multicentric trial. Br J Cancer. 2003;89:997–1001. doi: 10.1038/sj.bjc.6601226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Köhne CH, Van Cutsem E, Wils J, et al. Phase III study of weekly high-dose infusional fluorouracil plus folinic acid with or without irinotecan in patients with metastatic colorectal cancer: European Organisation for Research and Treatment of Cancer Gastrointestinal Group Study 40986. J Clin Oncol. 2005;23:4856–65. doi: 10.1200/JCO.2005.05.546. [DOI] [PubMed] [Google Scholar]
- 23.Lavelle F, Bissery MC, André S, et al. Preclinical evaluation of CPT-11 and its active metabolite SN-38. Semin Oncol. 1996;23(1 Suppl 3):11–20. [PubMed] [Google Scholar]
- 24.Farhat FS. A general review of the role of irinotecan (CPT11) in the treatment of gastric cancer. Med Oncol. 2007;24:137–46. doi: 10.1007/BF02698032. [DOI] [PubMed] [Google Scholar]
- 25.Enzinger PC, Kulke MH, Clark JW, et al. A phase II trial of irinotecan in patients with previously untreated advanced esophageal and gastric adenocarcinoma. Dig Dis Sci. 2005;50:2218–23. doi: 10.1007/s10620-005-3038-2. [DOI] [PubMed] [Google Scholar]
- 26.Stickel F, Jüngert B, Brueckl V, et al. Weekly high-dose 5-fluorouracil as 24-h infusion and folinic acid (AIO) plus irinotecan as second- and third-line treatment in patients with colorectal cancer pre-treated with AIO plus oxaliplatin. Anticancer Drugs. 2003;14:745–49. doi: 10.1097/00001813-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Dank M, Zaluski J, Barone C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19:1450–57. doi: 10.1093/annonc/mdn166. [DOI] [PubMed] [Google Scholar]
- 28.Moehler M, Eimermacher A, Siebler J, et al. Randomised phase II evaluation of irinotecan plus high-dose 5-fluorouracil and leucovorin (ILF) vs. 5-fluorouracil, leucovorin, and etoposide (ELF) in untreated metastatic gastric cancer. Br J Cancer. 2005;92:2122–28. doi: 10.1038/sj.bjc.6602649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolff K, Wein A, Reulbach U, et al. Weekly high-dose 5-fluorouracil as a 24-h infusion and sodium folinic acid (AIO regimen) plus irinotecan in patients with locally advanced nonresectable and metastatic adenocarcinoma or squamous cell carcinoma of the oesophagus: a phase II trial. Anticancer Drugs. 2009;20:165–73. doi: 10.1097/CAD.0b013e32831f8ec9. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. WHO handbook for reporting results for cancer treatment. 48. WHO Offset Publication; Geneva: 1979. [Google Scholar]
- 31.Greenwood M. Rep. Pub. Hlth. Med. Subj. 33. London: HM Stationary Office; 1926. The natural duration of cancer. [Google Scholar]
- 32.Hundahl SA, Menck HR, Mansour EG, Winchester DP. The National Cancer Data Base report on gastric carcinoma. Cancer. 1997;80:2333–41. doi: 10.1002/(sici)1097-0142(19971215)80:12<2333::aid-cncr15>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 33.Lehnert T, Rudek B, Buhl K, Golling M. Surgical therapy for loco-regional recurrence and distant metastasis of gastric cancer. Eur J Surg Oncol. 2002;28:455–61. doi: 10.1053/ejso.2002.1260. [DOI] [PubMed] [Google Scholar]
- 34.Ajani JA. Recent Developments in Cytotoxic Therapy for Advanced Gastric or Gastroesophageal Carcinoma: The Phase III Trials. Gastrointest Cancer Res. 2007;1(2 Suppl):16–21. [PMC free article] [PubMed] [Google Scholar]
- 35.Yilmaz U, Oztop I, Alacacioglu A, et al. Irinotecan combined with infusional 5-fluorouracil and high-dose leucovorin for the treatment of advanced gastric carcinoma as the first-line chemotherapy. Chemotherapy. 2006;52:264–70. doi: 10.1159/000094769. [DOI] [PubMed] [Google Scholar]
- 36.Pozzo C, Barone C, Szanto J, et al. Irinotecan in combination with 5-fluorouracil and folinic acid or with cisplatin in patients with advanced gastric or esophageal-gastric junction adenocarcinoma: results of a randomized phase II study. Ann Oncol. 2004;15:1773–81. doi: 10.1093/annonc/mdh473. [DOI] [PubMed] [Google Scholar]
- 37.Assersohn L, Brown G, Cunningham D, et al. Phase II study of irinotecan and 5-fluorouracil/leucovorin in patients with primary refractory or relapsed advanced oesophageal and gastric carcinoma. Ann Oncol. 2004;15:64–69. doi: 10.1093/annonc/mdh007. [DOI] [PubMed] [Google Scholar]
- 38.Thuss-Patience PC, Kretzschmar A, Deist T, et al. Irinotecan versus best supportive care (BSC) as second-line therapy in gastric cancer: A randomized phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) J Clin Oncol. 2009;27:15s. doi: 10.1016/j.ejca.2011.06.002. (Suppl; abstr 4540) [DOI] [PubMed] [Google Scholar]
- 39.Rivera F, Vega-Villegas ME, López-Brea MF. Chemotherapy of advanced gastric cancer. Cancer Treat Rev. 2007;33:315–24. doi: 10.1016/j.ctrv.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Lorenzen S, Weigert N, Heinemann V, et al. Docetaxel, cisplatin and leucovorin/fluorouracil in first-line advanced gastric cancer and adenocarcinoma of the esophagogastric junction: Results of the phase II GASTRO-TAX-1 trial. Journal of Clinical Oncology; 2007 ASCO Annual Meeting Proceedings Part I; Jun 20, 2007. p. 4561. Supplement. [Google Scholar]
- 41.Ajani JA, Phan A, Ho L, et al. Phase I/II trial of docetaxel plus oxaliplatin and 5-fluorouracil (D-FOX) in patients with untreated, advanced gastric or gastroesophageal cancer. Journal of Clinical Oncology; 2007 ASCO Annual Meeting Proceedings Part I; Jun 20, 2007. p. 4612. Supplement. [Google Scholar]
- 42.Al-Batran S, Hartmann JT, Hofheinz R, et al. Modified FOLFOX in combination with docetaxel for patients with metastatic adenocarcinoma of the stomach or gastroesophageal junction: A multicenter phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Journal of Clinical Oncology; 2007 ASCO Annual Meeting Proceedings Part I; Jun 20, 2007. p. 4545. Supplement. [Google Scholar]
- 43.Al-Batran SE, Hartmann JT, Hofheinz R, et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2008;19:1882–87. doi: 10.1093/annonc/mdn403. [DOI] [PubMed] [Google Scholar]
- 44.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 45.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Cancer. 1996;77:1254–62. [PubMed] [Google Scholar]
- 46.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–21. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ambiru S, Miyazaki M, Ito H, et al. Benefits and limits of hepatic resection for gastric metastases. Am J Surg. 2001;181:279–83. doi: 10.1016/s0002-9610(01)00567-0. [DOI] [PubMed] [Google Scholar]
- 48.Miyazaki M, Itoh H, Nakagawa K, et al. Hepatic resection of liver metastases from gastric carcinoma. Am J Gastroenterol. 1997;92:490–93. [PubMed] [Google Scholar]
- 49.Wein A, Riedel C, Köckerling F, et al. Impact of surgery on survival in palliative patients with metastatic colorectal cancer after first line treatment with weekly 24-hour infusion of high-dose 5-fluorouracil and folinic acid. Ann Oncol. 2001;12:1721–27. doi: 10.1023/a:1013521430755. [DOI] [PubMed] [Google Scholar]
- 50.Masi G, Loupakis F, Pollina L, et al. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg. 2009;249:420–25. doi: 10.1097/SLA.0b013e31819a0486. [DOI] [PubMed] [Google Scholar]
- 51.Folprecht G, Grothey A, Alberts S, et al. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol. 2005;16:1311–19. doi: 10.1093/annonc/mdi246. [DOI] [PubMed] [Google Scholar]
- 52.Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 53.Koucky K, Boxberger F, Albrecht H, et al. Downsizing after palliative systemic chemotherapy with weekly high-dose 5-fluorouracil (5-FU) as a 24h-infusion and sodium folinic acid (AIO regimen) plus irinotecan in patients with metastatic adenocarcinomas of the stomach or the gastro-esophageal junction followed by secondary metastatic resection. J Clin Oncol. 2009;27 (Suppl; abstr e15585) [Google Scholar]
- 54.Roth AD, Fazio N, Stupp R, et al. Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2007;25:3217–23. doi: 10.1200/JCO.2006.08.0135. [DOI] [PubMed] [Google Scholar]