Summary
Coexistent carotid artery stenosis (CS) and multivessel coronary artery disease (CAD) is not infrequent. One in 5 patients with multivessel CAD has a severe CS, and CAD incidence reaches 80% in those referred for carotid revascularization. We reviewed treatment strategies for concomitant severe CS and CAD.
We performed a literature search (MEDLINE) with terms including carotid artery stenting (CAS), coronary artery bypass grafting (CABG), carotid endarterectomy (CEA), stroke, and myocardial infarction (MI).
The main therapeutic option for CS-CAD has been (simultaneous or staged) CEA-CABG. This, however, is associated with a high risk of MI (in those with CEA prior to CABG) or stroke (CABG prior to CEA), and the cumulative major adverse event rate (MAE – death, stroke or MI) reaches 10–12%. With increasing adoption of CAS, a sequential strategy of CAS followed by CABG has emerged. Registries (usually single-centre) indicate an MAE rate of ≈7% for CAS followed by CABG (frequently after >30 days, due to double antiplatelet therapy). Recently, 1-stage CAS-CABG has been introduced. This involves different antiplatelet regimens and, in some centers, preferred off-pump CABG, with a cumulative MAE of 1.4–4.5%.
No randomized trial comparing different treatment strategies in CS-CAD has been conducted, and thus far reported series are prone to selection/reporting bias. In addition to the established surgical treatment (CEA-CABG, sequential/simultaneous), hybrid revascularization (CAS-CABG) is emerging as a viable therapeutic option. Larger, preferably multi-centre, studies are required before this can become widely applied.
Keywords: carotid artery stenosis, coronary artery disease, carotid endarterectomy, carotid artery stenting, coronary artery bypass grafting, myocardial infarction, stroke
Coexistent Carotid And Coronary Disease – the Problem
Severe carotid, vertebral or subclavian stenosis is found in 16.6% of patients with 3-vessel coronary artery disease (CAD) [1], whereas multivessel coronary disease and significant carotid stenosis only coexists in 2.8–22% of patients undergoing coronary artery bypass grafting (CABG) [2,3]. The frequency of carotid artery disease (stenosis >50%) increases from 5% in patients with 1-vessel CAD up to 40% in patients with left main coronary artery stenosis [4]. On the other hand, among patients who underwent elective carotid stenting, concomitant CAD is found in 66% [5] – 77% [6] of cases. The majority of these patients required coronary revascularization, either percutaneous coronary intervention (PCI) or CABG (7%), and the rest were treated conservatively, while the others (16%) already had a history of coronary revascularization [6]. Severe coronary disease is found in up to 37% of carotid endarterectomy (CEA) patients [7], and myocardial infarction (MI) is the most common cause of death after CEA [7,8].
The risk of stroke associated with cardiac surgery is estimated at about 2% in patients without significant (<50%) carotid stenosis and it increases with the severity of carotid disease [9]. The risk doubles in patients with unilateral carotid stenosis (50–99%), triples in those with bilateral carotid stenosis, and increases to almost 12% in patients with unilateral occlusion [9]. The stroke risk during CABG increases with age and rises to 9% in patients above age 80 [9]; in the elderly the CABG-associated mortality reaches 13% [10]. Another independent risk factor for neurological complications associated with CABG is female sex; the risk of neurological complications might be 1.6-fold higher for women than for men [11].
Another important issue is that about 60% of strokes associated with cardiac surgery are of embolic origin [12] as determined from examination of material from carotid plaques, aortic arch and ascending aorta (most probably mobilized during aortic clamping). In addition, systemic hypotension during on-pump CABG, combined with stenoses of extracranial arteries, may lead to brain hypoperfusion, which is responsible for 8.8% of strokes [12].
How to Treat a Patient with Severe Coronary and Carotid Disease?
Concomitant carotid and coronary disease is a common problem and an important risk factor of CABG-associated stroke. The optimal treatment strategy in coexistent severe carotid and coronary disease remains undefined, and this is reflected in the guidelines on CABG [13], carotid endarterectomy (CEA) [14] and carotid artery stenting (CAS) [15,16].
CEA and CABG: the Data
CEA and CABG can be performed either at one stage (synchronous/simultaneous CEA-CABG) or as a staged (two-staged) procedure with CEA preceding CABG or CABG preceding CEA. In addition, CABG performed off-pump may have advantages in context of required carotid revascularization.
Current surgical guidelines [13] indicate that CEA is usually recommended before or concomitant with CABG in patients with a symptomatic carotid stenosis or asymptomatic carotid stenosis of 80% or more (Class IIa/C) [13], and it is recommend that the revascularization strategy should be based on the individual risk profile of each patient [14].
The risk of stroke associated with synchronous CEA and CABG is twice (3.9% vs. 1.7%) that of isolated CABG (without coexistent carotid disease) [17].
A systematic review of 97 studies in 8972 patients treated with CEA and CABG (years 1972–2002) revealed that the risk of stroke or death is highest when both procedures are performed simultaneously (8.7%) and lowest when these procedures are staged (6.1%). However, the risk of myocardial infarction is highest in staged procedures, estimated at 6.5%. In the analyzed studies, no matter whether the procedures (CEA and CABG) were synchronous or staged, the overall rate of stroke, death or MI was 10.2–11.5% at 30 days [18]. CEA and OPCAB seem to be associated with the lowest complication rate (3.6%) of stroke, MI and death at 30-day observation [19].
These results indicate the clinical need for alternative (safer) revascularization methods for patients with coexistent severe carotid and coronary disease. As far as carotid revascularization alone is concerned, CAS with use of neuroprotection system devices (NPDs) seems to be a safe and effective alternative to CEA, especially for high surgical risk patients [20]. CAS and CEA have similar short- and long-term outcomes [21].
CAS and CABG: the Data
CEA/CAS guidelines [14,15] are unclear about the role of carotid artery stenting prior to CABG; it remains to be shown whether CAS could be the therapy of choice for patients with coronary and carotid artery disease when treated simultaneously[14]. This is indicated as an option for patients in whom CABG can be deferred for 4–5 weeks (because of clopidogrel therapy after CAS) [15]. Clopidogrel should be withdrawn 5–7 days before CABG. Dual antiplatelet therapy: acetylsalicylic acid (continued lifelong) and clopidogrel is recommended for at least 4 weeks and preferably 3 months after CAS, because of the stent endothelization, which takes 28–96 days [14–16].
Recently published guidelines [16] recommend that revascularization, either by CEA or CAS with embolic protection, is a reasonable procedure for use before or concurrent with CABG in symptomatic patients with carotid stenosis >80% (Class IIa/C), but the need for simultaneous or staged carotid and coronary revascularization in asymptomatic patients remains to be proven (Class IIb/C).
Two possible hybrid revascularization strategies are staged CAS-CABG (1st stage CAS and then CABG in at least 5 weeks, Figures 1, 2), and simultaneous 1-stage CAS-CABG (CAS and then CABG on the same day, Figures 3–6).
Figure 1.
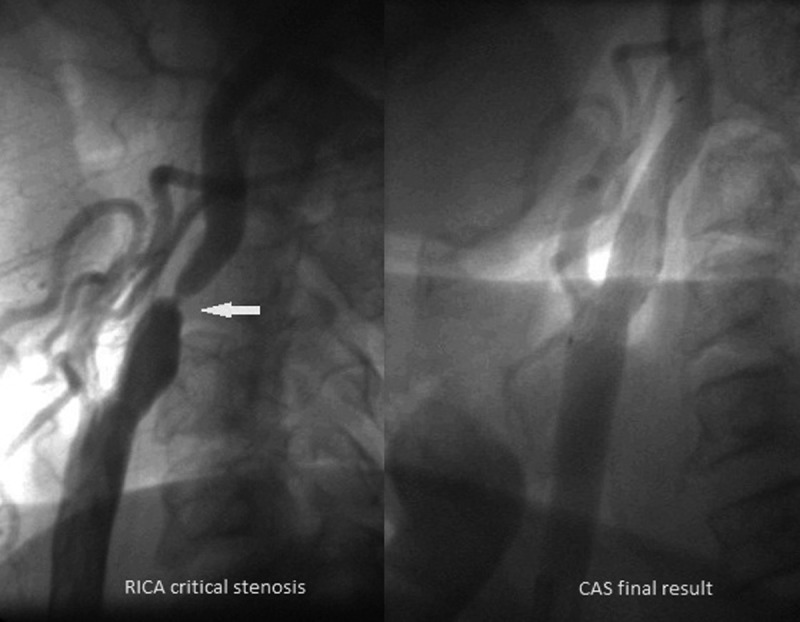
An example of the patient with critical RICA stenosis before and after successful CAS procedure in a patient after left hemisphere stroke (occluded LICA) accepted for staged CAS – CABG strategy.
Figure 2.
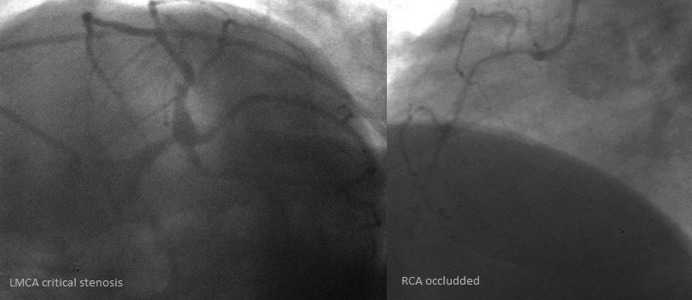
Coronary angiogram of the same patient (CCS class II angina), a critical stenosis of the left main coronary artery (LMCA) and occlusion of the right coronary artery (RCA).
Figure 3.
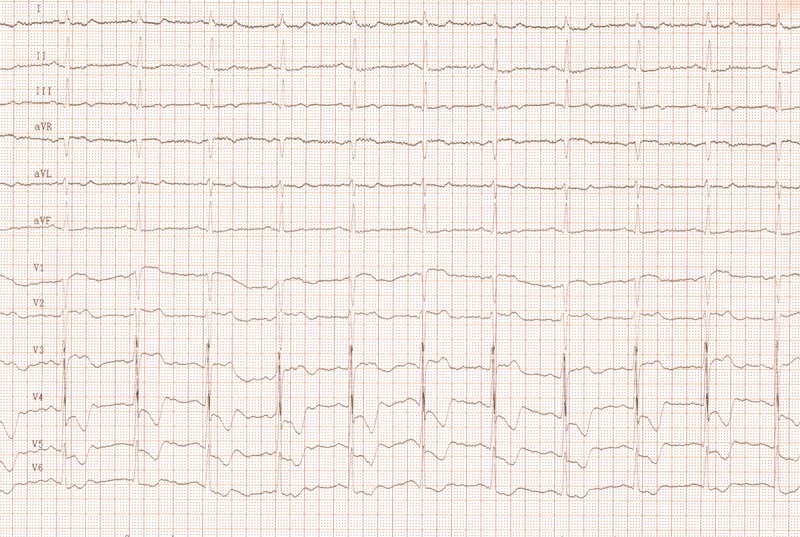
An example of a coronary unstable patient with multivessel coronary artery disease and recurrent TIAs. An electrocardiogram performed during CAS. CAS was immedaitelly followed by CABG.
Figure 6.
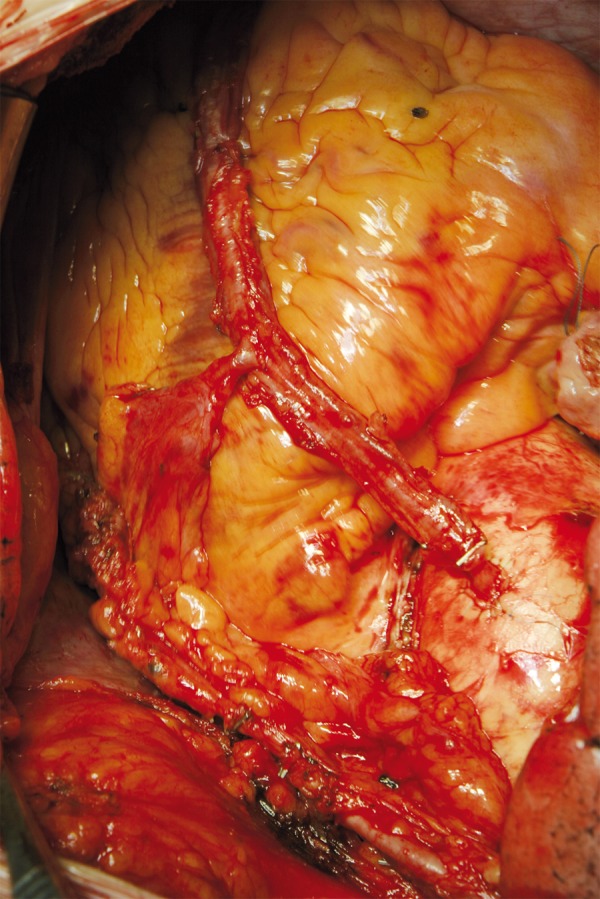
The patient was operated off pump, a total arterial revascularization (TAMR) was performed: LIMA-LAD, Radial Artery (RA): Y-anastomosis.
In a single-center registry of staged CAS-CABG including 356 neurologically asymptomatic patients, the overall rate of death, stroke and myocardial infarction was 6.7% at 30 days after CABG [22]. In the waiting period (mean 22 days) between CAS and CABG, there were 1 major stroke (intracranial hemorrhage), 4 (1.1%) ipsilateral minor strokes, and 8 (2.2%) transient ischemic attacks (TIA). The cardiac complication rate was 2.3%: 1 cardiac death (0.3%), 2 (0.6%) MIs, and 5 (1.4%) episodes of unstable angina. In this study [22] NPDs were used in only 40% of cases.
A meta-analysis of 11 studies including 760 patients (356 from a single center) [23], who were mainly (87%) asymptomatic revealed a 30-day outcome of 9.4% (death, stroke and myocardial infarction) for staged CAS-CABG (both on- and off-pump procedures); not significantly different from synchronous or staged surgical strategies (10.2–11.5%) [18]. The period between CAS and CABG ranged between 2 and 70 days in studies included in the meta-analysis [23]. The overall 30-day outcome (from CAS to 30 days after CABG) suggests that this form of hybrid revascularization might be a viable alternative to CEA-CABG because it provides similar safety profiles to ‘conventional’ surgical strategies. CAS combined with CABG could assume a much greater role in the treatment of patients with coexistent carotid and coronary disease due to its less invasive nature [23]. Nevertheless, CAS patients undergoing CABG are suspected to be at increased risk of bleeding complications because of dual antiplatelet therapy (acetylsalicylic acid indefinitely and clopidogrel for at least a month after CAS). The studies included in the meta-analysis [23] were heterogeneous in terms of antiplatelet regimen (different timing of dual antiplatelet therapy: aspirin+ clopidogrel or aspirin+ticlopidine or additional GPIIbIIIa infusion, clopidogrel was either stopped prior to CABG or continued during CABG) [23]. However, in this meta-analysis [23] only 7 major bleedings (0.9%) in 760 patients (1 intracranial hemorrhage, 4 chest bleedings, 1 cardiac tamponade, 1 groin hemorrhage) were reported. In the analyzed studies the NPDs were not routinely used; this might have overestimated the stroke rate. In another observation of 20 patients undergoing CAS before CABG, the time interval between procedures ranged from 1–62 days, and antiplatelet strategies were different; on long-term observation only 1 stroke occurred (677 days after procedure), and no deaths or myocardial infarcts were noted [24].
In the SHARP study [25], in 4 high-volume centers, 101 consecutive patients with severe coronary and carotid disease underwent simultaneous CAS and CABG. The 30-day cumulative incidence of stroke, MI or death was 4%: 2 patients (2%) had stroke immediately after CAS before CABG, and another 2 patients died (2%) in the post-operative period. A further 3 patients died between the 31st day and the 12th month after the procedure. The very first observation [26] (during years 1995–2005) of simultaneous CAS – CABG (including redo-CABG procedures in 10% of patients) performed in a group of 30 extremely high surgical risk patients indicated that the in-hospital rate for stroke and death of 10% [26] and myocardial infarction of 3% [26] was comparable to the cumulative outcome of simultaneous surgical strategy (11.5%) derived from the meta-analysis of CEA-CABG [18]. In another registries of simultaneous CAS – CABG performed within 1 day, the 30-day complication rate of 1.4–4.5% [27,28] (Table 1) was even lower. These initial results of simultaneous hybrid CAS-CABG indicate that this form of revascularization might be associated with the lowest event rate in comparison with the surgical approach or staged CAS-CABG.
Table 1.
Combined outcome of simultaneous CAS-CABG.
Another possible method to reduce neurological complications during simultaneous hybrid procedures is the combination of CAS with OPCAB. In the analyzed studies, OPCAB was performed in 13–17% of simultaneous CAS – CABG procedures [27–29]; in the largest patient series, the SHARP study [25], all procedures were on-pump. OPCAB can reduce complications associated with aortic clamping (embolization risk) and extracorporeal circulation (blood pressure drops, cardioplegia, hemodynamic instability, systemic inflammatory response and clotting disorders) [30,31], but it is more technically demanding, which is probably why it is still performed less frequently. OPCAB patients, in comparison with those undergoing CABG, seem to have lower stroke rate (1.0% vs. 2.4%, p<0.01), stroke related mortality (0.1% vs. 0.9%, p<0.01), and lower myocardial infarction rate (1.6% vs. 3.0%, p<0.01) [30]. Moreover, OPCAB showed better outcomes among high surgical risk and elderly patients, faster postoperative recovery, lower mortality rate, and shorter post-operative in-hospital stay compared to CABG [30–32]. As simultaneous CEA and OPCAB is associated with a low MI, stroke and death rate of 3.6% [19], similarly, 1-stage CAS and OPCAB could be associated with a low outcome, but this strategy has not yet been evaluated in larger studies.
The surgical approach and a hybrid revascularization are hard to compare because of the lack of evidence from randomized trials and patient selection in registries, depending on individual risk profile, lesion morphology in coronary and carotid arteries, and co-morbidities.
In the registry of 27,084 concurrent carotid revascularizations and CABGs performed during the same hospitalization over a 5-year period [33], 96.7% of patients were treated with CEA-CABG, whereas only 3.3% (887) had CAS-CABG. Patients undergoing CAS-CABG had significantly lower rates of postoperative stroke (2.4% vs. 3.9%, p<0.001) [33] and slightly lower rates of combined stroke and death (6.9 vs. 8.6%, p=0.1) [33] compared with patients who underwent CEA-CABG; however, in-hospital death rates were similar for both strategies (5.2% vs. 5.4%, respectively) [33]. The results of this study [33] indicate that asymptomatic patients who undergo CAS-CABG have a lower stroke rate compared with those undergoing CEA-CABG, but have similar in-hospital mortality; furthermore, according to this observation, CAS-CABG may be an alternative strategy in high-risk asymptomatic patients. Finally, this study suggests that the role of CAS-CABG is still unclear in symptomatic patients (the group of symptomatic patients undergoing CAS-CABG was very small and NPD usage rate was not reported, with a possible effect on the incidence of periprocedural stroke). Another limitation of this study is that the authors did not asses MI rate after CAS-CABG vs. CEA-CABG.
Our initial experience with 21 patients treated with hybrid revascularization (CAS-CABG) includes 8 with simultaneous CAS-CABG and 13 with a staged CAS-CABG (Table 2). No major adverse events (death, stroke, MI) at 30-day observation were observed. All CAS procedures were performed using NPDs (either proximal or distal) and stent type (closed/open-cell stent) according to the ‘tailored’ CAS algorithm [5]. The procedures were performed in a high-volume endovascular and surgical center performing over 200 CASs and over 1400 CABGs per year.
Table 2.
Our initial experience of simultaneous/staged CAS-CABG.
| Two staged CAS-CABG (13 pts) | Simultaneous hybrid CAS-CABG (8 pts) | |
|---|---|---|
| Age | 66.7±6.6, min. 56, max. 74 years | 70.5±4.4, min. 62, max. 77 years |
| Male gender | 9 (70%) | 6 (75%) |
| CCS IV | 2 (15%) | 4 (50%) |
| Symptomatic stenosis (stroke/TIA) | 10 (77%) | 5 (62%) |
| Ejection fraction | 60±4.2% | 53±7.1% |
| Left main stenosis | 5 (38%) | 2 (25%) |
| 3 vessel CAD | 10 (77%) | 6 (75%) |
| Mean ICA stenosis | 83 ±10% min. 60%, max. 90% | 85±15% min. 60%, max. 99% |
| Proximal NPDs | 6 (46%) | 5 (62%) |
| Closed cell stent | 10 (77%) | 5 (62%) |
| euroSCORE | 4.7 (±1.1) min. 3, max. 6 | 5.3 (±0.5) min. 5, max. 6 |
| OPCAB | 2 (15%) | 3 (37.5%) |
| TAMR* | 4 (30%) | 6 (75%) |
TAMR – total arterial myocardial revascularization.
Discussion
In patients with significant asymptomatic carotid stenosis of 80% or symptomatic carotid stenosis of 50% and coexistent multivessel coronary artery disease, several therapeutic options exist. Considering the patient risk profile, lesion morphology and symptom status, either surgical strategy or hybrid CAS-CABG revascularization may be considered. CEA-CABG (staged or synchronous) is associated with an event rate of 10.2–11.5% at 30-day observation (MI/stoke/death) [16]. The outcome of staged CAS-CABG appears comparable to CEA-CABG (cumulative risk of stroke/death/myocardial infarction of 9.4%) [23] and in experienced centers the complication rate can be even lower 6.5% [22]. Based on this data, staged CAS-CABG might be a feasible alternative to ‘conventional’ surgical strategy or might even be an option associated with lower complication rate. A novel simultaneous 1-stage CAS-CABG seems to be associated with the lowest event rate of all therapeutic methods. Further development of CAS, especially the strategy of fitting neuroprotection and stent type to the lesion morphology, stenosis severity and symptom status (‘tailored CAS’) [5], may lead to a reduction in peri- and post-procedural event rates. However, in published studies on staged or synchronous CAS-CABG [22–29], distal NPD was the only system device used during CAS procedure (in all SHARP study patients, but only 40% of 356 staged CAS-CABG patients) [25,22]. Usage of NPD (either proximal or distal) in all cases, as well as off-pump cardiac surgery (“aorta no touch” technique) could probably minimize neurological complications of CABG.
The main goal of performing carotid revascularization before cardiac surgery is stroke prevention or at least reduction of the stroke rate, as stroke remains the major noncardiac complication of CABG. [34] After successful carotid revascularization (by CAS) the incidence of any stroke after CABG decreases to 2.2% [35], which is the ‘usual’ stroke risk associated with CABG without coexistent carotid disease.
With the presently available, limited, evidence, it is hard to compare the surgical and hybrid strategies due to the heterogeneity of patients included in the reported groups. An ideal randomized trial should compare isolated CABG with CAS-CABG and CEA-CABG both in symptomatic and asymptomatic patients [34], but such a study is unlikely to ever be conducted. A recent report included over 650 patients divided in 3 groups: surgical (CEA-CABG), endovascular (CAS and PCI), and hybrid (CAS-CABG) revascularization, but the patient characteristics were not comparable [36]. Because of this heterogeneity (anatomic severity of disease and symptom status) the outcome of the endovascular strategy (CAS and 1-vessel PCI) cannot be compared with the outcome of a patient with severe CAD and carotid disease undergoing either surgical or hybrid strategy. The primary end point (death, stroke, MI) of about 10% for hybrid CAS-CABG performed in a minority of patients (n=68) [36] remains in contrast with emerging data from other high-volume centers [25,27–29].
Conclusions
A single ideal revascularization strategy for patients with coexisting severe carotid and coronary artery disease is unlikely to exist, and an individualized approach is mandatory, but this is ill-defined. The emerging CAS-CABG strategy may be a viable alternative to CEA-CABG, particularly if performed by experienced surgeons in high-volume centers. Single- center results of simultaneous 1-stage CAS-CABG are promising (both for cardiac and neurological complications), but more data and larger patient series are needed before widespread use of this strategy is possible.
Figure 4.
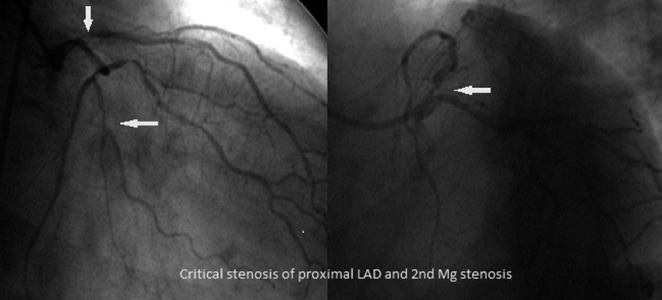
Coronary angiogram of the same patient: a critical stenosis of proximal left anterior descending artery (LAD) and stenosis of second marginal branch (Mg). RCA was without significant stenosis after PCI performed 7 years before.
Figure 5.
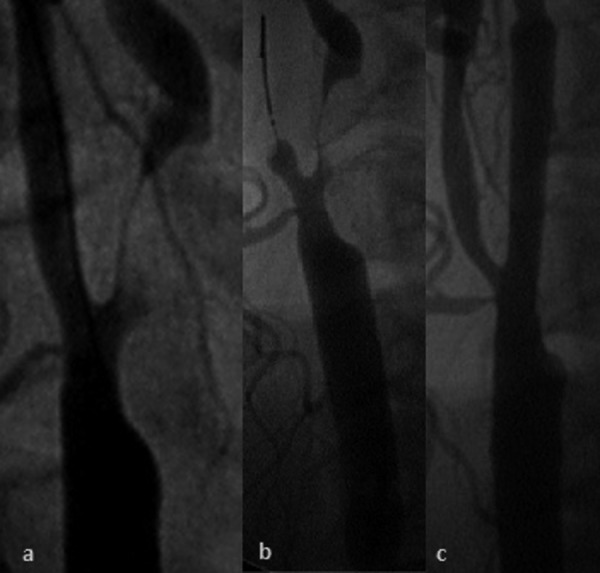
(A) Subtotal LICA stenosis of the same coronary unstable patient (recent amaurosis fugax of the left eye), (B) CAS performed with usage of proximal NPD (flow reversal), (C) final result after stent implantation.
Footnotes
Source of support: Self financing
References
- 1.Kablak-Ziembicka A, Tracz W, Przewlocki T, et al. Association of increased carotid intima-media thickness with the extent of coronary artery disease. Heart. 2004;90(11):1286–90. doi: 10.1136/hrt.2003.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hertzer NR, Loop FD, Beven EG, et al. Surgical staging for simultaneous coronary and carotid disease: a study including prospective randomization. J Vasc Surg. 1989;9(3):455–63. doi: 10.1067/mva.1989.vs0090455. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz LB, Bridgman AH, Kieffer RW, et al. Asymptomatic carotid artery stenosis and stroke in patients undergoing cardiopulmonary bypass. J Vasc Surg. 1995;21(1):146–53. doi: 10.1016/s0741-5214(95)70253-9. [DOI] [PubMed] [Google Scholar]
- 4.Kallikazaros I, Tsioufis C, Sideris S, et al. Carotid artery disease as a marker for the presence of severe coronary artery disease in patients evaluated for chest pain. Stroke. 1999;30:1002–7. doi: 10.1161/01.str.30.5.1002. [DOI] [PubMed] [Google Scholar]
- 5.Pieniazek P, Musialek P, Kablak-Ziembicka A, et al. Carotid artery stenting with patient- and lesion-tailored selection of the neuroprotection system and stent type: early and 5-year results from a prospective academic registry of 535 consecutive procedures (TARGET-CAS) J Endovasc Ther. 2008;15:249–262. doi: 10.1583/07-2264.1. [DOI] [PubMed] [Google Scholar]
- 6.Hofman R, Kypta A, Steinweider C, et al. Coronary angiography in patients undergoing carotid artery stenting shows a high incidence of significant coronary disease. Heart. 2005;91:1438–41. doi: 10.1136/hrt.2004.050906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertzer NR, Young JR, Beven EG, et al. Coronary angiography in 506 patients with extracranial cerebrovascular disease. Arch Intern Med. 1985;145(5):849–52. [PubMed] [Google Scholar]
- 8.Cohen SN, Hobson RW, II, Weiss DG, Chimowitz M. Death associated with asymptomatic carotid artery stenosis: long-term clinical evaluation. VA Cooperative Study 167 Group. J Vasc Surg. 1993;18(6):1002–9. discussion 1009–11. [PubMed] [Google Scholar]
- 9.Naylor AR, Mehta Z, Rothwell PM, Bell PR. Carotid artery disease and stroke during coronary artery bypass: a critical review of the literature. Eur J Vasc Endovasc Surg. 2002;23(4):283–94. doi: 10.1053/ejvs.2002.1609. [DOI] [PubMed] [Google Scholar]
- 10.Islamoglu F, Reyhanoglu H, Berber O, et al. Predictors of outcome after coronary artery bypass grafting in patients older than 75 years of age. Med Sci Monit. 2003;9(8):CR369–76. [PubMed] [Google Scholar]
- 11.Dziuba M, Jander S, Zwolinski R, Chizynski K. Surgical revascularization of myocardium in population of young women. Is it a group of increased operative risk? Arch Med Sci. 2009;5(2):135–40. [Google Scholar]
- 12.Likosky DS, Marrin CA, Caplan LR, et al. Determination of etiologic mechanisms of strokes secondary to coronary artery bypass graft surgery. Northern New England Cardiovascular Disease Study Group. Stroke. 2003;34(12):2830–34. doi: 10.1161/01.STR.0000098650.12386.B3. [DOI] [PubMed] [Google Scholar]
- 13.Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery) Circulation. 2004;110(14):e340–437. [PubMed] [Google Scholar]
- 14.Liapis CD, Bell PR, Mikhailidis D, et al. ESVS guidelines. Invasive treatment for carotid stenosis: indications, techniques. Eur J Vasc Endovasc Surg. 2009;37(Suppl 4):1–19. doi: 10.1016/j.ejvs.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Bates ER, Babb JD, Casey DE, Jr, et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 Clinical Expert Consensus Document on Carotid Stenting A Report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents (ACCF/SCAI/SVMB/SIR/ASITN Clinical Expert Consensus Document Committee on Carotid Stenting) J Am Coll Cardiol. 2007;49(1):126–70. doi: 10.1016/j.jacc.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Brott TG, Halperin JL, Abbara S et al: 2011 ASA /ACCF /AHA /AANN /AANS/ACR /ASNR /CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS Guideline on the Management of Patients With Extracranial Carotid and Vertebral Artery Disease A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery Developed in Collaboration With the American Academy of Neurology and Society of Cardiovascular Computed Tomography Stroke published online Jan 31, 2011
- 17.Ricotta JJ, Char DJ, Cuadra SA, et al. Modeling stroke risk after coronary artery bypass and combined coronary artery bypass and carotid endarterectomy. Stroke. 2003;34(5):1212–17. doi: 10.1161/01.STR.0000069263.08070.9F. [DOI] [PubMed] [Google Scholar]
- 18.Naylor AR, Cuffe RL, Rothwell PM, Bell PR. A systematic review of outcomes following staged and synchronous carotid endarterectomy and coronary artery bypass. Eur J Vasc Endovasc Surg. 2003;25(5):380–89. doi: 10.1053/ejvs.2002.1895. [DOI] [PubMed] [Google Scholar]
- 19.Fareed KR, Rothwell PM, Mehta Z, Naylor AR. Synchronous carotid endarterectomy and off-pump coronary bypass: an updated, systematic review of early outcomes. Eur J Vasc Endovasc Surg. 2009;37(4):375–78. doi: 10.1016/j.ejvs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Gurm HS, Nallamothu BK, Yadav J. Safety of carotid artery stenting for symptomatic carotid artery disease: a meta-analysis. Eur Heart J. 2008;29(1):113–19. doi: 10.1093/eurheartj/ehm362. [DOI] [PubMed] [Google Scholar]
- 21.Mantese VA, Timaran CH, Chiu D CREST Investigators. The Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST): Stenting Versus Carotid Endarterectomy for Carotid Disease. Stroke. 2010;41(Suppl 10):S31–34. doi: 10.1161/STROKEAHA.110.595330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Heyden J, Suttorp MJ, Bal ET, et al. Staged Carotid Angioplasty and Stenting Followed by Cardiac Surgery In Patients With Severe Asymptomatic Carotid Artery Stenosis: Early and Long-Term Results. Circulation. 2007;116:2036–42. doi: 10.1161/CIRCULATIONAHA.106.658625. [DOI] [PubMed] [Google Scholar]
- 23.Naylor AR, Mehta Z, Rothwell PM. A systematic review and meta-analysis of 30-day outcomes following staged carotid artery stenting and coronary bypass. Eur J Vasc Endovasc Surg. 2009;37(4):379–87. doi: 10.1016/j.ejvs.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Ranaweera PS, Bigelow BC, Leary MC, et al. Endovascular carotid artery stenting and early coronary artery bypass grafting for asymptomatic carotid artery stenosis: long-term outcomes and neurologic events. Catheter Cardiovasc Interv. 2009;73(2):139–42. doi: 10.1002/ccd.21824. [DOI] [PubMed] [Google Scholar]
- 25.Versaci F, Reimers B, Del Giudice C, et al. Simultaneous hybrid revascularization by carotid stenting and coronary artery bypass grafting: the SHARP study. JACC Cardiovasc Interv. 2009;2(5):393–401. doi: 10.1016/j.jcin.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Mendiz O, Fava C, Valdivieso L, et al. Synchronous Carotid Stenting and Cardiac Surgery: An Initial Single-Center Experience. Catheter Cardiovasc Interv. 2006;68(3):424–28. doi: 10.1002/ccd.20883. [DOI] [PubMed] [Google Scholar]
- 27.Velissaris I, Kiskinis D, Anastasiadis K. One stage carotid artery stenting and open heart surgery: a novel approach. J Cardiovasc Surg. 2009;50 [PubMed] [Google Scholar]
- 28.Palombo G, Stella N, Faraglia V, et al. Safety and effectiveness of combining carotid artery stenting with cardiac surgery – preliminary results of a single center experience. J Cardiovasc Surg. 2009;50:49–54. [PubMed] [Google Scholar]
- 29.Guerra M, Mota JC, Veloso M, et al. Combined carotid stenting and urgent coronary artery surgery in unstable angina patients with severe carotid stenosis. Interact Cardioasc Thorac Surg. 2009;9(2):278–81. doi: 10.1510/icvts.2009.204354. [DOI] [PubMed] [Google Scholar]
- 30.Brizzio ME, Zapolanski A, Shaw RE, et al. Stroke-Related Mortality in Coronary Surgery Is Reduced by the Off-Pump Approach. Ann Thorac Surg. 2010;89:19–23. doi: 10.1016/j.athoracsur.2009.07.076. [DOI] [PubMed] [Google Scholar]
- 31.Ngaage DL. Off-pump coronary artery bypass grafting: simple concept but potentially sublime scientific value. Med Sci Monit. 2004;10(3):RA47–54. [PubMed] [Google Scholar]
- 32.Hirose H, Amano A, Takahashi A. Side clamp used during off-pump coronary artery bypass does not increase the risk of stroke. Med Sci Monit. 2002;8(4):CR235–40. [PubMed] [Google Scholar]
- 33.Timaran CH, Rosero EB, Smith ST, et al. Trends and outcomes of concurrent carotid revascularization and coronary bypass. J Vasc Surg. 2008;48(2):355–60. doi: 10.1016/j.jvs.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 34.Van der Heyden J, Suttorp MJ, Schepens MA. Revascularization strategy in patients with severe concurrent carotid and coronary artert disease: “Failure to move forward is reason to regress”. J Cardiovasc Surg. 2009;50:55–62. [PubMed] [Google Scholar]
- 35.Guzman LA, Costa MA, Angiolillo DJ, et al. A systematic review of outcomes in patients with staged carotid artery stenting and coronary artery bypass graft surgery. Stroke. 2008;39(2):361–65. doi: 10.1161/STROKEAHA.107.495010. [DOI] [PubMed] [Google Scholar]
- 36.Ribichini F, Tomai F, Reimers B. Clinical outcome after endovascular, surgical or hybrid revascularisation in patients with combined carotid and coronary artery disease: the Finalised Research In ENDovascular Strategies Study Group (FRIENDS) EuroIntervention. 2010;6(3):328–35. doi: 10.4244/EIJV6I3A55. [DOI] [PubMed] [Google Scholar]