Summary
Background
The role of somatostatin analogues in advanced hepatocellular carcinoma (HCC) remains controversial. The aim of this study was to examine the effect of octreotide on the survival of patients with advanced HCC.
Material/Methods
Electronic databases including Medline, Embase, Cochrane controlled trials register, Web of Science and PubMed (updated to Dec 2010) and manual bibliographical searches were conducted. A meta-analysis of all randomized controlled trials (RCTs) comparing octreotide versus placebo or no treatment was performed.
Results
Eleven RCTs including 802 patients were assessed and 9 were included in the meta-analysis. Meta-analysis showed that the 6-mo and 12-mo survival rates in the octreotide group were significantly higher than those of the control group (6-mo: RR 1.41, 95%CI 1.12–1.77, P=0.003; 12-mo: RR 2.66, 95%CI 1.30–5.44, P=0.008). When including the studies using no treatment as control, with high quality, being performed in China, including >50 patients and with follow-up >2 years, the sensitivity analyses tended to confirm the primary meta-analysis. Whereas, when including the studies using placebo as control or being performed in western countries, the difference was not significant.
Conclusions
This meta-analysis demonstrates that octreotide could improve the survival of patients with advanced HCC, but possibly not in western countries. The role of detecting SSTR expression in the administration of octreotide in advanced HCC needs further investigation.
Keywords: octreotide, advanced hepatocellular carcinoma, meta-analysis
Background
Hepatocellular carcinoma (HCC) is the fifth most common malignant neoplasm in the world, and the third most common cause of cancer-related death [1]. More than 500, 000 new cases are currently diagnosed yearly, with an age-adjusted worldwide incidence of 5.5–14.9 per 100,000 population [2,3]. In addition, in the past three decades there has been a substantial increase in the incidence of HCC in developed countries [4].
Surgical resection or liver transplantation is the first choice of treatment for patients without portal vein involvement or distant metastasis. Recently, local ablation, especially radiofrequency ablation, has also been considered as the first line modality for small HCC [5]. However, these therapies are not suitable for patients with advanced HCC. Thus, systemic therapy remains the only option for advanced HCC [6–8].
Octreotide, an analogue of the cyclic peptide hormone somatostatin, has been evaluated for his potential efficacy in treatment of HCC in a number of studies. Over 40% of HCC express specific somatostatin receptors (SSTR), and in vitro data show a direct antitumor effect of octreotide in HCC [9,10]. Somatostatin analogues exert regulatory or suppressive effects against various tumors, principally by reducing symptomatic hormonal secretion and by a direct antineoplastic effect [11]. The molecular mechanisms involved in the antineoplastic activity of somatostatin are related to direct and indirect growth inhibition mediated by SSTR expressed on the target tissues [12].
Moreover, there have been a few clinical reports exploring octreotide in the treatment of HCC. Nevertheless, the role of octreotide in advanced HCC remains controversial [13]. In 1998, Kouroumalis et al. [14] performed the first randomized trial for the treatment of advanced HCC with octreotide in 58 patients, demonstrating significant improvement in survival of HCC patients treated with short-acting octreotide (median survival 13 mo and 4 mo, respectively). A non-randomized study with long-acting octreotide from the same author group confirmed the results using historical controls [15]. Stimulated by these results and the fact that there was no established systemic treatment available, further studies on octreotide were conducted. However, in 2002, the trial by Yuen et al. failed to demonstrate any survival benefit compared with placebo (1.93 mo vs. 1.97 mo) [16]. Subsequently, another 2 trials from France and Germany also failed to demonstrate any benefit on survival [17,18].
There have been 2 meta-analyses evaluating the effect of octreotide in advanced HCC, which only included 3 and 4 trials in the final meta-analysis (involving 238 and 373 patients, respectively) [19,20]. However, to date there have been nearly 10 RCTs assessing the effect of octreotide in advanced HCC; the largest RCT, by Barbare et al. [18], which involved 272 patients, was not included in the 2 meta-analyses. Thus, the conclusions of the 2 meta-analyses are flawed because of their failure to include several important RCTs, and this may have led to publication bias. Therefore, we performed an updated systematic review and meta-analysis to examine the effect of octreotide on the survival of patients with advanced HCC.
Material and Methods
Identification and selection of studies
Relevant studies were identified and selected by searching the databases Medline (1966 to Dec 2010), Embase (1980 to Dec 2010), Cochrane controlled trials register (Cochrane Library Issue 4, 2010), Web of Science (1981 to Dec 2010) and PubMed (updated to Dec 2010) under the search terms “hepatocellular carcinoma” or “liver cancer”, “octreotide” and “somatostatin analogues”. We also did a full manual search from the bibliographies of each peer-reviewed paper selected. No language or date limitations were imposed.
The following selection criteria were applied: 1) study design – RCT comparing octreotide versus placebo or no treatment; 2) study population – patients with advanced HCC. Duplicate publications were excluded. The decision to include or exclude any trial was made by 2 researchers acting independently. The 2 lists were compared and discrepancies were resolved.
Data extraction
Data were independently abstracted from each study by 2 researchers, and disagreement was resolved by consensus. Data were extracted from each study with a pre-designed review form. Data to be extracted were as follows: 6-mo survival rate, 12-mo survival rate and 24-mo survival rate.
Quality of methodology
The methodological quality of studies included in the meta-analysis was scored with the Jadad composite scale [21,22]. This is a 5-point quality scale, with low quality studies having a score of ≤2 and high quality studies a score of ≥3 [22,23]. Methodological quality assessment was independently performed by 2 of the present authors. Each study was given an overall quality score based on the above criteria, which was then used to rank studies. Any disagreement was resolved by consensus.
Statistical methods
The data analysis was performed using the random-effect model of DerSimonian and Laird method with the meta-analysis software Review Manager Software (RevMan 5.0, Cochrane Collaboration, Oxford, England) [21,22]. The risk ratio (RR) for the results was presented with 95% confidence interval (CI). We tested heterogeneity between trials with χ2 tests, with P≤0.1 indicating significant heterogeneity. Publication bias was tested with funnel plots.
Results
Description of the selected studies
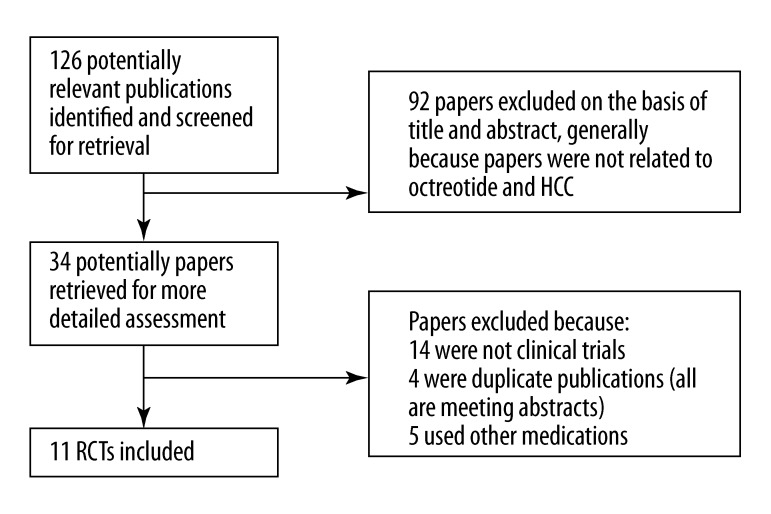
The search strategy generated 126 studies. From these, we identified 11 RCTs (involving 802 patients) comparing octreotide with placebo or no treatment, which fulfilled the criteria for consideration in this systematic review (Figure 1) [14,16–18,24–30]. Six studies were published in English, and 5 were in Chinese. All the studies were published as peer-reviewed articles. The baseline characteristics of the 11 trials are listed in Table 1.
Figure 1.
Flowchart showing selection of studies for inclusion in meta-analysis.
Table 1.
Baseline characteristics of trials.
| Study year | Country | Treated vs. control | Octreotide regimen | Control | Child-Pugh Classification | Okuda stage | Cirrhosis (%) | Portal thrombosis (%) |
|---|---|---|---|---|---|---|---|---|
| Kouroumalis 1998 | Greece | 28 vs. 30 | Octreotide 250 μg twice daily | No treatment | O (A 1, B 10, C 13) C (A 2, B 12, C 16) |
O (I 2, II 13, III 13) C (I 3, II 10, III 17) |
O 86 C 77 |
NR |
| Farooqi 2000 | Pakistan | 6 vs. 7 | Octreotide 250 μg twice daily | No treatment | NR | NR | O 83 C 86 |
NR |
| Wu 2001 | China | 12 vs. 13 | Octreotide 200 μg thrice daily | No treatment | NR | O (III 12) C (III 13) |
NR | NR |
| Yuen 2002 | Hong Kong | 35 vs. 35 | Octreotide 250 μg twice daily for 2 weeks + LAR 30 mg once every 4 weeks for 6 doses | Placebo | O (A 18, B 14, C 3) C (A 12, B 22, C 1) |
O (I 6, II 23, III 6) C (I 3, II 26, III 6) |
NR | O 48.6 C 60 |
| Yang 2003 | China | 32 vs. 33 | Octreotide 200 μg twice daily | No treatment | O (B-C 32) C (B-C 33) |
O (II-III 32) C (II-III 33) |
NR | NR |
| Zhang 2004 | China | 20 vs. 25 | Octreotide 100 μg thrice daily | No treatment | NR | NR | NR | NR |
| Becker 2007 | Germany | 60 vs. 59 | LAR 30 mg once every 4 wk | Placebo | O (A 53, B 35, C 12) C (A 53, B 37, C 10) |
O (I 30, II 65, III 5) C (I 32, II 58, III 10) |
O 95 C 91 |
O 44 C 54 |
| Dimitroulopoulos 2007 | Greece | 30 vs. 30 | Octreotide 0.5 mg every 8 h for 6 wk; at the end of wk 4–8 LAR 20 mg; at the end of wk 12 and every 4 wk LAR 30 mg | Placebo | O (A 15, B 15) C (A 11, B 19) |
NR | NR | O 0 C 0 |
| Ou 2007 | China | 16 vs. 14 | Octreotide 200 μg twice daily | No treatment | NR | O (III 16) C (III 14) |
NR | NR |
| Barbare 2009 | France | 135 vs. 137 | LAR 30 mg once every 4 wk | Placebo | O (A 90, B 34, C 1) C (A 93, B 32, C 2) |
NR | O 79 C 77 |
O 21 C 23 |
| Zhang 2010 | China | 21 vs. 24 | Octreotide 100 μg thrice daily | No treatment | NR | NR | NR | NR |
O – the octreotide group; C – the control group; LAR – long-acting octreotide; NR – not reported.
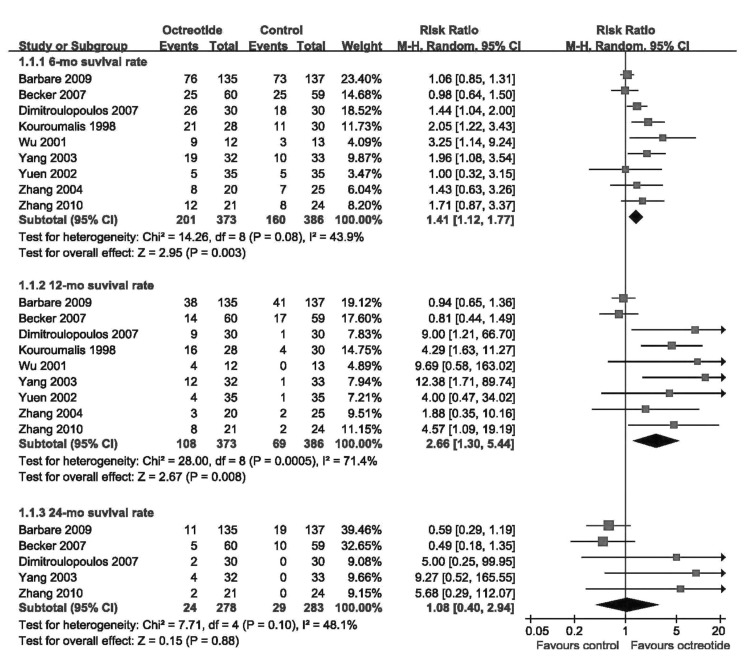
Nine trials reported 6-mo and 12-mo survival rates, and 5 trials reported 24-mo survival rate. Thus, the 9 trials were included in the meta-analysis, which involved 759 patients (373 were randomized to the octreotide group and 386 to the control group). Four studies were placebo-controlled, and 7 were untreated-controlled. The mean age ranged from 54.8 years to 69.5 years. In 1 trial [28], the patients of Child-Pugh stage C were excluded, and only the patients of SSTR(+) were included into the randomized trial. The methodological-quality scores ranged from 2 to 5 (Table 2).
Table 2.
Jadad quality score of the trials.
| Study, year | Randomization method | Blinding | Withdrawals or dropouts | Total |
|---|---|---|---|---|
| Kouroumalis 1998 | 2 | 0 | 1 | 3 |
| Farooqi 2000 | 2 | 0 | 1 | 3 |
| Wu 2001 | 1 | 0 | 1 | 2 |
| Yuen 2002 | 2 | 0 | 1 | 3 |
| Yang 2003 | 1 | 0 | 1 | 2 |
| Zhang 2004 | 2 | 0 | 1 | 3 |
| Becker 2007 | 2 | 2 | 1 | 5 |
| Dimitroulopoulos 2007 | 2 | 1 | 1 | 4 |
| Ou 2007 | 1 | 0 | 1 | 2 |
| Barbare 2009 | 2 | 2 | 1 | 5 |
| Zhang 2010 | 1 | 0 | 1 | 2 |
Meta-analysis of survival rates
Characteristics of the included trials are detailed in Table 3. Nine studies reported the 6-mo and 12-mo survival rates, and only 5 reported the 24-mo survival rate. The median survival of patients treated with octreotide ranged from 1.93 months to 13.0 months, and the median survival of patients in the control group ranged from 1.97 months to 7.03 months.
Table 3.
Survival of the patients in the trials.
| Study year | Median survival (mo) | 6-mo survival rate (%) | 12-mo survival rate (%) | 24-mo survival rate (%) |
|---|---|---|---|---|
| Kouroumalis 1998 | O 13.0 C 4.0 |
O 75 C 37 |
O 56 C 13 |
NR |
| Farooqi 2000 | NR | NR | NR | NR |
| Wu 2001 | O 5.7 C 1.6 |
O 75 C 23 |
O 33 C 0 |
O 0 C 0 |
| Yuen 2002 | O 1.93 C 1.97 |
O 14.2 C 14.2 |
O 10.5 C 3.3 |
NR |
| Yang 2003 | O 11.6 C 5.6 |
O 59 C 31 |
O 38 C 3 |
O 12 C 0 |
| Zhang 2004 | O 7 C 4 |
O 40 C 28 |
O 15 C 8 |
NR |
| Becker 2007 | O 4.7 C 5.3 |
O 41 C 42 |
O 23 C 28 |
O 9 C 17 |
| Dimitroulopoulos 2007 | O 11.4 C 6.5 |
O 87 C 60 |
O 30 C 3 |
O 7 C 0 |
| Ou 2007 | O 7 C 2.5 |
NR | NR | NR |
| Barbare 2009 | O 6.53 C 7.03 |
O 56 C 53 |
O 28 C 30 |
O 8 C 14 |
| Zhang 2010 | O 8 C 3 |
O 57 C 33 |
O 38 C 8 |
O 10 C 0 |
O – the octreotide group; C – the control group; NR – not reported.
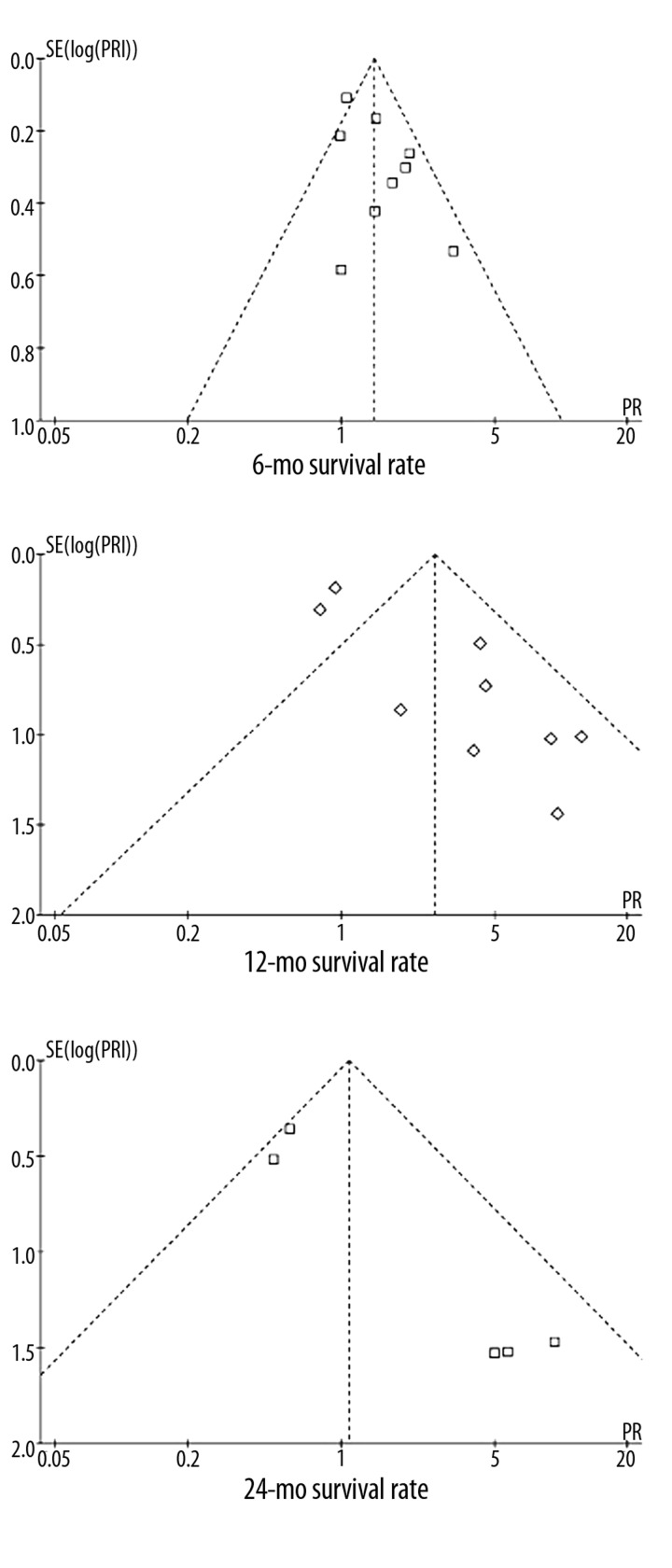
Meta-analysis showed that the 6-mo and 12-mo survival rates in the octreotide group were significantly higher than those of the control group (6-mo: 53.89% vs. 41.45%, RR 1.41, 95%CI 1.12–1.77, P=0.003; 12-mo: 28.95% vs. 17.88%, RR 2.66, 95%CI 1.30–5.44, P=0.008) (Figure 2). The 24-mo survival rate was not different between the 2 groups (RR 1.08, 95%CI 0.40–2.94, P=0.88) (Figure 2). The funnel plots for the results showed obvious asymmetry, suggesting the possibility of bias (Figure 3).
Figure 2.
Meta-analysis of the effects of octreotide on survival rates.
Figure 3.
Funnel plots of the included trials.
Sensitivity analysis
A sensitivity analysis was performed only including the studies administrating placebo as control (Table 2). We found no difference in 6-mo, 12-mo, or 24-mo survival rates between the octreotide group and the control group (6-mo: RR 1.13, 95% CI 0.95–1.35, P=0.16; 12-mo: RR 1.25, 95% CI 0.63–2.48, P=0.52; 24-mo: RR 0.60, 95% CI 0.33–1.10, P=0.10).
Another sensitivity analysis was performed only including the studies using no treatment as control (Table 2). The 6-mo and 12-mo survival rates in the octreotide group were significantly higher than those of the control group (6-mo: RR 1.94, 95% CI 1.44–2.61, P<0.0001; 12-mo: RR 4.51, 95% CI 2.33–8.73, P<0.00001). The 24-mo survival rate was also higher in the octreotide group, but difference was not significant (RR 7.32, 95% CI 0.92–58.14, P=0.06).
Six trials were of high quality (Jadad score ≥3), and a sensitivity analysis was conducted based on these trials (Table 2). The analysis showed higher 6-mo survival rate in the octreotide group (RR 1.25, 95% CI 1.00–1.57, P=0.05). The 6-mo and 12-mo survival rates in the 2 groups were not different (6-mo: RR 1.80, 95% CI 0.89–3.64, P=0.10; 12-mo: RR 0.60, 95% CI 0.33–1.10, P=0.10).
Four trials were performed in western countries, and a sensitivity analysis was carried out including these studies (Table 2). We found no difference in 6-mo, 12-mo, or 24-mo survival rates between the octreotide group and the control group (6-mo: RR 1.27, 95% CI 0.96–1.67, P=0.09; 12-mo: RR 1.69, 95% CI 0.74–3.87, P=0.22; 24-mo: RR 0.60, 95% CI 0.33–1.10, P=0.10).
Five trials were conducted in China, the country with the highest incidence of HCC, and a sensitivity analysis was made including these trials (Table 2). The 6-mo and 12-mo survival rates in the octreotide group were significantly higher than those of the control group (6-mo: RR 1.78, 95% CI 1.25–2.52, P=0.001; 12-mo: RR 4.59, 95% CI 1.99–10.59, P=0.0003). The 24-mo survival rate was also higher in the octreotide group, but the difference was not significant (RR 7.32, 95% CI 0.92–58.14, P=0.06).
Three trials included fewer than 50 patients. After excluding these 3 trials, sensitivity analysis showed that the 6-mo and 12-mo survival rates in the octreotide group were significantly higher than those of the control group (6-mo: RR 1.32, 95% CI 1.03–1.70, P=0.03; 12-mo: RR 2.37, 95% CI 1.02–5.48, P=0.04) The 24-mo survival rates of the 2 groups was not different (RR 0.87, 95% CI 0.33–2.32, P=0.78).
Five trials had follow-up longer than 2 years. A final sensitivity analysis was carried out including these 5 trials. The 6-mo survival rate in the octreotide group was significantly higher than that of the control group (6-mo: RR 1.27, 95% CI 1.01–1.61, P=0.05). The 12-mo survival rate was also higher in the octreotide group, but the difference was not significant (RR 2.14, 95% CI 0.89–5.15, P=0.09). The 24-mo survival rates of the 2 groups was not different (RR 1.08, 95% CI 0.40–2.94, P=0.88).
Discussion
There is currently no effective systemic therapy for advanced HCC except sorafenib [31–33]. The role of octreotide in advanced HCC is still uncertain. Since the first RCT study by Kouroumalis et al. [14] concluded that octreotide could significantly improve survival of patients with advanced HCC, octreotide has been commonly administrated in advanced HCC. However, a subsequent trial failed to show any benefit on survival [16]. In a study by Yuen et al. [16], the octreotide group and the control group had surprisingly poor survival rates of about 1.9 months. In their study, 82% of these patients were Okuda stage I and II, which were expected to have better survival according to the original Okuda study. The poor survival of 1.9 months was probably related to the fact that 48–60% and 14–20% of these patients had portal vein thromboses and distant metastases, respectively. Such poor survival might weaken the value of the study, because octreotide could not exert this effect in such a short time. Nevertheless, 2 recent well-designed trials, which had large size and long follow-up intervals, also failed to show any benefit on survival [17,18]. Indeed, In an RCT of 272 patients with advanced HCC, Barbare et al. [18] reported a median survival of 6.5 months in the octreotide arm, which was even shorter than that in the placebo arm (7.3 months).
Cebon et al. [34] reported that 41% of the HCC tissue samples overexpressed SSTR with high affinity for octreotide. Further studies found high detection rates of SSTR 2, 3 and 5 in HCC cells, although with high heterogenicity even in the same tumor.11,35In vitro studies have shown a direct antitumor effect of octreotide in HCC [11,12]. Thereby, it is presumed that the patients of SSTR(+) may have better response to octreotide than the patients of SSTR(−). A recent trial by Dimitroulopoulos et al. [22], based on scintigraphy with111 indium-labeled octreotide for SSTR expression and only including the patients of SSTR(+), reported a median survival of 7.7 mo in the octreotide group compared with 4 mo in the control group. However, the size of this trial was small, including only 60 patients.
Two meta-analyses have been performed to evaluate the effect of octreotide on the survival of patients with advanced HCC [19,20]. These 2 studies only included 3 and 4 trials in the final meta-analysis, and did not include the largest RCT. Thus, it is difficult to interpret findings from meta-analysis if the analysis includes insufficient numbers of trials, patients, and events.
In this systematic review, 11 trials were found and 9 were finally included in the meta-analysis. The primary meta-analysis showed that the 6-mo and 12-mo survival rates in the octreotide group were significantly higher than those of the control group, but no difference was found in the meta-analysis of 24-mo survival rates. Because most patients with advanced HCC have survival of no longer than 12 mo, for the clinical trials of advanced HCC the 6-mo and 12-mo survival rates are more important than the 24-mo rate. Thus, this study focused on the 6-mo and 12-mo survival rates.
However, the funnel plots showed obvious asymmetry, indicating the possibility of bias. The results of sensitivity analyses were also inconsistent. When including the studies using no treatment as control, with high quality, being performed in China, including >50 patients and with follow-up >2 years, the sensitivity analyses tended to confirm the primary meta-analysis; however, when including the studies using placebo as control or being performed in western countries, the difference was not significant. Importantly, these 2 sensitivity analyses both only included 4 trials, which might be an insufficient number for a meta-analysis.
The trial by Dimitroulopoulos et al. [28], the only study detecting SSTR expression and including SSTR(+) patients, documented that octreotide could significantly prolong the survival of the patients with advanced HCC. However, no other study detected SSTR expression before administrating octreotide. We found a marked difference between the studies from western countries and those from China. China is a hyper epidemic area for hepatitis B virus and hepatitis C virus infection and accounts for 45% of the deaths from HCC worldwide [3]. Among the trials included in this systematic review, 6 were performed in China and 5 were published in Chinese. Nevertheless, the relationship of the difference of the effect of octreotide on the survival of western and Chinese patients and the SSTR expression remains unknown and requires further investigation.
There were some limitations of this study. First, the funnel plots showed obvious asymmetry, suggesting the possibility of bias. Second, most studies did not detect SSTR expression before including patients. Third, whether there is difference in SSTR expression between western and Chinese patients remains unknown.
Conclusions
On the basis of the evidence we evaluated in this meta-analysis, we conclude that octreotide could improve the survival of patients with advanced HCC, but perhaps not in western countries. The role of detecting SSTR expression in the administration of octreotide in advanced HCC needs further investigation.
Table 4.
Sensitivity analysis of the included trials.
| Number of studies | RR (95%CI) | P | |
|---|---|---|---|
| Studies using placebo | 4 | ||
| 6-mo survival rate | 4 | 1.13 (0.95–1.35) | 0.16 |
| 12-mo survival rate | 4 | 1.25 (0.63–2.48) | 0.52 |
| 24-mo survival rate | 3 | 0.60 (0.33–1.10) | 0.10 |
| Studies using no treatment | 5 | ||
| 6-mo survival rate | 5 | 1.94 (1.44–2.61) | <0.0001 |
| 12-mo survival rate | 5 | 4.51 (2.33–8.73) | <0.00001 |
| 24-mo survival rate | 2 | 7.32 (0.92–58.14) | 0.06 |
| High-quality studies | 6 | ||
| 6-mo survival rate | 6 | 1.25 (1.00–1.57) | 0.05 |
| 12-mo survival rate | 6 | 1.80 (0.89–3.64) | 0.10 |
| 24-mo survival rate | 3 | 0.60 (0.33–1.10) | 0.10 |
| Studies in western countries | 4 | ||
| 6-mo survival rate | 4 | 1.27 (0.96–1.67) | 0.09 |
| 12-mo survival rate | 4 | 1.69 (0.74–3.87) | 0.22 |
| 24-mo survival rate | 3 | 0.60 (0.33–1.10) | 0.10 |
| Studies in China | 5 | ||
| 6-mo survival rate | 5 | 1.78 (1.25–2.52) | 0.001 |
| 12-mo survival rate | 5 | 4.59 (1.99–10.59) | 0.0003 |
| 24-mo survival rate | 2 | 7.32 (0.92–58.14) | 0.06 |
| Studies including >50 patients | 6 | ||
| 6-mo survival rate | 6 | 1.32 (1.03–1.70) | 0.03 |
| 12-mo survival rate | 6 | 2.37 (1.02–5.48) | 0.04 |
| 24-mo survival rate | 4 | 0.87 (0.33–2.32) | 0.78 |
| Studies with follow-up > 2 years | 5 | ||
| 6-mo survival rate | 5 | 1.27 (1.01–1.61) | 0.05 |
| 12-mo survival rate | 5 | 2.14 (0.89–5.15) | 0.09 |
| 24-mo survival rate | 5 | 1.08 (0.40–2.94) | 0.88 |
Footnotes
Potential competing interests: There are no competing interests in this study.
Source of support: Self financing
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–56. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 3.Shi J, Zhu L, Liu S, et al. A meta-analysis of case-control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer. 2005;92:607–12. doi: 10.1038/sj.bjc.6602333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Serag HB, Davila JA, Petersen NJ, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–23. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 5.Cho YK, Kim JK, Kim MY, et al. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453–59. doi: 10.1002/hep.22648. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48:520–37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 8.Cheng AL, Kang YK, Chen ZD, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 9.Liu HL, Huo L, Wang L. Octreotide inhibits proliferation and induces apoptosis of hepatocellular carcinoma cells. Acta Pharmacol Sin. 2004;25:1380–86. [PubMed] [Google Scholar]
- 10.Xidakis C, Kolios G, Valatas V, et al. Effect of octreotide on apoptosis-related proteins in rat Kupffer cells: a possible anti-tumour mechanism. Anticancer Res. 2004;24:833–41. [PubMed] [Google Scholar]
- 11.Lamberts SW, van der Lely AJ, de Herder WW, et al. Octreotide. New Engl J Med. 1996;334:246–54. doi: 10.1056/NEJM199601253340408. [DOI] [PubMed] [Google Scholar]
- 12.Samonakis DN, Notas G, Christodoulakis N, et al. Mechanisms of action and resistance of somatostatin analogues for the treatment of hepatocellular carcinoma: a message not well taken. Dig Dis Sci. 2008;53:2359–65. doi: 10.1007/s10620-007-0175-9. [DOI] [PubMed] [Google Scholar]
- 13.Slijkhuis WA, Stadheim L, Hassoun ZM, et al. Octreotide therapy for advanced hepatocellular carcinoma. J Clin Gastroenterol. 2005;39:333–38. doi: 10.1097/01.mcg.0000155136.35315.de. [DOI] [PubMed] [Google Scholar]
- 14.Kouroumalis E, Skordilis P, Thermos K, et al. Treatment of hepatocellular carcinoma with octreotide: a randomized controlled study. Gut. 1998;42:442–47. doi: 10.1136/gut.42.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samonakis DN, Moschandreas J, Arnaoutis T, et al. Treatment of hepatocellular carcinoma with long acting somatostatin analogues. Oncol Rep. 2002;9:903–7. [PubMed] [Google Scholar]
- 16.Yuen MF, Poon RT, Lai CL, et al. A randomized placebo-controlled study of long-acting octreotide for the treatment of advanced hepatocellular carcinoma. Hepatology. 2002;36:687–91. doi: 10.1053/jhep.2002.35071. [DOI] [PubMed] [Google Scholar]
- 17.Becker G, Allgaier HP, Olschewski M, et al. Long-acting octreotide versus placebo for treatment of advanced HCC: a randomized controlled double-blind study. Hepatology. 2007;45:9–15. doi: 10.1002/hep.21468. [DOI] [PubMed] [Google Scholar]
- 18.Barbare JC, Bouche O, Bonnetain F, et al. Treatment of advanced hepatocellular carcinoma with long-acting octreotide: A phase III multicentre, randomised, double blind placebo-controlled study. Eur J Cancer. 2009;45:1788–97. doi: 10.1016/j.ejca.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Guo TK, Hao XY, Ma B, et al. Octreotide for advanced hepatocellular carcinoma: a meta-analysis of randomized controlled trials. J Cancer Res Clin Oncol. 2009;135:1685–92. doi: 10.1007/s00432-009-0615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia WD, Zhang CH, Xu GL, et al. Octreotide therapy for hepatocellular carcinoma: a systematic review of the evidence from randomized controlled trials. Hepatogastroenterology. 2010;57:292–99. [PubMed] [Google Scholar]
- 21.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 22.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med. 2001;135:982–89. doi: 10.7326/0003-4819-135-11-200112040-00010. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609–13. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 24.Farooqi JI, Farooqi RJ. Efficacy of octreotide in cases of inoperable hepatocellular carcinoma: a clinical trial. J Coll Physicians Surg Pak. 2000;10:258–60. [Google Scholar]
- 25.Wu P, Gu XY, Jiang Z, et al. Efficacy of octreotide in advanced hepatocellular carcinoma: A clinical trial. Chin J Hepatobiliary Surg. 2001;7:766–68. [Google Scholar]
- 26.Yang MN, Xiao B, Wang XL, et al. Effects of octreotide in elderly patients with advanced primary hepatic cancer. J Clin Med in Pract. 2003;7:302–4. [Google Scholar]
- 27.Zhang L, Jiang Z, Li SY, et al. Clinical Study of octreodide for advanced primary Liver Cancer. Chin Clin Oncol. 2004;9:514–17. [Google Scholar]
- 28.Dimitroulopoulos D, Xinopoulos D, Tsamakidis K, et al. Long acting octreotide in the treatment of advanced hepatocellular cancer and overexpression of somatostatin receptors: randomized placebo-controlled trial. World J Gastroenterol. 2007;13:3164–70. doi: 10.3748/wjg.v13.i23.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ou SQ, Chen ZQ, Ma YL. Clinical study of octreotide for advanced hepatocellular carcinoma. Hainan Med J. 2007;18:19–20. [Google Scholar]
- 30.Zhang B, Xu F. The clinical observation of octreotide in the treatment of 45 patients with advanced primary liver carcinoma. J Basic Clin Oncol. 2010;23:52–54. [Google Scholar]
- 31.El-Din HG, Ghafar NA, Saad NE, et al. Relationship between codon 249 mutation in exon 7 of p53 gene and diagnosis of hepatocellular carcinoma. Arch Med Sci. 2010;6:348–55. doi: 10.5114/aoms.2010.14254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bassiouny AE, Abou-Shousha T, Moussa M, et al. Hepatic mRNA expression of histone (H3): an early predictor of tumorgenic changes in chronic hepatitis C. Arch Med Sci. 2009;5:506–12. [Google Scholar]
- 33.Bassiouny AE, El-Hassan SA, Moussa M, et al. Down-regulation of intrahepatic CD16+ and CD56+ immune cells in chronic Hepatitis C virus infection and HCV-related hepatocellular carcinoma. Arch Med Sci. 2009;5:321–28. [Google Scholar]
- 34.Cebon J, Findlay M, Hargreaves C, et al. Somatostatin receptor expression, tumour response, and quality of life in patients with advanced hepatocellular carcinoma treated with long-acting octreotide. Br J Cancer. 2006;95:853–61. doi: 10.1038/sj.bjc.6603325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynaert H, Rombouts K, Vandermonde A, et al. Expression of somatostatin receptors in normal and cirrhotic human liver and in hepatocellular carcinoma. Gut. 2004;53:1180–89. doi: 10.1136/gut.2003.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]