Summary
The most prevalent forms of bone cancer are osteosarcoma, chondrosarcoma, and Ewing’s sarcoma. Although chemotherapy and radiotherapy have replaced traditional surgical treatments, survival rates have undergone only marginal improvements. Current knowledge of the molecular pathways involved in each type of cancer has led to better approaches in cancer treatment. A number of cell signaling molecules are involved in tumorigenesis, and specific targets have been identified based on these signal transducers. This review highlights some of the important cellular pathways and potential therapeutic targets, tumor site-specific irradiation techniques, and novel drug delivery systems used to administer these drugs.
Keywords: chondrosarcoma, Ewing’s sarcoma, osteosarcoma, treatment
Background
Primary bone tumors most commonly originate in the bone. The malignant forms of these tumors manifest as osteosarcoma, chondrosarcoma, Ewing’s sarcoma, malignant fibrous histiocytoma, fibrosarcoma, and some other sarcoma types [1]. Gaining an understanding of various factors such as tumor environment, mechanisms of apoptosis and cell-cycle control, and cellular and molecular pathways could help in identifying novel molecular markers and targeted drug therapies for treatment of these tumors. In this review, we present recent developments in the treatment strategies of the 3 most prevalent forms of primary bone cancer: osteosarcoma, chondrosarcoma, and Ewing’s sarcoma.
Relevant literature searches for this review were carried out using PubMed and Google Scholar. The search terms included “osteosarcoma,” “chondrosarcoma,” and “Ewing’s sarcoma.” Subsequently, we conducted searches with MESH terminology to determine the current trends in each type of bone tumor. These included diagnosis and treatment and related current trials and preclinical research.
Osteosarcoma
Although bone tumors are rare neoplasms [1], many of them, including osteosarcoma, affect young children and have their origin in the metaphyseal areas of long bones [2, 3]. Osteosarcoma has been reported to be the sixth most common cancer in children and adolescents [3], and the 5-year survival rate is about 65% [4]. Osteosarcoma mostly occurs in adolescents – an age group in which bone growth is rapid. Therefore, a direct relationship may exist between rapid bone growth and osteosarcoma occurrence in this age group [3,5]. The skeletal growth period is longer in males than in females; hence, osteosarcoma is generally more common in males than in females [6].
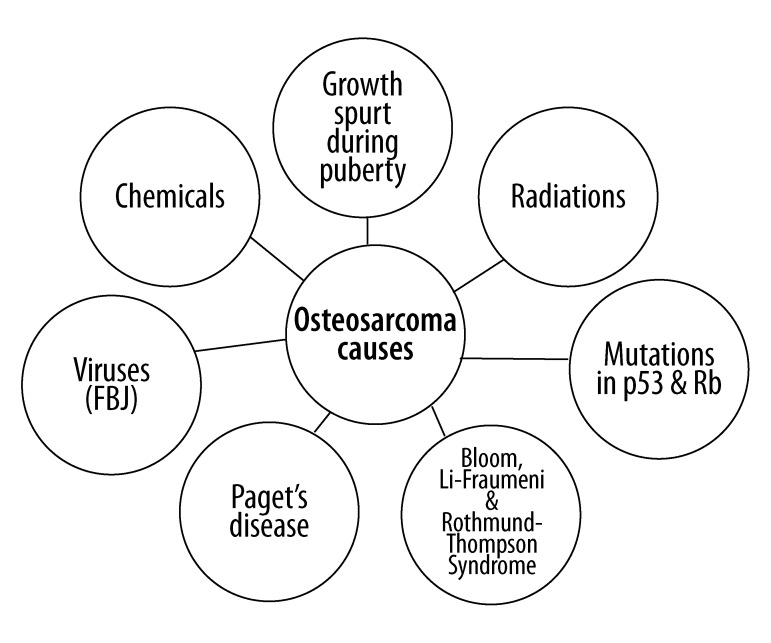
Fuchs and Pritchard [7] have suggested that certain agents contribute to the pathogenesis of osteosarcoma by altering the genetic make-up. These include radiation, chemical agents like beryllium, and viruses such as Finkel-Biskis-Jinkins (FBJ) virus containing the src oncogene. Figure 1 depicts the various causes of osteosarcoma.
Figure 1.
The causative agents contributing to the pathogenesis of osteosarcoma by altering the genetic make-up include the mutations in p53 and Rb tumor suppressor pathways, teenage growth spurt, Paget’s disease, chemical agents such as beryllium, radiations, and viruses such as Finkel-Biskis-Jinkins (FBJ) containing the src oncogene.
Osteosarcoma is generally fatal due to its tendency to metastasize to the lungs. Therefore, the survival of patients with osteosarcoma is a significant challenge despite the use of aggressive chemotherapy and radiotherapy. Attempts to modify chemotherapy and surgical procedures, accompanied by the application of radiotherapy, have made limb salvage (bone replacement) procedures possible, thereby increasing survival rates to a certain extent [8]. However, these efforts have not led to any major improvements in treatment outcome and quality of life of osteosarcoma patients. The current management of osteosarcoma is still associated with radical surgery involving excision of the primary tumor and/or amputation of the affected part, and painful and expensive chemotherapy [8].
Grading of osteosarcoma
Histological grading of sarcomas was introduced to improve the prediction of disease prognosis and to facilitate patient treatment decisions. It also plays an important role in comparing patient outcomes in clinical trials [9]. There are several grading systems for osteosarcoma. The Musculoskeletal Tumor Society staging system [10,11] for osteosarcoma is based on tumor grade (I = low-grade; II = high-grade), tumor extension (A = intra-compartmental; B = extra-compartmental), and presence of macroscopic distant metastases (III). Localized high-grade osteosarcoma is classified as stage IIA or IIB. The presence of metastatic disease, regardless of the extent of the primary lesion, represents stage III disease. The American Joint Committee on Cancer Staging System is similar to the Musculoskeletal Tumor Society Staging System; however, it classifies stage III as any tumor with skip metastases [14]. Tumor nodules growing outside the reactive rim but within the same bone or across a neighboring joint are termed “skip lesions” and represent regional intraosseous or transarticular metastases, respectively [12,13]. Stages I and II are subdivided into categories A and B based on tumor size being greater or less than 8 cm in any dimension, respectively, rather than tumor extension. This system also uses an extra stage IV, which is divided into IVA or M1a describing pulmonary metastases, and IVB or M1b describing other metastases [14]. The European and American Osteosarcoma Study Group uses the Enneking system to stage bone sarcomas, which is based on the grade of the tumor (G), the local extent of the primary tumor (T), and metastasis of the tumor to regional lymph nodes or other organs (M) [15]. Tumors are classified as either low grade (G1) or high grade (G2), and the extent of the primary tumor is classified as either localized intra-compartmental (T1) or extended extra-compartmental (T2). Tumors that have not spread to the lymph nodes or other organs are classified as M0, while those that have spread are M1 [15]. In addition to clinical grading, Meister et al characterized osteosarcomas as ranging from + to +++ by semi-quantitative evaluation of histological features based on cellular atypism and mitotic activity [16].
Diagnosis
Laboratory findings
Increased levels of erythrocyte sedimentation rate (ESR), alkaline phosphatase (AP) and lactate dehydrogenase (LDH) are indicators of osteosarcoma [4].
Radiographic studies
Preoperatively, radiographic studies are useful for determining the location of the primary tumor and for detecting metastasis. The current conventional and novel methods for radiographic diagnosis include plain radiographs, computed tomography (CT) scans, and magnetic resonance imaging (MRI). CT scans and MRI are employed to investigate the extent of the tumors and to study the involvement of surrounding structures such as blood vessels, nerves, and soft tissues in the development of bone cancer [4,13,17]. CT is often used to improve assessment, as it can delineate tumors in inaccessible sites. The 3-dimensional anatomy of the pelvis is difficult to interpret with conventional radiology; CT is particularly helpful in such situations [10]. CT can reveal the extent of both intra- and extra-osseous spread, as well as metastasis [18]. Vanel et al have shown that CT is the best technique to detect and enumerate pulmonary metastases originating from an osteosarcoma [19]. However, O’Flanagan et al argue that since MRI best demonstrates soft-tissue extension and bone marrow involvement, it should be preferred over CT [20]. CT has limited value in the assessment of intra-articular tumor growth [21]. Eftekhari et al. state that MRI is the most specific modality for staging [22]. According to Schima et al, MRI is highly sensitive for detecting joint invasion in patients with osteosarcoma; however, they also note that false-positive diagnoses might lead to overstaging of the tumor, resulting in unnecessarily radical surgical procedures [23]. In a review of bone sarcomas, Lietman and Joyce report that MRI is the primary imaging technique for evaluating the proximity of bone lesions to neurovascular structures [24]. MRI can determine the relationship between the tumor and the neurovascular bundle, which helps in estimating the need for resection [13]. Another technology that is proving increasingly useful in the preoperative planning of pelvic tumors is magnetic resonance angiography [10].
Recently, radionuclides or radiopharmaceuticals have been used to assess the metabolic activity of tumors. These chemicals can localize to specific organs and are thus helpful in monitoring the site and spread of the tumor. Positron emission tomography-CT (PET-CT) is emerging as an important tool for assessing the response of tumors to neoadjuvant chemotherapy [25–27]. Hawkins et al demonstrated that [F-18]-fluorodeoxy-D-glucose (FDG)-PET is a successful noninvasive imaging tool that can be used for accurate discrimination between responding and nonresponding osseous tumors [25]. Bone scintigraphy involves a total body scan, and it helps in determining the intra-osseous extension of tumors and the sites of bone metastasis [13,17]. This method also identifies axial and appendicular skeletal metastases [13]. Thallium scintigraphy is particularly used for detecting local recurrence and for determining tumor response to neoadjuvant (preoperative) chemotherapy when MRI is not helpful [13].
Genetics of osteosarcoma
Osteosarcoma tumorigenesis is associated with vital genetic alterations, including the inactivation of tumor suppressor genes; gain, loss, or rearrangement of chromosomal regions; and misregulated signal transduction pathways [4,28,29]. Gaining an understanding of the molecular pathogenesis of osteosarcoma could expand research horizons for the development of prognostic biomarkers and targeted therapies in the management of osteosarcoma. Some important molecular pathways and associated mutations are explained below.
The retinoblastoma susceptibility gene (RB1) and p53 tumor suppressor gene are involved in the cell-cycle regulation [27,28] and pathogenesis of osteosarcoma [28]. The other altered genes that are involved in the alteration of RB1 and p53 pathways include the overexpressed murine double minute gene 2 (MDM2), cyclin-dependent kinase 4 (CDK4), and tumor growth factor β (TGF β) [30–32].
Overexpression of HER2/neu, alteration in receptor activator of NF-kB (RANK) signaling in pagetic patients, and mutations in the RECQL4 gene in Rothmund-Thomson syndrome patients are linked with the occurrence of osteosarcoma [28,29,33]. RANK is an important gene involved in the regulation of bone remodeling [34,35]. Further, alterations in RANK signaling are associated with Paget’s disease of the bone [29].
Surgery and radiotherapy
Cancer therapy aims to increase disease-free survival. The primary objective of cancer surgery is complete tumor excision; conservation of the limb is always the secondary objective, while amputation is the last choice [13]. Limb salvage (bone replacement) is also possible by careful resection of the tumor, followed by the reconstruction of a viable, functional limb, thereby leading to a better quality of life for the patient [4,13]. However, with the development of effective surgical procedures and chemotherapy regimens, the disease-free survival rate has substantially increased and limb removal is seldom necessary [8,13].
In the case of metastatic tumors, more aggressive chemotherapy and surgical regimens should be adopted for achieving higher disease-free survival [4]. Radiotherapy along with surgical resection is also recommended in the treatment program. It is generally used to treat lesions situated in inaccessible sites. Preoperative radiotherapy could be given before the surgery to increase the success rates of limb-amputation techniques and reduce the risk of recurrence of the tumor [4].
High-dose photon irradiation (50–70 Gy) can be used in combination with aggressive chemotherapy when tumors are located in inaccessible sites such as the pelvic bone, vertebral column, and base of the skull. This irradiation is also useful in patients who do not consent to surgery [28,36].
An innovative approach of carrying out intraoperative extracorporeal irradiation to the bone was recommended by Anacak et al. [37]. The affected bone was irradiated at 50 Gy and was then reimplanted into the body. No local recurrence or symptoms of graft failure were detected in the irradiated bone during the mean follow-up period of 22 months [37].
Chemotherapy
Before the 1970’s, when the treatment for osteosarcoma was mainly limb amputation, the 5-year survival rate of patients was 10–20% [3,36]. However, the combination of surgery and modern multiagent, dose-intensive chemotherapy drastically increased the 5-year survival rates of patients to about 60–70% [4,8,36,38]. The current protocols for osteosarcoma typically include neoadjuvant (preoperative) therapy, followed by adjuvant (postoperative) therapy, if required [13]. The most commonly administered chemotherapy drugs include cisplatin, doxorubicin, ifosfamide, and high-dose methotrexate with leucovorin calcium rescue [13,38].
Currently, attempts are being made to predict the responses of patients to preoperative chemotherapy based on their genetic profiles. One such attempt was made by Man et al. [39]. They developed a multigene classifier that could predict the response of osteosarcoma to preoperative chemotherapy at the time of diagnosis. Forty-five genes were identified, and poor responders had overexpression of these genes [40]. Despite aggressive chemotherapy involving high doses of methotrexate, relapse and pulmonary metastases have been found to be very common in these patients [4].
The therapeutic potential of bisphosphonates such as zoledronic acid, minodronate, risedronate, and alendronate has gained wide recognition in recent years due to their inhibitory effect (antitumor effects) on the growth of human osteosarcoma cells [40–44]. Additionally, Horie et al demonstrated the antitumor effects of zoledronic acid along with paclitaxel (PAC) or gemcitabine (GEM) in mouse osteosarcoma cells [45].
Molecular targets for therapy
Early assessment of disease is vital to study the physiology of tumor progression. As discussed earlier, surgical removal of the tumor may be combined with chemotherapy regimens, radiotherapy and immunotherapy. Toxicity is a common problem associated with aggressive chemotherapy. Due to low therapeutic indices of chemotherapeutic treatments, efficient molecular target drugs are currently being employed. They are promising from the viewpoint of maximizing the treatment potential for cancer with limited toxic effects. The important feature of these targeted drugs is their ability to locate and destroy tumor cells without harming the normal cells [46].
Interferon-γ is known to enhance the sensitivity of osteosarcoma to a number of chemotherapeutic drugs, and it plays an important role in angiogenesis [4]. Studies conducted by Yuan et al have demonstrated that a combination of interferon-γ and conventional chemotherapeutic agents such as doxorubicin may be used for the management of osteosarcoma with functional p53 [47]. It has also been demonstrated that interferon-γ sensitizes osteosarcoma cell lines via the upregulation of Fas receptors and caspase-8 [48].
Interleukins (ILs) have been adopted as immunotherapeutic molecules against osteosarcoma cell lines. Interleukin-2 (IL-2) is known to facilitate the generation of immunoglobulins and induce the differentiation of natural killer (NK) cells [4]. In a study on pre- and postoperative IL-2 and chemotherapy regimens for childhood osteosarcoma, Luksch et al. demonstrated the magnitude of NK cell activity. It was found that the NK cell count significantly correlated with the clinical outcome and IL-2-induced immune activation despite intensive chemotherapy [49]. Gaining an understanding of the role of receptor tyrosine kinase (RTK) in tumor growth factor signaling and pathogenesis of osteosarcoma has led to the development of target antibodies and inhibitors [50].
Clinically, targeted drug therapy with trastuzumab (Herceptin) has been very successful in breast cancer patients with human epidermal growth factor receptor 2 (HER2/neu) overexpression [51]. Hughes et al demonstrated that the expression of HER2 with epidermal growth factor receptors can contribute to osteosarcoma pathogenesis, thus providing another therapeutic target [52].
Insulin-like growth factors (IGFs) are involved in cell differentiation and growth, and their association with osteosarcoma with regard to rapid bone growth has been clearly delineated [50]. An overexpression of IGF-1 and IGF-2 was found to be present in a number of cancer cell lines, including primary sarcomas. The usefulness of the molecules that target the IGF-1 receptor is currently being investigated in a number of preclinical and clinical studies. OSI-906 (OSI Pharmaceuticals, NY, USA), which specifically targets the IGF-1 receptor, has entered phase 1 trials [50]. The molecule exhibited inhibitory activity in an IGF-1 receptor-dependent xenograft model in preclinical studies [53].
The importance of signal transduction in a number of cancer types has led to the development of drugs targeting various signal transduction pathways. Currently, research is being conducted to understand the signal transduction pathways that are activated by binding of the growth factors to the receptors. One such promising approach is the use of mammalian target of rapamycin (mTOR) inhibitors in osteosarcoma [54]. mTOR is a key component of the AKT signaling pathway, which plays an important role in cell growth and proliferation. The inhibitory activity of rapamycin against tumor cells has been demonstrated in murine osteosarcoma models and in pediatric clinical studies [55,56]. Gazzit et al observed that rapamycin strongly inhibits cell growth by increasing the G1-phase and decreasing the S-phase of the cell cycle, which also results in the downregulation of cyclin D1 and m-TOR [57].
The role of vascular endothelial growth factor (VEGF), which supports angiogenesis by the inhibition of apoptosis, has also been implicated in the treatment of osteosarcoma. Recently, a study on AZD2171, a specific inhibitor of the VEGF receptor, has shown encouraging results, with AZD2171 showing an inhibitory effect on solid osteosarcoma tumor xenografts [58].
Pigment epithelium-derived factor (PEDF), in contrast to VEGF, is a potent inhibitor of angiogenesis, and a decreased level of PEDF is associated with many types of tumors [59]. Studies conducted on osteosarcoma cell lines have demonstrated that PEDF inhibits angiogenesis by the suppression of VEGF and induction of apoptosis of endothelial cells via the Fas/FasL death pathway [60,61]. However, to our knowledge, the exact correlation between PEDF and osteosarcoma has not yet been studied. A recent study conducted by Choong et al demonstrated the in vivo and in vitro effects of overexpressed recombinant PEDF (rPEDF) in orthotopic models of osteosarcoma [62]. It revealed the multi-modal antitumor activity of PEDF via the inhibition of angiogenesis, cell proliferation, differentiation, and metastatic activity. The receptor activator of the nuclear factor-κB ligand (RANKL) is known to play an important role in osteoclast biology by controlling osteoclastogenesis and osteoclast activity [34]. Therefore, the inhibitors of RANKL are potential drug candidates, as they have been shown to exhibit antitumor effects with fewer adverse effects [50].
Denosumab, a monoclonal antibody inhibiting the activity of RANKL, has shown promising results in the treatment of patients with breast cancer-related bone metastases [63]. Its potential benefit in osteosarcoma is yet to be investigated. Therefore, clinical studies that investigate the role of RANKL inhibitors in osteosarcoma are envisaged.
The delivery of transferrin receptors, which are expressed by cancer cells, can be used to enhance the effectiveness of gene therapy for cancer. Nakase et al examined the efficacy of the p53 gene by introducing it into a transferrin receptor expressing a human osteosarcoma cell line (HOSM-1) [64]. Transferrin-mediated liposomes were used for the delivery. The results demonstrated the inhibitory effect of transferrin-mediated liposomes on tumor cells, suggesting an effective strategy for the treatment of osteosarcoma.
Rexin-G is the first gene therapy vector that has a cytocidal cyclin G1 construct [65]. It is the only broad-spectrum, tumor-targeted, systematically engineered genetic medicine that has been clinically adopted for cancer [65]. Chawla et al studied the activity of Rexin-G simultaneously in phase I and phase II clinical trials for chemotherapy-resistant metastatic sarcoma, pancreatic cancer, and breast cancer [66]. They also studied chemotherapy-resistant osteosarcoma in phase II trials. Their findings indicated that Rexin-G could control tumor growth and the progression of metastasis, thereby improving the survival rates of patients with chemotherapy-resistant osteosarcoma.
Table 1 depicts the other promising molecular markers that may help in the prognosis of osteosarcoma and may serve as potential treatment targets [67–69].
Table 1.
Examples of molecular markers in osteosarcoma prognosis.
| Tenascin-C | Cytochrome-C 1 (CYC-1) | CDH 11 gene | |
|---|---|---|---|
| Technique employed for gene identification | Microarray data and gene ontology analysis. | Surface Enhanced Laser Desorption/Ionization-Time of Flight-Mass Spectrometry (SELDI-TOF-MS). | Use of osteosarcoma xenografts. |
| Activity observed | The gene belongs to extracellular matrix (ECM). The gene is differentially distributed amongst cells with different metastatic potentials. |
The gene plays an important role in mitochondrial respiratory chain and apoptosis. High levels of CYC-1 observed among osteosarcoma patients; surgical removal of the tumor reduces the levels. |
The gene is critical for cell-cell adhesion. Activity of the gene is lower amongst osteosarcoma patients. |
Novel drug delivery systems
Novel drug delivery mechanisms have the potential to overcome the resistance exhibited by the tumor to conventional chemotherapeutic agents. They achieve this by maximizing the efficacy and minimizing the toxicity levels. The drug delivery systems employ synthetic polymers, microcapsules, cells, lipoproteins, and liposomes for efficient and controlled release of the drugs. Of these, gene therapy is the most promising modality that is being studied. The novel drug delivery systems have the potential to improve the management of inaccessible tumors, which require very high doses of chemotherapy. Most of these agents are currently under phase II or phase III of clinical testing [70].
Few therapeutic options are available for osteosarcoma patients with pulmonary metastases. One such system is the aerosolized drug formulation of cisplatin, which is currently in phase Ib/IIa trials. This system has been proven to be a useful model for the delivery of high concentrations of the drug in osteosarcoma patients with pulmonary relapse. The inhaled drug was well tolerated, with limited associated systemic adverse effects, and minimum discontinuation was required [70].
Stem cell therapy
Numerous studies have been conducted to investigate the effect of combining autologous stem cells with high doses of chemotherapy in the treatment of relapsed osteosarcoma [71,72]. Although these studies failed to demonstrate an improvement in survival rate, the combination of stem cell transplantation and high-dose chemotherapy remains an important therapeutic area for further exploration.
Chondrosarcoma
Chondrosarcoma is a diverse group of malignant bone tumors that have some relationship with the cartilage phenotype. The cells of different types of chondrosarcoma produce a cartilaginous (chondroid) matrix [73]. Chondrosarcoma is the third most common type of primary bone tumor (after myeloma and osteosarcoma) and accounts for 20–27% of all primary malignant osseous tumors [74]. The incidence in males and females is almost equal, and the mean age at diagnosis is 30–60 years [75]. Due to their extracellular matrix, low percentage of dividing cells, and poor vascularity, these tumors have a tendency to be chemo- and radiotherapy resistant. However, chondrosarcomas grow slowly and rarely metastasize [75].
Types of chondrosarcoma
Chondrosarcoma can be primary or secondary depending on whether the tumor has developed de novo or has superimposed on preexisting benign cartilaginous neoplasms such as enchondroma or osteochondroma. Based on the location and histologic characteristics, primary chondrosarcoma is categorized further into conventional intramedullary, clear cell, juxtacortical, myxoid, mesenchymal, extraskeletal, and dedifferentiated [75,76].
Chondrosarcoma is most frequently found in the bones that elongate due to endochondral ossification. The most common sites include proximal femur, proximal humerus, distal femur, and ribs [78]. The difference between the tumor types can be observed when they are compared histologically. Resting chondrocytes are found in mesenchymal chondrosarcoma, while hypertrophic chondrocytes are found in clear cell chondrosarcoma [77,78]. Osteochondroma recapitulates all the different levels of a growth plate and occurs at the surface of the bone [79]. In contrast, conventional peripheral and central chondrosarcomas generally contain proliferating chondrocytes lying in small lacunae [80].
Enchondromas, which are benign cartilage tumors, are generally found incidentally, most commonly in the bones of the hand and feet. Radiographically, they appear as small cartilage nests (generally <5 cm in diameter) with multiple intralesional calcifications. Occasionally, very mild endosteal scalloping occurs; however, true cortical invasion and the involvement of adjacent soft tissues are rare. Histologically, the islands of normal hyaline cartilage are surrounded by the lamellar bone. On rare occasions, enchondromas become symptomatic or lead to a pathologic fracture and the patient then needs surgical treatment [81]. Juxtacortical chondrosarcoma arises on the surface of the bone and is histologically identical to conventional intramedullary chondrosarcoma [82].
Mesenchymal chondrosarcomas are highly aggressive tumors that are radiographically and histologically distinct from the conventional and de-differentiated types. They are eccentrically located in the bone and commonly extend into soft tissues. This variant of chondrosarcoma is characterized by a bimorphic pattern that is composed of highly undifferentiated small round cells (similar to Ewing’s sarcoma) and islands of well-differentiated hyaline cartilage. This tumor generally affects young adults and teenagers and shows a widespread distribution in the skeleton. The craniofacial bones, ribs, ileum, and vertebrae are the most common sites [83].
Approximately 10% of all chondrosarcomas are of the de-differentiated type. The most common sites of involvement are the pelvic bones, femur, and humerus; however, they may also occur in the head, spine, breast, and prostate. These tumors form a distinct variety of chondrosarcoma – they contain 2 clearly defined components: a well-differentiated cartilage tumor (enchondroma or chondrosarcoma grade I and II) that is juxtaposed to a high-grade non-cartilaginous sarcoma. Histologically, there is a typical abrupt transition between the cartilaginous and non-cartilaginous components. Both tumor components are evident in varying proportions. The malignant non-cartilaginous component is most frequently a malignant fibrous histiocytoma, osteosarcoma, or fibrosarcoma, although other malignant tumors have been reported as the differentiated component. The cartilaginous and non-cartilaginous components are often adjacent, and the term “collision of two tumors” has been applied to this lesion. Radiographically, the tumor produces an ill-defined, lytic, and intra-osseous lesion associated with cortical disruption and extending into the soft tissues [84].
Grading of chondrosarcoma
Conventional chondrosarcoma is divided into 4 histologic grades based on its appearance under a microscope. The grading is primarily based on the nuclear size of tumor cells, nuclear staining (hyperchromasia or darker staining of nuclear material), and cellularity [85]. Grade I (or low-grade) tumors closely resemble normal cartilage; however, they may surround areas of lamellar bone (this is not seen in benign lesions) or may show atypical cells, including binucleate forms (cells with 2 nuclei). It is difficult to distinguish between enchondroma and central grade I chondrosarcoma, and conventional radiography is not reliable [86]. Grade II (or intermediate-grade) tumors are more cellular, with a greater degree of nuclear atypia, hyperchromasia, and nuclear size [87]. Grade III (or high-grade) tumors have significant areas of marked pleomorphism, large cells with more hyperchromatic nuclei than grade II tumors, occasional giant cells, and abundant necrosis. Mitosis is frequently detected. Myxoid changes or chondroid matrix liquefaction is a common feature of chondrosarcoma, particularly in grade II and grade III lesions. Grade I and grade II tumors have the highest occurrence, whereas grade III tumors are rare and are thought to arise from 1 of the other 3 histologic subtypes or from a benign precursor [74]. De-differentiated chondrosarcomas are highly malignant, particularly aggressive (they grow rapidly and disturb surrounding tissues), and have a poor prognosis [84].
Diagnosis
Benign cartilaginous tumors are asymptomatic, whereas malignant tumors almost always produce symptoms such as local swelling and pain. Diagnosis should be made in a multidisciplinary setting, based on clinical, radiological, and histological aspects. CT and MRI are vital adjunct tools for accurate tumor characterization [88]. It is particularly advised that CT be performed in the pelvis and other flat bones where it may be difficult to discern the pattern of bone destruction and the presence of matrix mineralization. MRI is required to define the extent of intra-osseous and soft tissue involvement [75].
Surgery and radiotherapy
Surgical resection remains the mainstay of treatment of chondrosarcomas. The extent of surgical resection and adjuvant therapy is dependent on the clinical and histologic characteristics of the lesions. While wide, en-bloc excision is ideal for intermediate- and high-grade chondrosarcoma, in low-grade chondrosarcoma, extensive intralesional curettage followed by local adjuvant treatment (e.g., phenolization or cryosurgery) and filling the cavity with bone graft has shown promising long-term clinical results and satisfactory local control [89,90]. Recently, Okada et al. treated 2 cases of grade 1 chondrosarcoma by curettage with pasteurization in situ, and long-term follow-up showed excellent oncological results. In addition, there was no functional deficit in either case [91]. Local adjuvant treatment can be successfully used only in lesions confined to the bone [90].
Chondrosarcomas are considered relatively radiotherapy resistant. Radiotherapy can be considered after incomplete resection, aiming at maximal local control (curative) and in situations where resection is not feasible or would cause unacceptable morbidity (palliative). For curative intentions, doses >60 Gy are required to achieve local control [75]. Due to the limitations of conventional radiotherapy, the role of photon radiotherapy in chondrosarcoma has been tested. This therapy is advantageous in that the exit dose after energy deposition in the target volume leads to better sparing of critical structures close to the tumor. It has been found that proton radiotherapy is beneficial in incompletely resected chondrogenic tumors of the skull base and axial skeleton. Local control rates of 85–100% with mixed photon-proton or proton-only protocols (doses up to 79 cobalt Gray equivalents) have been reported by several authors [92–95].
Radiotherapy with carbon ions or other charged particles represents another attractive radiation modality, which combines the physical advantages of protons with a higher radiobiological activity. Schulz-Ertner et al. recently reported the effectiveness and toxicity of carbon ion radiotherapy in chondrosarcomas of the skull base [96].
Chemotherapy
Chemotherapy is generally not effective in chondrosarcoma, particularly in the most frequently observed conventional type and the rare (low-grade) clear-cell variant. An explanation for chemotherapy resistance may be the expression of the multidrug-resistance 1 gene P-glycoprotein, resulting in resistance to doxorubicin in vitro[97]. The effect of chemotherapy on grade II and III chondrosarcomas is difficult to assess because the number of reported cases is very low and the reported series are mostly retrospective. There is an urgent need for the inclusion of these patients in prospective trials. Due to the lack of clear evidence, the role of adjuvant chemotherapy in de-differentiated chondrosarcoma remains unclear, and the standard use of adjuvant chemotherapy outside a clinical protocol should be reconsidered [75].
However, chemotherapy appears to be effective in the treatment of mesenchymal chondrosarcoma. Huvos et al. reported a retrospective series of mesenchymal chondrosarcoma patients treated with preoperative chemotherapy; they observed 3 complete and 3 partial responses (all T-10 or T-11 protocols) and 3 nonresponders (all high-dose methotrexate monotherapy) among 9 patients treated with preoperative chemotherapy [98]. The T-10 protocol and its variants comprise a multi-agent regimen with high-dose methotrexate, doxorubicin, cisplatin, and a combination of bleomycin, cyclophosphamide, and dactinomycin [98–100]. The histologic response to neoadjuvant chemotherapy using the T-10 protocol is an important prognostic factor in patients with non-metastatic disease [100,101]. This observation has influenced recent treatment regimens [101]. The T11 protocol of the Memorial Sloan-Kettering Cancer Center is also a multi-drug chemotherapy regimen, including actinomycin, doxorubicin, cyclophosphamide, vincristine, methotrexate, and bleomycin [102]. Nooij et al observed 1 of 2 good pathological responses in the primary tumor after preoperative doxorubicin-cisplatin combination therapy; however, only a limited effect was observed in 4 patients treated with the same regimen in a metastatic setting (2 with stable diseases) [103]. Thus, patients with mesenchymal chondrosarcoma could be considered for adjuvant chemotherapy.
Molecular targets for therapy
Recently, substantial new insights into molecular biology, cytogenetics, and immunopathology have helped researchers gain a better understanding of the disease, and this should help in the development of targeted treatments. The new therapeutic options available include agents targeting the molecular pathways of the disease, hormonal therapy, and antiangiogenesis therapy.
The exostosin (EXT) genes have been implicated in hereditary multiple exostoses – an autosomal dominant disorder characterized by multiple osteochondromas. The 3 genes related to this disease are EXT1, EXT2, and EXT3. These tumor suppressor genes code for proteins called glycosyltransferases and are involved in the biosynthesis of heparan sulfate proteoglycans. Heparan sulfate proteoglycans bind and create gradients of Indian hedgehog (IHH), parathyroid related protein (PThRP), and fibroblast growth factor (FGF), all of which are involved in cartilage growth and maturation. Thus, the loss of a binding protein for growth regulating proteins results in abnormal growth [104].
The IHH/PThRP pathway is active in multiple enchondromatosis and potentially the development of chondrosarcoma. In the growth plate, IHH maintains chondrocytes in the proliferative phase, and PThRP causes the downregulation of IHH. Preclinical studies have confirmed the role of IHH/PThRP in tumorigenesis [105,106]. This has led researchers to propose IHH blocking compounds as promising therapy for cartilaginous neoplasms [106–108]. A study by Miyaji et al revealed that monoclonal antibody against PTHLH induced differentiation and apoptosis of chondrosarcoma cells in vitro; this occurred because of downregulation of its downstream target BCL2 – the antiapoptotic protein [108].
More traditional suppressor genes such as p16 and p53 have also been implicated in the progression of chondrosarcoma to higher grades, although results for the latter are inconsistent [109–112]. Sex hormones, particularly estrogen, are important in the regulation of longitudinal skeletal growth that results from chondrocyte proliferation. They also play an important role in the differentiation in the epiphyseal growth plates of long bones. In chondrosarcoma, the receptor for estrogen signaling is present in the nucleus, as revealed by immunohistochemistry, thereby suggesting that estrogen signaling is active [113]. Further, most chondrosarcomas have been shown to express aromatase and have specific activity in primary cultures and in an established chondrosarcoma cell line. These observations suggest that antiestrogen treatment could be used for the treatment of chondrosarcoma.
Hypoxia-induced hypoxia-inducible factor-1a (HIF-1a) expression is consistent with the hypothesis that hypoxia is a driving force behind the angiogenic activity in chondrosarcoma [114]. The most important angiogenic cytokine activated by HIF-1a is VEGF, which alleviates the hypoxic condition by stimulating vascular ingrowth. Inhibiting the expression of HIF-1a by using the strategy of short inhibitory RNA sequences complementary to HIF-1a caused an upregulation of VEGF mRNA [115]. This confirms the role of HIF-1a as an antiangiogenic target.
Other markers of angiogenesis – cysteine proteinases (cathepsins) and matrix metalloproteinases (MMP) – are involved in the destruction of the extracellular matrix. Overexpression of the proteolytic activity of MMP 1, 2, 9, and 13 and cathepsin B and L correlated with a high histological grade in chondrosarcomas. Thus, there is a potential role for cathepsin and MMP inhibitors in the treatment of chondrosarcoma [116]. Additionally, prostaglandin G/H synthase 2 (COX2), a mediator of angiogenesis, was shown to be expressed in chondrosarcoma [117]. Selective COX2 inhibitors can therefore be effective in the treatment of chondrosarcoma.
Studies have reported incidental responses of chondrosarcomas to novel agents, including recombinant human Apo2L/tumor necrosis factor receptor apoptosis-inducing ligand (TRAIL) and VEGF antisense [118,119]. A study by Sulzbacher et al reported the occurrence of platelet-derived growth factor receptor α (PDGFα) in chondrosarcoma [120]. The study also showed that an increase in the expression of PDGFα was associated with increasing histological grade and shorter survival. If the role of PDGFα is confirmed, imatinib could serve as a possible treatment for chondrosarcoma [121].
Ewing’s Sarcoma
Ewing’s sarcoma (ES) is the most common primary bone tumor malignancy occurring in childhood and the second most common form of primary bone cancer. ES is typically characterized by a small round-cell tumor arising in the bones in approximately 85% of cases. It occurs less commonly in the soft tissues, including the mouth [122]. Caucasians are more prone to developing ES as compared to Asians, while the disease is non-existent in Africans or African-Americans [124]. Pathologically, this form of bone cancer is not a single condition – it is often a combination of several clinically related disorders with similar molecular alterations, (i.e., expression of tumor-specific chimeric oncoproteins). These oncoproteins are expressed through balanced chromosomal translocations that involve the EWS gene. Therefore, all ES-related disorders are collectively referred to as the Ewing sarcoma family of tumors (ESFT) [124]. This family comprises ES, extra-osseous ES, Askin tumor, and primitive neuroectodermal tumor (pNET).
The symptoms of ES include pain at the site of the mass and soft tissue swelling around the mass. Patients with metastatic disease may experience general symptoms such as non-specific signs of inflammation, anorexia, fever, malaise, fatigue, and weight loss. ES is predominantly a childhood malignancy – approximately 80% of ES cases occur in patients <20 years of age [123].
Grading of Ewing’s sarcoma
As described by Kotilingam, the AJCC soft tissue sarcoma staging system includes tumor (T), grade (G), node (N), and metastasis (M), with “a” indicating superficial (those lacking involvement of the superficial muscular fascia) and “b” indicating deep designations. Stages I to III describe localized soft tissue sarcoma, whereas soft tissue sarcoma that has metastasized to lymph nodes and/or other distant sites is classified as Stage IV. ES is always considered a high-grade lesion [125]. Costa et al described the NCI system, which depends on the histological features of the lesion. Grade 1 lesions include myxoid and well-differentiated liposarcomas and deep-seated dermatofibrosarcoma protuberans, leiomyosarcoma, malignant hemangiopericytoma, malignant schwannoma, and chordoid sarcoma or myxoid chondrosarcoma; Grade 2 includes lesions with absent or minimal necrosis (up to 15%); and Grade 3 includes lesions with moderate or marked necrosis [126]. ES is categorized as a Grade 3 lesion with poor prognosis [126]. Shortly after Costa et al published their initial grading system, the French Federation of Cancer Centers (FNCLCC) also proposed a 3-tier grading system (the French system). From the criteria examined, 3 independent prognostic factors (necrosis, mitotic activity, and degree of differentiation) were identified. Points were assigned for each category: 0–2 depending on the amount of necrosis (0%, ≤50%, and >50%), 1–3 for mitotic figures per 10 high-power fields (0–9, 10–19, and ≥20), and 1–3 according to the degree to which tumor cells resembled the normal counterpart [9,127]. Nomograms, which incorporate clinical, histological, and demographic findings, have proved accurate in predicting disease-specific survival in sarcomas. The FNCLCC system requires significant amounts of tissue for diagnosis and grading. Using core needle biopsies has decreased the amount of material available to type and grade sarcomas and has reduced the cost of out-patient care [127].
Diagnosis
Diagnostically, age is a very important factor in ES. This is because ES is normally associated with other small round-cell tumors such as small-cell carcinoma and large-cell lymphoma in patients older than 30 years of age. In patients younger than 5 years of age, ES may be associated with metastatic neuroblastoma. Therefore, depending on the age of the patient, these conditions need to be ruled out prior to treatment initiation.
Positive immunohistochemical staining of the MIC2 gene product is reported in 90% of cases. Although diagnostic imaging is often carried out to determine the disease, biopsy is considered the most definitive method [124]. As observed histologically, ES is composed of a homogeneous population of small round cells with a high nucleus-to-cytoplasm ratio. The cytoplasm in these cells is very limited, pale, vacuolated, and characterized by faded outer boundaries. Immunohistochemical and cytogenetic studies are often employed for differentiating between ES and other small cell tumors.
The most common genetic diagnostic factor, occurring in 85% of the cases, is the translocation of t(11;22)(q24: q12). Recently, translocations on chromosome 22, t(21;22)(q22;q12) and t(7;22)(p22;q12), involving the EWS gene have also been identified as diagnostic factors [124]. The CD99 antigen is consistently expressed in all the cells belonging to ESFT. They also serve as markers to distinguish ESFT from other small blue round cell tumors [128,129].
Surgery and radiotherapy
ES patients are usually receptive to chemotherapy as well as radiation therapy. Therefore, surgical techniques are generally employed only in severe cases. Large unresectable tumors are normally treated with radiation. Intralesional procedures have not shown any significant advantages over radiotherapy [130]. Conventionally, chemotherapy is followed by limb salvage and postoperative adjuvant chemotherapy. Local resections and reconstruction procedures have considerable advantages over the traditional amputation procedures and do not affect survival rates [130,131]. In a comprehensive analysis of a large series of patients with ESFT treated at the St. Jude Children’s Research Hospital over the last 2 decades, Rodrıguez-Galindo et al studied factors that influence local and distant control [132]. They found that optimal systemic and local treatment modalities are intertwined, and a combined therapeutic approach helps in the successful treatment of ESFT (both local and distant disease).
Chemotherapy
Prognosis is very poor in children with metastatic/relapsed disease; therefore, there exists a constant need for targeted and effective therapy. There have been numerous advances in the treatment strategies for ES; however, there has been very little improvement in the survival rates. It has been proposed that the treatment of ES should include chemotherapy to treat distant metastases regardless of initial staging.
Combination chemotherapy has been more useful in the treatment of ES in comparison to mono therapy. The most effective drugs are doxorubicin, cyclophosphamide, vincristine, actinomycin-D, ifosfamide, and etoposide. The Italian Association for Pediatric Hematology-Oncology and the National Council of Research conducted a study to assess the overall survival rate and event-free survival. They concluded that a combination of ifosfamide and etoposide used in addition to the standard chemotherapy with vincristine, actinomycin D, doxorubicin, and cyclophosphamide can improve the prognosis of ES. The authors suggested an improvement in event-free survival, which could be due to a change in the treatment strategy that accentuated tumor control. The new chemotherapeutic regime proposed by this group is suggested to be more conducive to surgery as well as to better prognosis [133]. A 5-year study conducted on patients with localized ES revealed that the 5-year event-free survival rate was 69% in patients receiving etoposide in addition to the standard 4-drug VACD (vincristine, actinomycin D, cyclophosphamide, and doxorubicin) therapy, while patients receiving VACD alone had a rate of 54% [134].
Molecular targets for therapy
The t(11;22)(q24: q12) translocation results in the formation of the EWS-Fli1 fusion gene constituting the 5′ end of the EWS gene (chromosome 22) fused with the 3′ end of the Fli1 gene (chromosome 11) [135,136,47]. The EWS-Fli1 fusion gene is normally detected in peripheral blood samples and is often employed as a diagnostic method. The active role of the EWS-Fli1 fusion gene in the oncogenetic process suggests that a mediator affecting the pathway could serve as a valuable molecular chemotherapeutic target. The inhibition of EWS-Fli1 by antisense oligonucleotides or small interfering RNAs (SiRNAs) has proven to be useful in arresting cell growth in vivo. However, the inhibitory potential was limited due to the rapid degradation and poor penetration abilities of the oligonucleotides and SiRNAs [137–140].
A study has also indicated that the immunomodulatory drug rapamycin has the potential to downregulate the EWS-Fli1 fusion gene [141]. This effect of rapamycin is mediated via the inhibition of the protein kinase mTOR that acts downstream of the phosphatidylinositol 3-kinase (PI3K) activation pathway [142].
The EWS-Fli1 fusion gene product regulates the expression of many cell cycle regulatory molecules that are involved in the control of the G1–S phase transition. It causes the upregulation of G1 cyclins (cyclin D1 and E) and induces the downregulation of CDK inhibitors (p16, p14, p21, and p27) [143–145]. The p53 tumor suppressor pathway is responsible for the positive regulation of p21, and it has been observed that the expression of p21 is lost in 55% of all ESFT cases [144,146]. The suppression of p21 by the EWS-Fli1 fusion gene results in the downregulation of histone acetyltransferase (HAT) activity, which in turn is responsible for the transcriptional regulation of the genes [147]. Histone acetylation modulates the chromatin structure and balance between HAT and histone deacetylases (HDAC) [148]. Thus, the treatment of ES cells with an HDAC inhibitor not only restores HAT activity via p21 induction, but also leads to the inhibition of cell growth and apoptosis [147,149]. The induction of apoptosis was also observed in wild-type p53 ESFT cells. Similar results have been observed following the overexpression of p27. The induction of p27 by using an adenoviral vector results in the inhibition of ESFT cell growth [150].
The formation of fusion proteins constituting the EWS and ETS family genes also affects important cellular pathways such as insulin growth factor 1 (IGF-1)/insulin-like growth factor 1 receptor (IGF-1R), epidermal growth factor (EGF)/human epidermal growth factor receptor 2 (HER2), and p53. Alterations in p53 have been observed in a majority of ESFT cell lines [151]. Lessnick et al constructed a primary human fibroblast model overexpressing the EWS-Fli1 gene [152]. These cells underwent growth arrest, thereby suggesting that growth arrest-abrogating collaborative mutations are essential for tumorigenesis. Further, gene expression profiling confirmed the upregulation of p53. Subsequent inhibition of p53 resulted in an improved growth arrest status, thus suggesting an active role of p53 in ES transcriptional profiling [152]. The mutations in p53 have been associated with a reduction in response to chemotherapy [153]. Adenoviral transfection of wild-type p53 in the ESFT cell line and RH1 containing mutp53 resulted in decreased viability and increased sensitivity to the chemotherapeutic agents cisplatin and doxorubicin [154].
A novel IGF-1R inhibitor, NVP-AEW541, has been shown to decrease the proliferation of ES cells by arresting the cells in the G1-phase and inducing apoptosis [155]. Treatment of HER2-overexpressing ES cell lines with trastuzumab, a humanized antibody, only moderately inhibited cell growth. However, when trastuzumab was combined with IGF-1R antibodies, growth inhibition of up to 60% was observed [156].
Another molecular target that has recently been identified is TRAIL. TRAIL binds to the receptors on the surface of cancer cells, thus initiating apoptotic signals. ES cells are highly susceptible (~80%) to TRAIL-mediated apoptosis in vitro[157–160]. Similar observations have been made in a mouse xenograft model [161]. On account of the significant apoptotic aspects of TRAIL in ES cells, several clinical studies employing TRAIL-R/R2 agnostic antibodies are currently underway [162,163]. ES cells express low levels of caspase-8 – an enzyme that is resistant to TRAIL-induced cell death. An in vitro study suggested that the addition of interferon-γ causes resistant cells to produce more caspase-8, thus increasing their sensitivity to TRAIL-induced death. A study conducted on human tumor tissues revealed that the administration of interferon-γ increases caspase-8 expression in deficient cells. Further, treatment with TRAIL resulted in cell death, thus indicating the role of caspase-8 in the restoration of cellular sensitivity to TRAIL. These results indicate the importance of interferon-γ and caspase-8 as therapeutic targets during ES [164].
Another study demonstrated that the addition of interferon-γ to ES cells increases the cellular sensitivity to TRAIL-mediated apoptosis via the upregulation of procaspase-8 [158–163]. Alterations in these pathways lead to enhanced tumor growth via the suppression of apoptosis. The inactivation of EWS/ETS could serve as a promising therapeutic target due to its role in tumorigenesis as well as its specificity for transformed cells alone. As mentioned earlier, CD99 is an important diagnostic factor for ESFT. In vitro studies suggest that the incubation of ES cells with an anti-CD99 antibody causes rapid cell aggregation and induces caspase-independent apoptosis. Additionally, anti-CD99 also increases the sensitivity of ESFT cells to chemotherapeutic agents [165,166]. Numerous small molecules targeting specific molecular pathways and kinases have been identified for the treatment of ES. These molecules have shown significant potential in various stages of the cell growth cycle. Combination treatment strategies with traditional chemotherapeutic agents and molecular targets have been suggested to synergistically arrest cell growth and reduce the chances of developing resistance among ES patients [167]. In a retrospective analysis of 72 patients diagnosed with ESFT, Kavalar et al. reported that the expression of EMA, NSE, CD99, TdT, bcl-2, p53, CD117, S-100, and CK MNF116 had no statistically significant impact on survival [168]. Tumor location was also not a significant prognostic factor. The age of the patient at the time of diagnosis and the presence of tumor necrosis were the only significant prognostic factors in the study [168].
Stem cell therapy
Recently, stem cells for ESFT have been identified and characterized. Suva et al. isolated a subpopulation of CD133+ tumor cells that displayed potential to initiate and sustain tumor growth [169]. This effect on tumor growth was possible via serial transplantation carried out in non-obese diabetic or severely immunocompromised mice. In vitro cell differentiation assays suggest that CD133+ cells retain the ability to distinguish between adipogenic, osteogenic, and chondrogenic lineages. These properties are characteristic of mesenchymal stem cells. Higher levels of OCT4 and NANOG were observed in CD133+ cells as compared to CD133− cells, as analyzed via quantitative real-time polymerase chain reaction studies [169].
Conclusions
Traditional chemo- and radio-therapeutic targets remain the main treatment modalities for these 3 types of bone cancer. However, several limitations exist, including development of drug resistance, toxicity associated with high-dose therapies, non-selective receptor targeting, and inaccessibility of tumor sites. Current research is targeted toward providing essential prognostic and therapeutic factors that could increase survival in patients with primary bone tumors. The progressive efforts are directed toward molecular pathogenesis, cell biology, signal transduction pathways, and targeted radiotherapy. Promising developments in these areas could reduce the need for surgical interventions, thus improving the quality of life of patients with bone tumors.
Footnotes
Source of support: Self financing
References
- 1.Unni KK, Dahlin DC. Grading of bone tumors. Semin Diagn Pathol. 1984;1:165–72. [PubMed] [Google Scholar]
- 2.Link MP, Gebhardt MC, Meyers PA. Osteosarcoma. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. Lippincott Williams and Wilkins; Philadelphia: 2002. pp. 1051–80. [Google Scholar]
- 3.Longhi A, Errani C, De Paolis M, et al. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006;32:423–36. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Ta HT, Dass CR, Choong PF, Dunstan DE. Osteosarcoma treatment: state of the art. Cancer Metastasis Rev. 2009;28:247–63. doi: 10.1007/s10555-009-9186-7. [DOI] [PubMed] [Google Scholar]
- 5.Cotterill SJ, Wright CM, Pearce MS, Craft AW. Stature of young people with malignant bone tumors. Pediatr Blood Cancer. 2004;42:59–63. doi: 10.1002/pbc.10437. [DOI] [PubMed] [Google Scholar]
- 6.Rytting M, Pearson P, Raymond AK, et al. Osteosarcoma in preadolescent patients. Clin Orthop Relat Res. 2000;373:39–50. doi: 10.1097/00003086-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs B, Pritchard DJ. Etiology of osteosarcoma. Clin Orthop Relat Res. 2002;397:40–52. doi: 10.1097/00003086-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson WS, Goorin AM. Current treatment of osteosarcoma. Cancer Invest. 2001;19:292–315. doi: 10.1081/cnv-100102557. [DOI] [PubMed] [Google Scholar]
- 9.Brown FM, Fletcher CD. Problems in grading soft tissue sarcomas. Am J Clin Pathol. 2000;114(Suppl):S82–89. doi: 10.1093/ppr/114.1.s82. [DOI] [PubMed] [Google Scholar]
- 10.Wolf RE. Sarcoma and metastatic carcinoma. J Surg Oncol. 2000;73:39–46. doi: 10.1002/(sici)1096-9098(200001)73:1<39::aid-jso11>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Picci P. Osteosarcoma (osteogenic sarcoma) Orphanet J Rare Dis. 2007;2:6–9. doi: 10.1186/1750-1172-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bickels J, Jelinek JS, Shmookler BM, et al. Biopsy of musculoskeletal tumors: current concepts. Clin Orthop. 1999;368:212–19. [PubMed] [Google Scholar]
- 13.Wittig JC, Bickels J, Priebat D, et al. Osteosarcoma: a multidisciplinary approach to diagnosis and treatment. Am Fam Physician. 2002;65:1123–32. [PubMed] [Google Scholar]
- 14.Comparison Guide: AJCC Cancer Staging Manual. Chicago: American Joint Committe on Cancer; Available from wwwcancerstagingorg. [Google Scholar]
- 15. [accessed on February 24, 2011]. http://www.ctu.mrc.ac.uk/euramos/about_o_grading_and_staging.asp.
- 16.Meister P, Konrad E, Lob G, et al. Osteosarcoma: histological evaluation and grading. Arch Orthop Trauma Surg. 1979;94:91–98. doi: 10.1007/BF00433573. [DOI] [PubMed] [Google Scholar]
- 17.Tan JZ, Schlicht SM, Powell GJ, et al. Multidisciplinary approach to diagnosis and management of osteosarcoma – a review of the St Vincent’s Hospital experience. Int Semin Surg Oncol. 2006;3:38–45. doi: 10.1186/1477-7800-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watt I. Radiology in the diagnosis and management of bone tumours. J Bone Joint Surg Br. 1985;67(4):520–29. doi: 10.1302/0301-620X.67B4.3875614. [DOI] [PubMed] [Google Scholar]
- 19.Vanel D, Henry-Amar M, Lumbroso J, et al. Pulmonary evaluation of patients with osteosarcoma: roles of standard radiography, tomography, CT, scintigraphy, and tomoscintigraphy. AJR Am J Roentgenol. 1984;143:519–23. doi: 10.2214/ajr.143.3.519. [DOI] [PubMed] [Google Scholar]
- 20.O’Flanagan SJ, Stack JP, McGee HM, et al. Imaging of intramedullary tumour spread in osteosarcoma. A comparison of techniques. J Bone Joint Surg Br. 1991;73:998–1001. doi: 10.1302/0301-620X.73B6.1955451. [DOI] [PubMed] [Google Scholar]
- 21.Soye I, Levine E, De Smet AA, Neff JR. Computed tomography in the preoperative evaluation of masses arising in or near the joints of the extremities. Radiology. 1982;143:727–32. doi: 10.1148/radiology.143.3.6952525. [DOI] [PubMed] [Google Scholar]
- 22.Eftekhari F. Imaging assessment of osteosarcoma in childhood and adolescence: diagnosis, staging, and evaluating response to chemotherapy. In: Rosen ST, editor. Cancer Treat Res. Vol. 152. Springer; 2009. pp. 33–62. [DOI] [PubMed] [Google Scholar]
- 23.Schima W, Amann G, Stiglbauer R, et al. Preoperative staging of osteosarcoma: efficacy of MR imaging in detecting joint involvement. AJR Am J Roentgenol. 1994;163:1171–75. doi: 10.2214/ajr.163.5.7976895. [DOI] [PubMed] [Google Scholar]
- 24.Lietman SA, Joyce MJ. Bone sarcomas: Overview of management, with a focus on surgical treatment considerations. Cleve Clin J Med. 2010;77(Suppl 1):S8–12. doi: 10.3949/ccjm.77.s1.02. [DOI] [PubMed] [Google Scholar]
- 25.Hawkins DS, Rajendran JG, Conrad EU, et al. Evaluation of chemotherapy response in pediatric bone sarcomas by F-18]-fluorodeoxy-D-glucose positron emission tomography. Cancer. 2002;94:3277–84. doi: 10.1002/cncr.10599. [DOI] [PubMed] [Google Scholar]
- 26.Brenner W, Bohuslavizki KH, Eary JF. PET imaging of osteosarcoma. J Nucl Med. 2003;44:930–42. [PubMed] [Google Scholar]
- 27.Huang TL, Liu RS, Chen TH, et al. Comparison between F-18-FDG positron emission tomography and histology for the assessment of tumor necrosis rates in primary osteosarcoma. J Chin Med Assoc. 2006;69:372–76. doi: 10.1016/S1726-4901(09)70275-8. [DOI] [PubMed] [Google Scholar]
- 28.Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9:422–41. doi: 10.1634/theoncologist.9-4-422. [DOI] [PubMed] [Google Scholar]
- 29.Hansen MF. Genetic and molecular aspects of osteosarcoma. J Musculoskelet Neuronal Interact. 2002;2:554–60. [PubMed] [Google Scholar]
- 30.Miller CW, Aslo A, Won A, et al. Alterations of the p53, Rb and MDM2 genes in osteosarcoma. J Cancer Res Clin Oncol. 1996;122:559–65. doi: 10.1007/BF01213553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wunder JS, Eppert K, Burrow SR, et al. Co-amplification and overexpression of CDK4, SAS and MDM2 occurs frequently in human parosteal osteosarcomas. Oncogene. 1999;18:783–88. doi: 10.1038/sj.onc.1202346. [DOI] [PubMed] [Google Scholar]
- 32.Franchi A, Arganini L, Baroni G, et al. Expression of transforming growth factor β isoforms in osteosarcoma variants: association of tgfβ1 with high-grade osteosarcomas. J Pathol. 1998;185:284–89. doi: 10.1002/(SICI)1096-9896(199807)185:3<284::AID-PATH94>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 33.Lindor NM, Furuichi Y, Kitao S, et al. Rothmund-Thomson syndrome due to RECQ4 helicase mutations: report and clinical and molecular comparisons with Bloom syndrome and Werner syndrome. Am J Med Genet. 2000;90:223–28. doi: 10.1002/(sici)1096-8628(20000131)90:3<223::aid-ajmg7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 34.Akiyama T, Dass CR, Choong PF. Novel therapeutic strategy for osteosarcoma targeting osteoclast differentiation, bone-resorbing activity, and apoptosis pathway. Mol Cancer Ther. 2008;7:3461–69. doi: 10.1158/1535-7163.MCT-08-0530. [DOI] [PubMed] [Google Scholar]
- 35.Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halperin EC. Osteosarcoma. In: Halperin EC, Constine L, Tarbell N, editors. Pediatric Radiation Oncology. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 291–318. [Google Scholar]
- 37.Anacak Y, Sabah D, Demirci S, Kamer S. Intraoperative extracorporeal irradiation and re-implantation of involved bone for the treatment of musculoskeletal tumors. J Exp Clin Cancer Res. 2007;26:571–74. [PubMed] [Google Scholar]
- 38.Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–52. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- 39.Man TK, Chintagumpala M, Visvanathan J, et al. Expression profiles of osteosarcoma that can predict response to chemotherapy. Cancer Res. 2005;65:8142–50. doi: 10.1158/0008-5472.CAN-05-0985. [DOI] [PubMed] [Google Scholar]
- 40.Ory B, Heymann MF, Kamijo A, et al. Zoledronic acid suppresses lung metastases and prolongs overall survival of osteosarcoma-bearing mice. Cancer. 2005;104:2522–29. doi: 10.1002/cncr.21530. [DOI] [PubMed] [Google Scholar]
- 41.Kubista B, Trieb K, Sevelda F, et al. Anticancer effects of zoledronic acid against human osteosarcoma cells. J Orthop Res. 2006;24:1145–52. doi: 10.1002/jor.20129. [DOI] [PubMed] [Google Scholar]
- 42.Kubo T, Shimose S, Matsuo T, et al. Inhibitory effects of a new bisphosphonate, minodronate, on proliferation and invasion of a variety of malignant bone tumor cells. J Orthop Res. 2006;24:1138–44. doi: 10.1002/jor.20177. [DOI] [PubMed] [Google Scholar]
- 43.Murayama T, Kawasoe Y, Yamashita Y, et al. Efficacy of the third-generation bisphosphonate risedronate alone and in combination with anticancer drugs against osteosarcoma cell lines. Anticancer Res. 2008;28:2147–54. [PubMed] [Google Scholar]
- 44.Cheng YY, Huang L, Lee KM, et al. Alendronate regulates cell invasion and MMP-2 secretion in human osteosarcoma cell lines. Pediatr Blood Cancer. 2004;42:410–15. doi: 10.1002/pbc.20019. [DOI] [PubMed] [Google Scholar]
- 45.Horie N, Murata H, Kimura S, et al. Combined effects of a third-generation bisphosphonate, zoledronic acid with other anticancer agents against murine osteosarcoma. Br J Cancer. 2007;96:255–61. doi: 10.1038/sj.bjc.6603548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vasir JK, Labhasetwar V. Targeted drug delivery in cancer therapy. Technol Cancer Res Treat. 2005;4:363–74. doi: 10.1177/153303460500400405. [DOI] [PubMed] [Google Scholar]
- 47.Yuan XW, Zhu XF, Huang XF, et al. Interferon-alpha enhances sensitivity of human osteosarcoma U2OS cells to doxorubicin by p53-dependent apoptosis. Acta Pharmacol Sin. 2007;28:1835–41. doi: 10.1111/j.1745-7254.2007.00662.x. [DOI] [PubMed] [Google Scholar]
- 48.Inaba H, Glibetic M, Buck S, et al. Interferon-γ sensitizes osteosarcoma cells to fas-induced apoptosis by up-regulating fas receptors and caspase-8. Pediatr Blood Cancer. 2004;43:729–36. doi: 10.1002/pbc.20151. [DOI] [PubMed] [Google Scholar]
- 49.Luksch R, Perotti D, Cefalo G, et al. Immunomodulation in a treatment program including pre- and post-operative interleukin-2 and chemotherapy for childhood osteosarcoma. Tumori. 2003;89:263–68. doi: 10.1177/030089160308900306. [DOI] [PubMed] [Google Scholar]
- 50.O’Day KG, Richard G. Novel therapeutic agents for osteosarcoma. Expert Rev Anticancer Ther. 2009;9:511–23. doi: 10.1586/era.09.7. [DOI] [PubMed] [Google Scholar]
- 51.Hudis CA. Trastuzumab-mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 52.Hughes DP, Thomas DG, Giordano TJ, et al. Cell surface expression of epidermal growth factor receptor and Her-2 with nuclear expression of Her-4 in primary osteosarcoma. Cancer Res. 2004;64:2047–53. doi: 10.1158/0008-5472.can-03-3096. [DOI] [PubMed] [Google Scholar]
- 53.Ji QS, Mulvihill M, Rosenfeld-Franklin M, et al. Preclinical characterization of OSI-906: a novel IGF-1R kinase inhibitor in clinical trials. Molecular Targets and Cancer Therapeutics Meeting Abstracts. 2007:Abstract C192. [Google Scholar]
- 54.Ferrari S, Palmerini E. Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr Opin Oncol. 2007;19:341–46. doi: 10.1097/CCO.0b013e328122d73f. [DOI] [PubMed] [Google Scholar]
- 55.Wan X, Mendoza A, Khanna C, Helman LJ. Rapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcoma. Cancer Res. 2005;65:2406–11. doi: 10.1158/0008-5472.CAN-04-3135. [DOI] [PubMed] [Google Scholar]
- 56.Houghton PJ, Morton CL, Kolb EA, et al. Initial testing (stage 1) of the mTOR inhibitor rapamycin by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:799–805. doi: 10.1002/pbc.21296. [DOI] [PubMed] [Google Scholar]
- 57.Gazitt Y, Kolaparthi V, Moncada K, et al. Targeted therapy of human osteosarcoma with 17AAG or rapamycin: characterization of induced apoptosis and inhibition of mTOR and Akt/MAPK/Wnt pathways. Int J Oncol. 2009;34:551–61. [PubMed] [Google Scholar]
- 58.Maris JM, Courtright J, Houghton PJ, et al. Initial testing of the VEGFR inhibitor AZD2171 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:581–87. doi: 10.1002/pbc.21232. [DOI] [PubMed] [Google Scholar]
- 59.Clark JC, Dass CR, Choong PF. A review of clinical and molecular prognostic factors in osteosarcoma. J Cancer Res Clin Oncol. 2008;134:281–97. doi: 10.1007/s00432-007-0330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cai J, Jiang WG, Grant MB, Boulton M. Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. J Biol Chem. 2006;281:3604–13. doi: 10.1074/jbc.M507401200. [DOI] [PubMed] [Google Scholar]
- 61.Volpert OV, Zaichuk T, Zhou W, et al. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat Med. 2002;8:349–57. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- 62.Choong P, Dass C, Ek E, Sim F. Pigment epithelium-derived factor inhibits osteosarcoma growth and metastasis2. J Bone Joint Surg Br. 2009;91(Suppl II):252. [Google Scholar]
- 63.Lipton A, Steger GG, Figueroa J, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25:4431–37. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 64.Nakase M, Inui M, Okumura K, et al. p53 gene therapy of human osteosarcoma using a transferrin-modified cationic liposome. Mol Cancer Ther. 2005;4:625–31. doi: 10.1158/1535-7163.MCT-04-0196. [DOI] [PubMed] [Google Scholar]
- 65.Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8:573–87. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chawla SP, Chua VS, Fernandez L, et al. Phase I/II and phase II studies of targeted gene delivery in vivo: intravenous Rexin-G for chemotherapy-resistant sarcoma and osteosarcoma. Mol Ther. 2009;17:1651–57. doi: 10.1038/mt.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiong W, Niu PY, Zhu WT, Chen J. Tenascin-C as a prognostic biomarker in osteosarcoma? Chin Med J (Engl) 2009;122:2737–43. [PubMed] [Google Scholar]
- 68.Li G, Zhang W, Zeng H, et al. An integrative multi-platform analysis for discovering biomarkers of osteosarcoma. BMC Cancer. 2009;9:150–60. doi: 10.1186/1471-2407-9-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bruheim S, Nakajima G, Malandsmo GM, et al. CDH11 is a potential survival biomarker for osteosarcoma. Proc Am Assoc Cancer Res. 2006;47:Abstract 1150. [Google Scholar]
- 70.Chou AJ, Bell MD, Mackinson C, et al. Phase Ib/IIa study of sustained release lipid inhalation targeting cisplatin by inhalation in the treatment of patients with relapsed/progressive osteosarcoma metastatic to the lung-ASCO Annual Meeting Proceedings Part I. J Clin Oncol. 2007;5:Abstract 18S 9525. [Google Scholar]
- 71.Fagioli F, Aglietta M, Tienghi A, et al. High-dose chemotherapy in the treatment of relapsed osteosarcoma: an Italian sarcoma group study. J Clin Oncol. 2002;20:2150–56. doi: 10.1200/JCO.2002.08.081. [DOI] [PubMed] [Google Scholar]
- 72.Sauerbrey A, Bielack S, Kempf-Bielack B, et al. High-dose chemotherapy (HDC) and autologous hematopoietic stem cell transplantation (ASCT) as salvage therapy for relapsed osteosarcoma. Bone Marrow Transplant. 2001;27:933–37. doi: 10.1038/sj.bmt.1703023. [DOI] [PubMed] [Google Scholar]
- 73.World Health Organization. Cartilage tumors. In: Fletcher CDM, Unni KK, Mertens F, editors. World Health Organization Classification of Tumors, Pathology and Genetics of tumors of soft tissue and bone. IARC Press; Lyon, France: 2002. pp. 234–57. [Google Scholar]
- 74.Bjornsson J, McLeod RA, Unni KK, et al. Primary chondrosarcoma of long bones and limb girdles. Cancer. 1998;83:2105–19. [PubMed] [Google Scholar]
- 75.Gelderblom H, Hogendoorn PC, Dijkstra SD, et al. The clinical approach towards chondrosarcoma. Oncologist. 2008;13:320–29. doi: 10.1634/theoncologist.2007-0237. [DOI] [PubMed] [Google Scholar]
- 76.Murphey MD, Walker EA, Wilson AJ, et al. From the archives of the AFIP: imaging of primary chondrosarcoma: radiologic-pathologic correlation. Radiographics. 2003;23:1245–78. doi: 10.1148/rg.235035134. [DOI] [PubMed] [Google Scholar]
- 77.Nakashima Y, Park YK, Sugano O. Mesenchymal chondrosarcoma. In: Flecther CDM, Unni KK, Mertens F, editors. World Health Organization classification of tumors, Pathology and genetics of tumors of soft tissue and bone. IARC Press; Lyon: 2002. pp. 255–56. [Google Scholar]
- 78.McCarthy EF, Freemount A, Hegendoorn PCW. Clear cell chondrosarcoma. In: Flecther CDM, Unni KK, Mertens F, editors. World Health Organization classification of tumors, Pathology and genetics of tumors of soft tissue and bone. IARC Press; Lyon: 2002. pp. 257–58. [Google Scholar]
- 79.Khurana J, Abdul-Karim F, Bovée JVMG. Osteochondroma. In: Fletcher CDM, Unni KK, Mertens F, editors. World Health Organization classification of tumors, Pathology and genetics of tumors of soft tissue and bone. IARC Press; Lyon: 2002. pp. 234–36. [Google Scholar]
- 80.Lucas DR, Bridge JA. Chondromas: enchondroma, periosteal chondroma, and enchondromatosis. In: Flecther CDM, Unni KK, Mertens F, editors. World Health Organization classification of tumors, Pathology and genetics of tumors of soft tissue and bone. IARC Press; Lyon: 2002. pp. 237–40. [Google Scholar]
- 81.Murphey MD, Flemming DJ, Boyea SR, et al. Enchondroma versus chondrosarcoma in the appendicular skeleton: differentiating features. Radiographics. 1998;18:1213–37. doi: 10.1148/radiographics.18.5.9747616. quiz 44–45. [DOI] [PubMed] [Google Scholar]
- 82.Murphey MD, Johnson DL, Bhatia PS, et al. Parosteal lipoma: MR imaging characteristics. AJR Am J Roentgenol. 1994;162:105–10. doi: 10.2214/ajr.162.1.8273646. [DOI] [PubMed] [Google Scholar]
- 83.Bertoni F, Picci P, Bacchini P, et al. Mesenchymal chondrosarcoma of bone and soft tissues. Cancer. 1983;52:533–41. doi: 10.1002/1097-0142(19830801)52:3<533::aid-cncr2820520325>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 84.Dickey ID, Rose PS, Fuchs B, et al. Dedifferentiated chondrosarcoma: The role of chemotherapy with updated outcomes. J Bone Joint Surg Am. 2004;86:2412–18. [PubMed] [Google Scholar]
- 85.Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977;40:818–31. doi: 10.1002/1097-0142(197708)40:2<818::aid-cncr2820400234>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 86.Skeletal Lesions Interobserver Correlation among Expert Diagnosticians (SLICED) Study Group. Reliability of histopathologic and radiologic grading of cartilaginous neoplasms in long bones. J Bone Joint Surg. 2007;89:Abstract 2113–23. doi: 10.2106/JBJS.F.01530. [DOI] [PubMed] [Google Scholar]
- 87.Schiller AL. Diagnosis of borderline cartilage lesions of bone. Semin Diagn Pathol. 1985;2:42–62. [PubMed] [Google Scholar]
- 88.Littrell LA, Wenger DE, Wold LE, et al. Radiographic, CT, and MR imaging features of dedifferentiated chondrosarcomas: a retrospective review of 174 de novo cases. Radiographics. 2004;24:1397–409. doi: 10.1148/rg.245045009. [DOI] [PubMed] [Google Scholar]
- 89.Fiorenza F, Abudu A, Grimer RJ, et al. Risk factors for survival and local control in chondrosarcoma of bone. J Bone Joint Surg Br. 2002;84:93–99. doi: 10.1302/0301-620x.84b1.11942. [DOI] [PubMed] [Google Scholar]
- 90.Leerapun T, Hugate RR, Inwards CY, et al. Surgical management of conventional grade I chondrosarcoma of long bones. Clin Orthop Relat Res. 2007;463:166–72. doi: 10.1097/BLO.0b013e318146830f. [DOI] [PubMed] [Google Scholar]
- 91.Okada K, Nagasawa H, Chida S, Nishida J. Curettage with pasteurization in situ for grade 1 chondrosarcoma – long-term follow up study of less invasive surgical procedure. Med Sci Monit. 2009;15(3):CS44–48. [PubMed] [Google Scholar]
- 92.Noel G, Habrand JL, Jauffret E, et al. Radiation therapy for chordoma and chondrosarcoma of the skull base and the cervical spine. Prognostic factors and patterns of failure. Strahlenther Onkol. 2003;179:241–48. doi: 10.1007/s00066-003-1065-5. [DOI] [PubMed] [Google Scholar]
- 93.Hug EB, Slater JD. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. Neurosurg Clin N Am. 2000;11:627–38. [PubMed] [Google Scholar]
- 94.Weber DC, Rutz HP, Pedroni ES, et al. Results of spot-scanning proton radiation therapy for chordoma and chondrosarcoma of the skull base: the Paul Scherrer Institut experience. Int J Radiat Oncol Biol Phys. 2005;63:401–9. doi: 10.1016/j.ijrobp.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 95.Rosenberg AE, Nielsen GP, Keel SB, et al. Chondrosarcoma of the base of the skull: a clinicopathologic study of 200 cases with emphasis on its distinction from chordoma. Am J Surg Pathol. 1999;23:1370–78. doi: 10.1097/00000478-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 96.Schulz-Ertner D, Nikoghosyan A, Hof H, et al. Carbon ion radiotherapy of skull base chondrosarcomas. Int J Radiat Oncol Biol Phys. 2007;67:171–77. doi: 10.1016/j.ijrobp.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 97.Wyman JJ, Hornstein AM, Meitner PA, et al. Multidrug resistance-1 and p-glycoprotein in human chondrosarcoma cell lines: expression correlates with decreased intracellular doxorubicin and in vitro chemoresistance. J Orthop Res. 1999;17:935–40. doi: 10.1002/jor.1100170619. [DOI] [PubMed] [Google Scholar]
- 98.Huvos AG, Rosen G, Dabska M, Marcove RC. Mesenchymal chondrosarcoma. A clinicopathologic analysis of 35 patients with emphasis on treatment. Cancer. 1983;51:1230–37. doi: 10.1002/1097-0142(19830401)51:7<1230::aid-cncr2820510710>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 99.Rosen G, Caparros B, Huvos AG, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49:1221–30. doi: 10.1002/1097-0142(19820315)49:6<1221::aid-cncr2820490625>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 100.Rao BN, Rodriguez-Galindo C. Intra-Arterial Cisplatin in Osteosarcoma: Same Question, Different Answer. Ann Surg Oncol. 2003;10:481–83. doi: 10.1245/aso.2003.04.903. [DOI] [PubMed] [Google Scholar]
- 101.Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: The Memorial Sloan-Kettering Experience. J Clin Oncol. 1992;10:5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 102.Wunder JS, Paulian G, Huvos AG, et al. The Histological Response to Chemotherapy as a Predictor of the Oncological Outcome of Operative Treatment of Ewing Sarcoma. J Bone Joint Surg Am. 1998;80:1020–33. doi: 10.2106/00004623-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 103.Nooij MA, Whelan J, Bramwell VH, et al. Doxorubicin and cisplatin chemotherapy in high-grade spindle cell sarcomas of the bone, other than osteosarcoma or malignant fibrous histiocytoma: a European Osteosarcoma Intergroup Study. Eur J Cancer. 2005;41:225–30. doi: 10.1016/j.ejca.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 104.Francannet C, Cohen-Tanugi A, Le Merrer M, et al. Genotype-phenotype correlation in hereditary multiple exostoses. J Med Genet. 2001;38:430–34. doi: 10.1136/jmg.38.7.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hopyan S, Gokgoz N, Poon R, et al. A mutant PTH/PTHrP type I receptor in enchondromatosis. Nat Genet. 2002;30:306–10. doi: 10.1038/ng844. [DOI] [PubMed] [Google Scholar]
- 106.Hopyan S, Nadesan P, Yu C, et al. Dysregulation of hedgehog signalling predisposes to synovial chondromatosis. J Pathol. 2005;206:143–50. doi: 10.1002/path.1761. [DOI] [PubMed] [Google Scholar]
- 107.Tiet TD, Alman BA. Developmental pathways in musculoskeletal neoplasia: involvement of the Indian Hedgehog-parathyroid hormone-related protein pathway. Pediatr Res. 2003;53:539–43. doi: 10.1203/01.PDR.0000054688.93486.18. [DOI] [PubMed] [Google Scholar]
- 108.Miyaji T, Nakase T, Onuma E, et al. Monoclonal antibody to parathyroid hormone-related protein induces differentiation and apoptosis of chondrosarcoma cells. Cancer Lett. 2003;199:147–55. doi: 10.1016/s0304-3835(03)00347-1. [DOI] [PubMed] [Google Scholar]
- 109.van Beerendonk HM, Rozeman LB, Taminiau AH, et al. Molecular analysis of the INK4A/INK4A-ARF gene locus in conventional (central) chondrosarcomas and enchondromas: indication of an important gene for tumour progression. J Pathol. 2004;202:359–66. doi: 10.1002/path.1517. [DOI] [PubMed] [Google Scholar]
- 110.Asp J, Sangiorgi L, Inerot SE, et al. Changes of the p16 gene but not the p53 gene in human chondrosarcoma tissues. Int J Cancer. 2000;85:782–86. doi: 10.1002/(sici)1097-0215(20000315)85:6<782::aid-ijc7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 111.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 112.Terek RM, Healey JH, Garin-Chesa P, et al. p53 mutations in chondrosarcoma. Diagn Mol Pathol. 1998;7:51–56. doi: 10.1097/00019606-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 113.Cleton-Jansen AM, van Beerendonk HM, Baelde HJ, et al. Estrogen signaling is active in cartilaginous tumors: implications for antiestrogen therapy as treatment option of metastasized or irresectable chondrosarcoma. Clin Cancer Res. 2005;11:8028–35. doi: 10.1158/1078-0432.CCR-05-1253. [DOI] [PubMed] [Google Scholar]
- 114.Maxwell PH, Pugh CW, Ratcliffe PJ. Activation of the HIF pathway in cancer. Curr Opin Genet Dev. 2001;11:293–99. doi: 10.1016/s0959-437x(00)00193-3. [DOI] [PubMed] [Google Scholar]
- 115.Lin PP, Brody RI, Hamelin AC, et al. Differential transactivation by alternative EWS-FLI1 fusion proteins correlates with clinical heterogeneity in Ewing’s sarcoma. Cancer Res. 1999;59:1428–32. [PubMed] [Google Scholar]
- 116.Rozeman LB, Hogendoorn PC, Bovee JV. Diagnosis and prognosis of chondrosarcoma of bone. Expert Rev Mol Diagn. 2002;2:461–72. doi: 10.1586/14737159.2.5.461. [DOI] [PubMed] [Google Scholar]
- 117.Sutton KM, Wright M, Fondren G, et al. Cyclooxygenase-2 expression in chondrosarcoma. Oncology. 2004;66:275–80. doi: 10.1159/000078327. [DOI] [PubMed] [Google Scholar]
- 118.Levine AM, Tulpule A, Quinn DI, et al. Phase I study of antisense oligonucleotide against vascular endothelial growth factor: decrease in plasma vascular endothelial growth factor with potential clinical efficacy. J Clin Oncol. 2006;24:1712–19. doi: 10.1200/JCO.2005.03.4801. [DOI] [PubMed] [Google Scholar]
- 119.Herbst RS, Mendolson DS, Ebbinghaus S. A phase I safety and pharmacokinetic (PK) study of recombinant Apo2L/TRAIL, an apoptosis-inducing protein in patients with advanced cancer. J Clin Oncol. 2006;24:Abstract 3013. [Google Scholar]
- 120.Sulzbacher I, Birner P, Trieb K, et al. Platelet-derived growth factor-alpha receptor expression supports the growth of conventional chondrosarcoma and is associated with adverse outcome. Am J Surg Pathol. 2001;25:1520–27. doi: 10.1097/00000478-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 121.Bovee JV, Cleton-Jansen AM, Taminiau AH, Hogendoorn PC. Emerging pathways in the development of chondrosarcoma of bone and implications for targeted treatment. Lancet Oncol. 2005;6:599–607. doi: 10.1016/S1470-2045(05)70282-5. [DOI] [PubMed] [Google Scholar]
- 122.Longtin R. Ewing’s sarcoma: a miracle drug waiting to happen? J Natl Cancer Inst. 2003;95:1574–76. doi: 10.1093/jnci/95.21.1574. [DOI] [PubMed] [Google Scholar]
- 123.Iwamoto Y. Diagnosis and treatment of Ewing’s sarcoma. Jpn J Clin Oncol. 2007;37:79–89. doi: 10.1093/jjco/hyl142. [DOI] [PubMed] [Google Scholar]
- 124.Thacker MM, Temple HT, Scully SP. Current treatment for Ewing’s sarcoma. Expert Rev Anticancer Ther. 2005;5:319–31. doi: 10.1586/14737140.5.2.319. [DOI] [PubMed] [Google Scholar]
- 125.Kotilingam D, Lev DC, Lazar AJ, Pollock RE. Staging soft tissue sarcoma: evolution and change. CA Cancer J Clin. 2006;56:282–91. doi: 10.3322/canjclin.56.5.282. quiz 314–15. [DOI] [PubMed] [Google Scholar]
- 126.Costa J, Wesley RA, Glatstein E, Rosenberg SA. The grading of soft tissue sarcomas. Results of a clinicohistopathologic correlation in a series of 163 cases. Cancer. 1984;53:530–41. doi: 10.1002/1097-0142(19840201)53:3<530::aid-cncr2820530327>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 127.Deyrup AT, Weiss SW. Grading of soft tissue sarcomas: the challenge of providing precise information in an imprecise world. Histopathology. 2006;48:42–50. doi: 10.1111/j.1365-2559.2005.02288.x. [DOI] [PubMed] [Google Scholar]
- 128.Ambros IM, Ambros PF, Strehl S, et al. MIC2 is a specific marker for Ewing’s sarcoma and peripheral primitive neuroectodermal tumors. Evidence for a common histogenesis of Ewing’s sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer. 1991;67:1886–93. doi: 10.1002/1097-0142(19910401)67:7<1886::aid-cncr2820670712>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 129.Kovar H, Dworzak M, Strehl S, et al. Overexpression of the pseudoautosomal gene MIC2 in Ewing’s sarcoma and peripheral primitive neuroectodermal tumor. Oncogene. 1990;5:1067–70. [PubMed] [Google Scholar]
- 130.Schuck A, Ahrens S, Paulussen M, et al. Local therapy in localized Ewing tumors: results of 1058 patients treated in the CESS 81, CESS 86, and EICESS 92 trials. Int J Radiat Oncol Biol Phys. 2003;55:168–77. doi: 10.1016/s0360-3016(02)03797-5. [DOI] [PubMed] [Google Scholar]
- 131.Bacci G, Ferrari S, Bertoni F, et al. Prognostic factors in nonmetastatic Ewing’s sarcoma of bone treated with adjuvant chemotherapy: analysis of 359 patients at the Istituto Ortopedico Rizzoli. J Clin Oncol. 2000;18:4–11. doi: 10.1200/JCO.2000.18.1.4. [DOI] [PubMed] [Google Scholar]
- 132.Rodriguez-Galindo C, Navid F, et al. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol. 2008;19:814–20. doi: 10.1093/annonc/mdm521. [DOI] [PubMed] [Google Scholar]
- 133.Rosito P, Mancini AF, Rondelli R, et al. Italian Cooperative Study for the treatment of children and young adults with localized Ewing sarcoma of bone: a preliminary report of 6 years of experience. Cancer. 1999;86:421–28. doi: 10.1002/(sici)1097-0142(19990801)86:3<421::aid-cncr10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 134.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 135.van der Woude HJ, Bloem JL, Hogendoorn PC. Preoperative evaluation and monitoring chemotherapy in patients with high-grade osteogenic and Ewing’s sarcoma: review of current imaging modalities. Skeletal Radiol. 1998;27:57–71. doi: 10.1007/s002560050339. [DOI] [PubMed] [Google Scholar]
- 136.Lin PP, Brody RI, Hamelin AC, et al. Differential transactivation by alternative EWS-Fli1 fusion proteins correlates with clinical heterogeneity in Ewing’s sarcoma. Cancer Res. 1999;59:1428–32. [PubMed] [Google Scholar]
- 137.Siligan C, Ban J, Bachmaier R, et al. EWS-FLI1 target genes recovered from Ewing’s sarcoma chromatin. Oncogene. 2005;24:2512–24. doi: 10.1038/sj.onc.1208455. [DOI] [PubMed] [Google Scholar]
- 138.Hahm KB, Cho K, Lee C, et al. Repression of the gene encoding the TGF-beta type II receptor is a major target of the EWS-FLI1 oncoprotein. Nat Genet. 1999;23:222–27. doi: 10.1038/13854. [DOI] [PubMed] [Google Scholar]
- 139.Nishimori H, Sasaki Y, Yoshida K, et al. The Id2 gene is a novel target of transcriptional activation by EWS-ETS fusion proteins in Ewing family tumors. Oncogene. 2002;21:8302–9. doi: 10.1038/sj.onc.1206025. [DOI] [PubMed] [Google Scholar]
- 140.Dohjima T, Lee NS, Li H, et al. Small interfering RNAs expressed from a Pol III promoter suppress the EWS/Fli-1 transcript in an Ewing sarcoma cell line. Mol Ther. 2003;7:811–16. doi: 10.1016/s1525-0016(03)00101-1. [DOI] [PubMed] [Google Scholar]
- 141.Mateo-Lozano S, Tirado OM, Notario V. Rapamycin induces the fusion-type independent downregulation of the EWS//FLI-1 proteins and inhibits Ewing’s sarcoma cell proliferation. Oncogene. 2003;22:9282–7. doi: 10.1038/sj.onc.1207081. [DOI] [PubMed] [Google Scholar]
- 142.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16:525–37. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 143.Sherr CJ. The INK4a/ARF network in tumor expression. Nat Rev Mol Cell Biol. 2001;2:731–37. doi: 10.1038/35096061. [DOI] [PubMed] [Google Scholar]
- 144.Pei XH, Xiong Y. Biochemical and cellular mechanisms of mammalian CDK inhibitors: a few unresolved issues. Oncogene. 2005;24:2787–95. doi: 10.1038/sj.onc.1208611. [DOI] [PubMed] [Google Scholar]
- 145.Matsumoto Y, Tanaka K, Nakatani F, et al. Downregulation and forced expression of EWS-Fli1 fusion gene results in changes in the expression of G(1)regulatory genes. Br J Cancer. 2001;84:768–75. doi: 10.1054/bjoc.2000.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Maitra A, Roberts H, Weinberg AG, Geradts J. Aberrant expression of tumor suppressor proteins in the Ewing family of tumors. Arch Pathol Lab Med. 2001;125:1207–12. doi: 10.5858/2001-125-1207-AEOTSP. [DOI] [PubMed] [Google Scholar]
- 147.Nakatani F, Tanaka K, Sakimura R, et al. Identification of p21WAF1/CIP1 as a direct target of EWS-Fli1 oncogenic fusion protein. J Biol Chem. 2003;278:15105–15. doi: 10.1074/jbc.M211470200. [DOI] [PubMed] [Google Scholar]
- 148.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 149.Sakimura R, Tanaka K, Nakatani F, et al. Antitumor effects of histone deacetylase inhibitor on Ewing’s family tumors. Int J Cancer. 2005;116:784–92. doi: 10.1002/ijc.21069. [DOI] [PubMed] [Google Scholar]
- 150.Matsunobu T, Tanaka K, Matsumoto Y, et al. The prognostic and therapeutic relevance of p27kip1 in Ewing’s family tumors. Clin Cancer Res. 2004;10:1003–12. doi: 10.1158/1078-0432.ccr-0788-3. [DOI] [PubMed] [Google Scholar]
- 151.Kovar H, Auinger A, Jug G, et al. Narrow spectrum of infrequent p53 mutations and absence of MDM2 amplification in Ewing tumours. Oncogene. 1993;8:2683–90. [PubMed] [Google Scholar]
- 152.Lessnick SL, Dacwag CS, Golub TR. The Ewing’s sarcoma oncoprotein EWS/FLI induces a p53-dependent growth arrest in primary human fibroblasts. Cancer Cell. 2002;1:393–401. doi: 10.1016/s1535-6108(02)00056-9. [DOI] [PubMed] [Google Scholar]
- 153.Rusch V, Klimstra D, Venkatraman E, et al. Aberrant p53 expression predicts clinical resistance to cisplatin-based chemotherapy in locally advanced non-small cell lung cancer. Cancer Res. 1995;55:5038–42. [PubMed] [Google Scholar]
- 154.Ganjavi H, Gee M, Narendran A, et al. Adenovirus-mediated p53 gene therapy in pediatric soft-tissue sarcoma cell lines: sensitization to cisplatin and doxorubicin. Cancer Gene Ther. 2005;12:397–406. doi: 10.1038/sj.cgt.7700798. [DOI] [PubMed] [Google Scholar]
- 155.Scotlandi K, Manara MC, Nicoletti G, et al. Antitumor activity of the insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal tumors. Cancer Res. 2005;65:3868–76. doi: 10.1158/0008-5472.CAN-04-3192. [DOI] [PubMed] [Google Scholar]
- 156.Scotlandi K, Manara MC, Hattinger CM, et al. Prognostic and therapeutic relevance of HER2 expression in osteosarcoma and Ewing’s sarcoma. Eur J Cancer. 2005;41:1349–61. doi: 10.1016/j.ejca.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 157.Mitsiades N, Poulaki V, Mitsiades C, Tsokos M. Ewing’s sarcoma family tumors are sensitive to tumor necrosis factor-related apoptosis-inducing ligand and express death receptor 4 and death receptor 5. Cancer Res. 2001;61:2704–12. [PubMed] [Google Scholar]
- 158.Kontny HU, Hammerle K, Klein R, et al. Sensitivity of Ewing’s sarcoma to TRAIL-induced apoptosis. Cell Death Differ. 2001;8:506–14. doi: 10.1038/sj.cdd.4400836. [DOI] [PubMed] [Google Scholar]
- 159.Kumar A, Jasmin A, Eby MT, Chaudhary PM. Cytotoxicity of tumor necrosis factor related apoptosis-inducing ligand towards Ewing’s sarcoma cell lines. Oncogene. 2001;20:1010–14. doi: 10.1038/sj.onc.1204154. [DOI] [PubMed] [Google Scholar]
- 160.Van Valen F, Fulda S, Truckenbrod B, et al. Apoptotic responsiveness of the Ewing’s sarcoma family of tumours to tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) Int J Cancer. 2000;88:252–59. doi: 10.1002/1097-0215(20001015)88:2<252::aid-ijc17>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 161.Merchant MS, Yang X, Melchionda F, et al. Interferon gamma enhances the effectiveness of tumor necrosis factor-related apoptosis-inducing ligand receptor agonists in a xenograft model of Ewing’s sarcoma. Cancer Res. 2004;64:8349–56. doi: 10.1158/0008-5472.CAN-04-1705. [DOI] [PubMed] [Google Scholar]
- 162.Cohen RB, Meropol NJ, Padavic K, et al. A phase I clinical trial of HGSETR1, an agonist monoclonal antibody to TRAIL-R1, in patients with advanced solid tumors. 16th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics; Geneva. 2004. [Google Scholar]
- 163.Fulda S, Kufer MU, Meyer E, et al. Sensitization for death receptor- or drug-induced apoptosis by re-expression of caspase-8 through demethylation or gene transfer. Oncogene. 2001;20:5865–77. doi: 10.1038/sj.onc.1204750. [DOI] [PubMed] [Google Scholar]
- 164.Lissat A, Vraetz T, Tsokos M, et al. Interferon-gamma sensitizes resistant Ewing’s sarcoma cells to tumor necrosis factor apoptosis-inducing ligand-induced apoptosis by up-regulation of caspase-8 without altering chemosensitivity. Am J Pathol. 2007;170:1917–30. doi: 10.2353/ajpath.2007.060993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cerisano V, Aalto Y, Perdichizzi S, et al. Molecular mechanisms of CD99-induced caspase-independent cell death and cell-cell adhesion in Ewing’s sarcoma cells: actin and zyxin as key intracellular mediators. Oncogene. 2004;23:5664–74. doi: 10.1038/sj.onc.1207741. [DOI] [PubMed] [Google Scholar]
- 166.Scotlandi K, Baldini N, Cerisano V, et al. CD99 engagement: an effective therapeutic strategy for Ewing tumors. Cancer Res. 2000;60:5134–42. [PubMed] [Google Scholar]
- 167.Kontny U. Regulation of apoptosis and proliferation in Ewing’s sarcoma-opportunities for targeted therapy. Hematol Oncol. 2006;24:14–21. doi: 10.1002/hon.766. [DOI] [PubMed] [Google Scholar]
- 168.Kavalar R, Pohar Marinsek Z, Jereb B, et al. Prognostic value of immunohistochemistry in the Ewing’s sarcoma family of tumors. Med Sci Monit. 2009;15(8):CR442–52. [PubMed] [Google Scholar]
- 169.Suva ML, Riggi N, Stehle JC, et al. Identification of cancer stem cells in Ewing’s sarcoma. Cancer Res. 2009;69:1776–81. doi: 10.1158/0008-5472.CAN-08-2242. [DOI] [PubMed] [Google Scholar]