Summary
Background
We studied the use of teriparatide in postmenopausal women with severe osteoporosis.
Material/Methods
Two groups (A and B) of patients affected by severe osteoporosis (T-score ⩽−2.5 at bone mineral density were analyzed and 2 vertebral fractures on radiograph).
Group A was treated for 18 months with 20 μg/day of teriparatide. Group B was treated with bisphosphonates 70 mg/week. Every woman assumed 1 g of calcium and 800 IU of vitamin D3 daily. We evaluated the effects of therapy after 18 months (T18) from the beginning with bone turnover markers (alkaline phosphatase, procollagen type 1 N-terminal propeptide, and N-telopeptide cross-links) and dual-energy X-ray absorptiometry.
Results
Group A, at T18 procollagen type 1 N-terminal propeptide levels, increased 127%; bone alkaline phosphatase levels increased to 65%; N-telopeptide cross-links levels increased to 110%.
Group B, at T18 procollagen type 1 N-terminal propeptide levels, decreased to 74%; bone alkaline phosphatase levels decreased to 41%; N-telopeptide cross-links levels decreased to 72%.
After 18 months, lumbar bone mineral density increased to 12.4% and femoral bone mineral density increased to 5.2% in group A. Group B lumbar bone mineral density increased to 3.85% and femoral bone mineral density increased to 1.99%. Only a new vertebral fracture occurred in group A (2.4%), whereas 6 fractures occurred in group B (15.7%).
The quality of life questionnaire of the European Foundation for Osteoporosis (QUALEFFO) revealed a significant improvement in daily living, performed domestic jobs, and locomotor function in groups A and B.
Conclusions
The use of rhPTH in patients with severe osteoporosis offers more protection against fractures and improves the QoL more than bisphosphonates.
Keywords: teriparatide, quality of life, severe osteoporosis
Background
Osteoporosis is a systemic disease leading to progressive decrease in bone mineral density, decreased bone strength, and increased risk of skeletal fractures [1]. Approximately 30% of women have sustained at least 1 vertebral fracture by the age of 75 [2]. These fractures are an important and common cause of morbidity in osteoporotic patients; moreover, fractures evidenced both clinically and at radiographic examination are associated with an increased mortality rate [3]. Approximately 75% of patients who present with a clinical vertebral fracture experience chronic pain. Back pain owing to vertebral fractures has a significant affect on osteoporotic patients [4–6], and the number and severity of these fractures also increases the risk of developing chronic back pain [7]. This has a marked negative impact on the quality of life (QoL) and functional impairment of the affected patients [8]. Conventional treatments for osteoporosis including bisphosphonates, selective estrogen receptor modulators (SERMs), and estrogen have been shown to reduce the rate of bone resorption and to preserve bone mass [9]. Another therapeutic option includes rhPTH, an agent that has been shown to increase both bone mass and bone strength.
When injected, teriparatide (rDNA origin), the amino-terminal fragment of human PTH (rhPTH 1–34), is a potent bone formation agent for the treatment of severe osteoporosis [10]. It increases osteoblast production/growth and prevents osteoblast apoptosis; at the same time, it enhances absorption of calcium from the intestine, renal reabsorption of calcium, while decreasing the excretion of phosphates in the kidney [11–14]. When administered once daily by subcutaneous injection, rhPTH increases bone density and improves trabecular architecture, cortical geometry, and strength [15,16]. Not only does rhPTH increase trabecular bone density by stimulating bone formation, but it also stimulates osteoclastic bone resorption [17–19].
In several studies, rhPTH has been shown to increase bone mineral density in postmenopausal osteoporosis, in senile osteoporosis in men, and in glucocorticoid-induced osteoporosis [10]. Teriparatide acts via the PTH-1 receptor on osteoblasts and bone marrow stromal cells to induce osteoblastic bone formation, that is, osteoid synthesis and accelerated mineralization [17]. This, in turn, results in reductions in skeletal fractures to levels equivalent to, or over, those obtained by using antiresorptive agents [15]. The increase in bone mineral density induced by rhPTH is substantial, ranging 10% to 15% over 2 to 3 years in most studies [10,20–24]. Moreover, rhPTH can cause demonstrable increases in bone mineral density and changes in markers of bone turnover within 3 months since the start of treatment [10,25].
Quality of life (QoL) can be measured to compare the effect of different treatments for osteoporosis. Measuring pain scores only for these patients would be inadequate, because apart from acute and chronic back pain, patients with vertebral fractures also experience anxiety, constant fear of falling, or suffering another fracture while their daily living activities are impaired. Most information has been collected thanks to the efforts made by some researchers to develop specific instruments to test the physical and emotional disability generated by the disease. Generic tools available for measuring QoL are useful to evaluate general health but they lack disease specificity. More recently, some specific instruments have been developed to measure the QoL in osteoporosis more accurately. The most widely used are the Osteoporosis quality of life questionnaire (OQLQ), the Osteoporosis Assessment Questionnaire (OPAQ), the Osteoporosis-targeted Quality of life Questionnaire (OPTQoL), and the quality of life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO-41) [26].
One of the first ones was Qualeffo-41, which has been translated and validated in different languages including Italian [27,28]. This questionnaire has proved to be repeatable, coherent, and able to discriminate between patients and controls. In the last few years, other specific questionnaires have been developed, but not all of them have been as extensively used and validated in different countries as Qualeffo-41.
The goal of this study was firstly to assess the validity of rhPTH treatment in a cohort of postmenopausal women with severe osteoporosis; secondly, to evaluate the improvement in QoL and pain symptoms after several months of rhPTH therapy. A follow-up Qualeffo-41 questionnaire was used to quantify the patient’s pain and the affect on QoL after rhPTH therapy.
Material and Methods
The study was a 18 months, randomized prospective cohort study conducted at Department of Molecular and Clinical Endocrinology and Oncology, University of Naples Federico II, Naples, Italy. Inclusion criteria for this study consisted of back pain, postmenopausal osteoporosis (T-score ⩽−2.5 at lumbar spine or femoral neck), the presence of 2 osteoporotic vertebral fractures, previous treatment for osteoporosis. The exclusion criteria were: an increased risk of osteosarcoma (ie, patients with Paget disease bone, previous skeletal exposure to external beam radiotherapy, or previous malignant neoplasm involving the skeleton), hypercalcemia, malignant neoplasms, impaired renal function, liver disease, history of diseases other than postmenopausal osteoporosis that affect bone metabolism, nephrolithiasis, alcohol or drug abuse.
Secondary osteoporosis was excluded in order to avoid the interference of the primitive disease with the patient’s quality of life. Informed consent was obtained from all subjects and the study protocol was approved by the Hospital/Science’s Ethical Committee.
Eighty-one postmenopausal women were enrolled and divided in two groups with no statistically significant differences in any of the considered variables: Group A – forty-two women (mean age 65±9 yrs; mean body mass index – BMI −24.5±2.6 kg/m2), with severe postmenopausal osteoporosis (mean lumbar BMD −3.88±0.70, mean femoral neck BMD −3.07±0.60 and with 2 vertebral atraumatic fractures), persistent back pain and previous treatment with biphosphonates for osteoporosis; Group B – thirty-nine women matched for age (60±14.4 yrs), BMI (22.8±8.8 Kg/m2), menopausal status, affected by back pain, severe postmenopausal osteoporosis (lumbar spine BMD −3.90±0.73, mean femoral neck BMD −3.02±0.61 and with 2 vertebral atraumatic fractures) previously treated for osteoporosis with biphosphonates. BMI, age at menopause, lifestyle habits (i.e., smoking, drinking, nutrition style), nutrition anamnesis (calcium intake), history of diseases other than osteoporosis, family history of osteoporosis were considered.
Groups were randomized to daily treatment with 20 μg s.c. of recombinant human parathyroid hormone (rhPTH 1–34), self-administered injections (group A) or 70 mg per os of alendronate every week (group B). All women received 1,000 mg elemental calcium daily and 800 IU of vitamin D daily for 18 months.
Biochemical markers of bone turnover were dosed in all the selected female patients: alkaline phosphatase (ALP: 35–104 U/L), N-terminal propeptide of type I procollagen (PINP: 19–102 μg/l), N-telopeptide crosslinks (NTx: 5–65 nmol/mmol Crea) were assessed at baseline, 3, 12 and 18 months (T0, T3, T12, T18).
The BMD of the lumbar spine and the proximal femur was measured by dual-energy X-ray absorptiometry (dual-energy X-ray absorptiometry QDR 1000; Hologic, Waltham, MA, USA) at baseline and at 18 months (T0, T18).
All women underwent anteroposterior and lateral radiography of thoracic and lumbar spine at T0 and T18.
The QoL questionnaire of the European Foundation for Osteoporosis (QUALEFFO) was administered at baseline and at the end of the study to evaluate the impact of rhPTH on health-related QoL.
Originally, the questionnaire consisted of 48 questions and 6 visual analogue scales. In the Qualeffo validation study, the number of items was reduced, and the visual analogue scales were removed. This resulted in the Qualeffo-41 [10,11], which consisted of 41 questions in 5 domains: pain, physical function, social function, general health perception, and mental function.
All answers were recorded so that all items range from 1 to 5, and all answers were standardized so that 1 represents the best and 5 the worst QoL, with the exception of questions E23-25 (questions with 3 answer options), questions E24-26-27-28 (4 answer options), and questions G33-34-35-37-39-40 (answer with reverse scores: 1 is the worst while 5 is the best).
Domain scores are calculated by averaging the answers of 1 domain and transforming the scores to a score from 0 to 100.
Data are expressed as mean ± SD or percentage; moreover, to assess the affect of teriparatide treatment on markers of bone turnover, bone mineral density, and on health-related QoL, Pearson correlation coefficients were computed in the 2 study groups. The level of significance was P value lower than .05. Therefore, percentage changes of biomarkers, bone mineral density, and Qualeffo results were calculated.
Results
Baseline characteristics of the subjects are summarized in Table 1. There was no significant difference in these characteristics between the 2 groups.
Table 1.
Clinical characteristics of postmenopausal women treated with teriparatide and controls.
| Baseline mean characteristics of the subjects | Group A | Group B |
|---|---|---|
| Age (years) | 65±9 | 60±14.4 |
| Height (cm) | 160±9 | 162±11 |
| Weight (kg) | 63±12.2 | 61±5.2 |
| BMI (kg/m2) | 24.5±2.6 | 22.8±8.8 |
| Tobacco smoking (pack-year) | 100±30 | 100±20 |
| History of fractures (#pts) | 42 | 38 |
| Previous osteoporosis therapy (#pts) | 42 | 38 |
| Back pain (#pts) | 42 | 38 |
In the group A, 39 (93%) out of the 42 recruited women completed the study: 2 patients discontinued the drug therapy because of lack of compliance with the study treatment; 1 patient discontinued because of a new vertebral atraumatic fracture.
The aim of this study was to evaluate percentage changes from baseline in biochemical markers of bone turnover, values of bone mineral density (measured at lumbar spine and proximal femur), and measurements of QoL.
Follow-up checks, where we also evaluated clinical conditions, adverse events, compliance to treatment and use of non-steroid anti-inflammatory drugs, were carried out at 3, 12 and 18 months since the beginning of treatment.
Markers of bone turnover
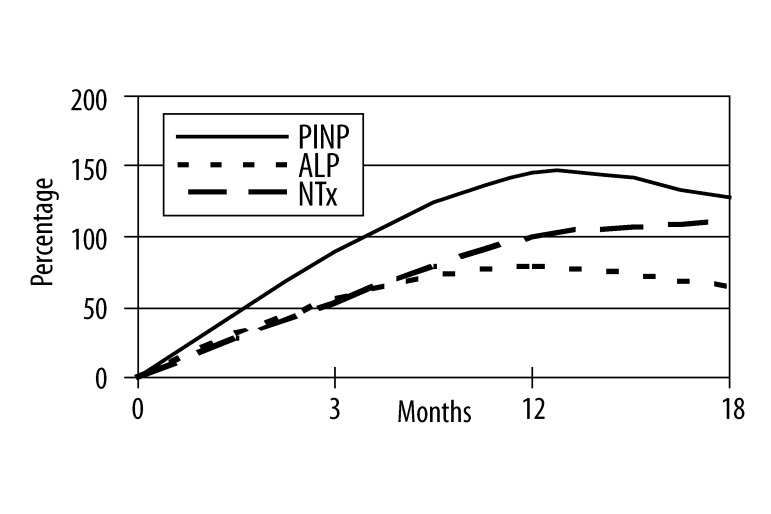
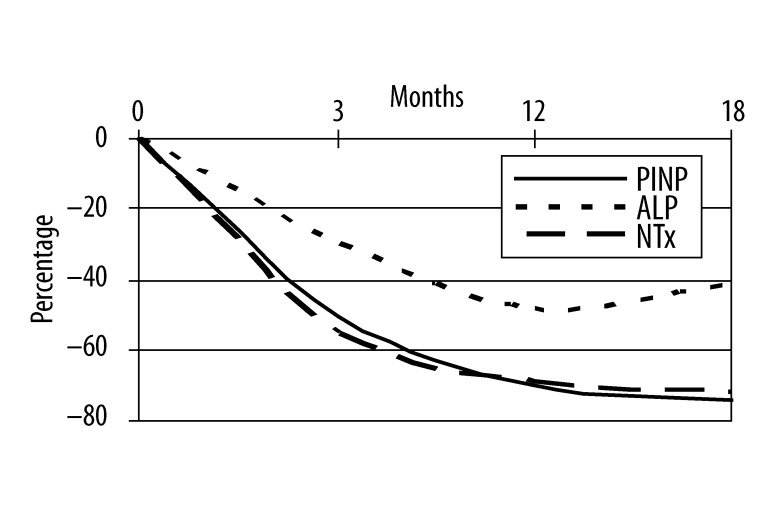
In group A serum levels of PINP increased of 90%, 145% and 127% at T3, T12, T18, respectively; bone ALP levels increased of 57%, 79% and 65%; NTx levels increased of 53% at T3, of 100% at T12, of 110% at T18. In group B percentage changes from baseline of serum levels of PINP were −50%, −70% and −74% at T3, T12, T18, respectively; bone ALP levels decreased of 30%, 48% and 41%; NTx levels were reduced by 55% at T3, of 69% at T12, of 72% at T18.
Mean percentage changes from baseline are shown over time for all 3 biochemical markers in Figures 1 and 2.
Figure 1.
Mean percentage changes of biochemical markers of bone turnover at baseline and at the end of the study in group A.
Figure 2.
Mean percentage changes of biochemical markers of bone turnover at baseline and at the end of the study in group B.
Mean PINP values were 42±6 μg/l at T0, 80±12 μg/l at T3, 103±34 μg/l at T12, 95±26 μg/l at T18 in group A (T0 vs T3 r: 0.81, p<0.001; T0 vs T12 r: 0.88, p<0.001; T0 vs T18 r: 0.86, p<0.001) and 78±16 μg/l, 39±5 μg/l, 23±13 μg/l, 20±8 μg/l at T0, T3, T12, T18 respectively (T0 vs T3 r: 0.67, p<0.001; T0 vs T12 r: 0.76, p<0.001; T0 vs T18 r: 0.82, p<0.001). In group A mean ALP value at T0 was 68±20 U/L, 107±20 U/L at T3, 122±45 U/L at T12 and 112±35 U/L at T18 (T0 vs T3 r: 0.72, p<0.001; T0 vs T12 r: 0.46, p<0.01; T0 vs T18 r: 0.85, p<0.001); instead, in group B mean ALP was 72 ±15 U/L at baseline, 50±15 after 3 months, 37±17 U/L at T12 and 42±7 U/L at the end of the study (T0 vs T3 r: 0.91, p<0.001; T0 vs T12 r: 0.53, p<0.001; T0 vs T18 r: 0.65, p<0.001). NTx mean values in group A were 31±7 nmol/mmol Crea, 47±21 nmol/mmol Crea, 62±11 nmol/mmol Crea, 65±23 nmol/mmol Crea at T0, T3, T12 and T18 respectively (T0 vs T3 r: 0.22, p>0.1; T0 vs T12 r: 0.57, p<0.001; T0 vs T18 r: 0.49, p<0.01) while in group B NTx mean values were 53±4 nmol/mmol Crea at T0, 29 ±11 nmol/mmol Crea at T3, 20±13 nmol/mmol Crea at T12 and 15±8 nmol/mmol Crea at T18 (T0 vs T3 r: 0.74, p<0.001; T0 vs T12 r: 0.58, p<0.001; T0 vs T18 r: 0.69, p<0.001).
Bone mineral density
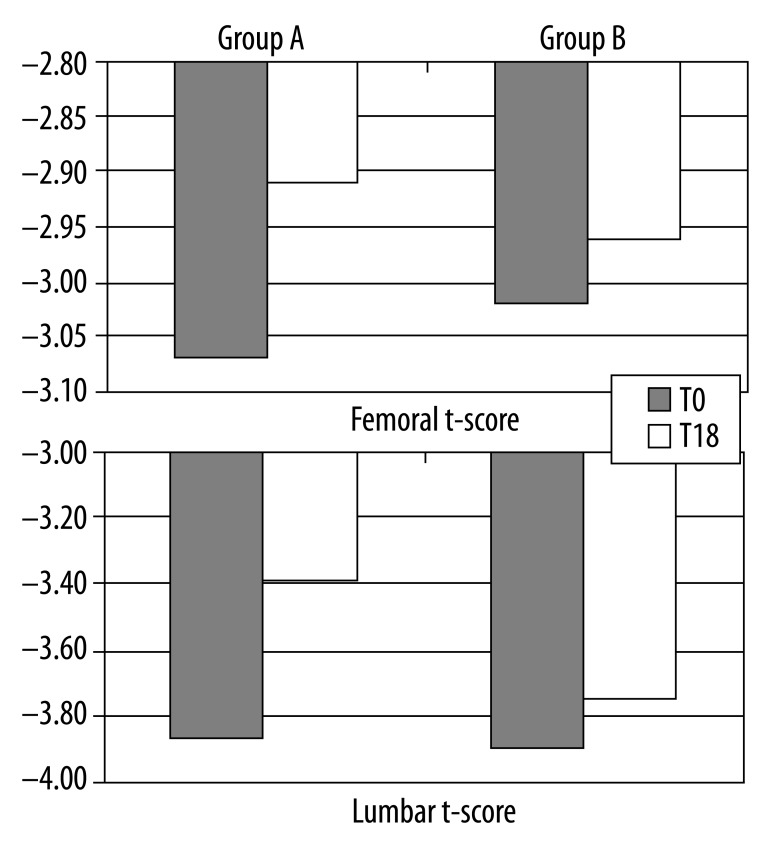
By the end of the study period, the BMD values expressed in terms of T-score in our total pool displayed important changes (Figure 3).
Figure 3.
BMD values expressed in terms of T-scored at baseline and at the end of the study.
At month 18, lumbar spine BMD increased by 12.4% in group A compared with group B in which it increased by 3.85%. Specifically, in group A mean T-score at T0 was −3.87±0.71 and mean T-score at T18 was −3.39±0.72 (r: 0.88; p<0.001); instead, in group B, mean T-score at T0 was −3.90±0.73 and mean T-score at T18: −3.75±0.72 (r: 0.98; p<0.001).
The BMD in the femur increased from baseline at month 18, in group A, by 5.2% and by 1.99% in group B. In group A, mean femoral neck T-score was −3.07±0.60 at baseline and mean T-score at T18 was −2.91±0.63 at the end of the study (r: 0.87; p<0.001); in group B, mean femoral T-score was −3.02±0.61 at T0 and −2.96±0.64 at T18 (r: 0.99; p<0.001).
X-ray evaluation
At T0, all patients of groups A and B showed baseline vertebral fractures. Patients treated with teriparatide were more protected against new fractures, compared with patients treated with bisphosphonates; in fact, only 1 new vertebral fracture occurred in group A (2.4%) at study endpoint (T18) vs 6 new vertebral fractures that occurred in group B (15.7%) at T18.
Adverse events
Teriparatide was safe and generally well-tolerated in most study subjects. The most-common reported adverse effects were back pain worsened in the first month of treatment that was reported by 14% of women; nausea, reported by 10%; headache and dizziness that were reported by only 1 and 2 women. Bisphosphonates were provided comparable in terms of tolerability to teriparatide. The reported adverse effects were abdominal pain in 9 patients, arthralgia in 4 patients, and dyspepsia in 1 patient.
Quality of life
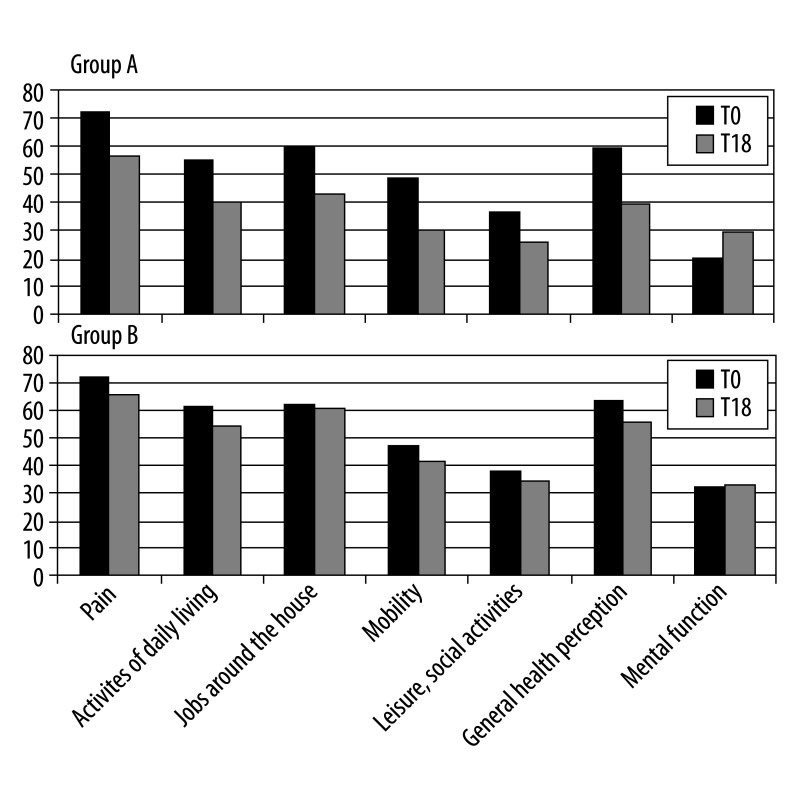
We evaluated the teriparatide impact on several aspects of QoL by administering the patients Qualeffo-41 test at T0 and T18 (Figure 4).
Figure 4.
Values of the dimensions of the QUALEFFO-41 in patients treated and not treated with teriparatide.
First domain (domain A) result indicated a serious reduction in pain (−22%) after treatment with rhPTH: mean scores measured was 72±9.2 at start and 56±14 after 18 months (r: 0.61; P<.001) compared with group B (−9.7%; 71±8.7 at T0 and 65±11 at T18; r: 0.53; P<.001).
For everyday activities (domain B), an average of 55±13.6 was measured at T0 and of 40–22.9 at T18 (r: 0.44; P<.01) in group A which had a total improvement of 27.3%, while the improvement was of 11% in group B (61.1±18 at T0 and 54.3±15.7 at T18; r: 0.68; P<.001).
The performed domestic job domain (domain C) showed an improvement of 29% in group A (60±10.7 at T0; 42.6±13.3 at T18; r: 0.68; P<.001) and of 2.9% in group B (62.1±13.3 at T0; 60.3±13.7 at T18; r: 0.88; P<.001).
The mean score of domain D (locomotor function) was of 48.4±10 at baseline and 30.2±14.3 at the end of the study that indicates a percentage change of 37.8% in group A compared to group B where the change was of 11.5% (46.8±8.6 at T0; 41.4±10.05 at T18; r: 0.69; P<.001).
The quality of free time and of the social activities (domain E) at the 2 time points they were 36.2±8.6 and 25.9±8.6, indicating a percentage change of 28.4% in group A (r: 0.50; P<.01); the values of group B were 38.2±14 at T0 and 34.2±14.3 at T18 reaching a percentage change of 10.5% only (r: 0.87; P<.001).
Patients taking teriparatide showed also an improvement of 33.9% in the self-perception of their health (domain F) (59±25 at T0; 39±16.7 at T18; r: 0.84; P<.001) versus 12.8% improvement of group B (63.2±28.2 at T0; 55.1±24.8 at T18; r: 0.90; P<.001).
In the mood domain (domain G), the Qualeffo-test revealed a mean value of 20.6±7.2 at T0 and 29.3±9.7 at T18 in group A (r: 0.71; P<.001). These data demonstrated a considerable improvement (29.7%) compared with group B (1.8%; 32.5±9.8 at T0; 33.1±9.9 at T18; r: 0.18; P>.1).
As a consequence of pain relief, the consumption of nonsteroidal anti-inflammatory drugs also decreased in 29 women of group A, while it did not decrease in group B.
Discussion
Our results demonstrate that daily injections of rhPTH for 18 months are an efficacious and generally well-tolerated therapy in postmenopausal women with severe osteoporosis. More importantly, in our experience rhPTH therapy results in decreased fractures and pain symptoms in patients with osteoporosis, and may be particularly beneficial to patients with a history of vertebral fractures.
Teriparatide is the first documented effective anabolic treatment of osteoporosis. Teriparatide is a bone-forming agent and its effect is demonstrated by increases in biochemical markers of bone turnover over the study period: bone formation markers showed more rapid and higher increases than resorption ones, suggesting an early imbalance of bone turnover in favor of formation; these data are in agreement with previous studies [29,30]. Treatment with teriparatide resulted in a greater increase in bone mineral density (BMD).
After 18 months of therapy with rhPTH, bone mineral density in the lumbar spine and of the proximal femur increased by 12.4% and 5.2%; these reported percentage increases are consistent with results of other studies [10,31]. At month 18 in patients treated with bisphosphonates, instead, lumbar spine increased only by 3.85% and the bone mineral density in the femur increased by 1.99%. The differences in the percentage increases between the 2 groups of patients can be explained with substantial differences between antiresorptive and anabolic therapeutic effects on bone mass and architecture as well as on bone mineral density. Moreover, rhPTH increases trabecular connectivity [32], whereas the majority of bone mineral density increases observed with bisphosphonate treatment are a result of increased mineralization of existing bone matrix [33].
Teriparatide has been referred to be an efficacious treatment against new fractures, making a significant change in the course of severe osteoporosis, which can lead rapidly not only to varying degrees of disability, the loss of self-sufficiency, and to institutionalization, but also to death [34–36]. In this study, we have confirmed that patients with osteoporosis treated with teriparatide experience improvements in pain symptoms.
In addition, use of teriparatide caused a considerable decrease of pain, accompanied by a consequent reduction of the need for nonsteroidal anti-inflammatory drugs by the patients; this was the main factor that assured an absolute compliance of the patients.
In severe osteoporosis vertebral fractures are an important cause of back pain owing to muscle weakness and altered posture, resulting in serious acute and chronic pain that contributes to further disabilities; in fact, vertebral fractures are the top health condition accounting for length of hospitalization, and added significantly to the length of hospitalization in patients admitted for other medical problems. Apart from the physical disabilities had by these patients, chronic back pain has a significant impact on the patient’s QoL. In fact, patients with vertebral fractures often experience impaired physical functions, limited activities of daily living, limited leisure and recreational activities, and significant emotional distress with loss of self-esteem and depression [34–36]. In this study, we have confirmed that patients with osteoporosis treated with teriparatide experience improvements in pain symptoms.
To analyze all these variables and to measure the values of QoL at baseline and at the end of the study, we used the Qualeffo-test, developed by Lips and associates in 1997 [27,28]. Specifically, we used Qualeffo-41, that represents a QoL questionnaire which is brief, easy to administer, and with adequate preliminary psychometric properties. We preferred this test to the generic ones (such as NHP, SIP, SF-36, EQ-SD) because of its specificity in the evaluation of QoL in people affected by osteoporosis with vertebral deformities; in fact, disadvantages of generic instruments are that they usually include irrelevant questions and that these questionnaires are less able to investigate those aspects that mostly influence QoL of osteoporotic patients [26–28]. This makes the Qualeffo-41 potentially useful during routine clinical practice and research for the treatment and the follow-up of postmenopausal women with severe osteoporosis.
By using this test, our study evidences that the use of rhPTH influences all considered domains of QoL; in fact, pain, physical and social functions, mood significantly improved in nearly all patients treated with rhPTH at the end of the study. Thus, the reduction of back pain observed from baseline of active treatment through posttreatment follow-up is consistent with the reduction of new vertebral painful fractures, that means improved QoL for this kind of patients. Our data clearly demonstrate that rhPTH is more effective than bisphosphonates in acting on back pain and all the domains of QoL. Adverse events (back pain, headache, nausea, dizziness) in our patients receiving rhPTH were mild and transient, with a percentage lower than in previous studies [10,36]. The adverse effects attributed to teriparatide did not stop the patients from continuing with the treatment.
Conclusions
Our results demonstrate that rhPTH increases bone mineral density considerably, reduces the occurrence of new fractures, and the need for analgesic therapy. Therefore, this study evidences that this anabolic agent represents a valid therapeutic option in severe postmenopausal osteoporosis; moreover, patients after this treatment experience, improvements not only in pain relief, but also in certain aspects of emotional functioning, activities of daily living, and leisure activities, improve QoL considerably.
Footnotes
Source of support: Departmental sources
References
- 1.NIH Consensus Development Panel on Osteoporosis Prevention. Diagnosis and Therapy 2001 Osteoporosis prevention, diagnosis and therapy. JAMA. 285:785–95. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 2.Nevitt M, Chen P, Kiel DP, et al. Reduced risk of back pain following teriparatide treatment: a meta-analysis. Osteopor Int. 2006;17:273–80. doi: 10.1007/s00198-005-2013-2. [DOI] [PubMed] [Google Scholar]
- 3.Chen P, Krege JH, Adachi JD, et al. CaMOS Research Group. Vertebral fracture status and the World Health Organization risk factors for predicting osteoporotic fracture risk. J Bone Miner Res. 2009;24(3):495–502. doi: 10.1359/jbmr.081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papaioannou A, Kennedy CC, Ioannidis G, et al. CaMos Study Group. The impact of incident fractures on health-related quality of life: 5 years of data from the Canadian Multicentre Osteoporosis Study. Osteoporos Int. 2009;20(5):703–14. doi: 10.1007/s00198-008-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haczynski J, Jakimiuk A. Vertebral fractures: a hidden problem of osteoporosis. Med Sci Monit. 2001;7(5):1108–17. [PubMed] [Google Scholar]
- 6.Hoesel LM, Pausch M, Schnettler R, Heiss C. The impact of osteoporosis on the classification of hip and wrist fractures. Med Sci Monit. 2008;14(3):HY1–8. [PubMed] [Google Scholar]
- 7.Boonen S, Singer AJ. Osteoporosis management: impact of fracture type on cost and quality of life in patients at risk for fracture I. Curr Med Res Opin. 2008;24(6):1781–88. doi: 10.1185/03007990802115796. [DOI] [PubMed] [Google Scholar]
- 8.Sedrine W, Radican L, Reginster JY. On conducting burden-of-osteoporosis studies: a reviw of the core concepts and practical issues. A study carried out under the auspices of a WHO Collaborating Center. Rheumatology. 2001;40:7–14. doi: 10.1093/rheumatology/40.1.7. [DOI] [PubMed] [Google Scholar]
- 9.Benhamou CL. Effects of osteoporosis medications on bone quality. Joint Bone Spine. 2007;74:39–47. doi: 10.1016/j.jbspin.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 11.Roberts MD, Santner TJ, Hart RT. Local bone formation due to combined mechanical loading and intermittent hPTH-(1-34) treatment and its correlation to mechanical signal distributions. J Biomech. 2009;42(15):2431–38. doi: 10.1016/j.jbiomech.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Jilka RL, Weinstein RS, Bellido T, et al. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–46. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jilka RL, O’Brien CA, Ali AA, et al. Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts. Bone. 2009;44(2):275–86. doi: 10.1016/j.bone.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Y, Zhao JJ, Mitlak BH, et al. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18(11):1932–41. doi: 10.1359/jbmr.2003.18.11.1932. [DOI] [PubMed] [Google Scholar]
- 15.Dempster DW, Cosman F, Kurland ES, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001;16:1846–53. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- 16.Gabet Y, Kohavi D, Müller R, et al. Intermittently administered parathyroid hormone 1-34 reverses bone loss and structural impairment in orchiectomized adult rats. Osteoporos Int. 2005;16(11):1436–43. doi: 10.1007/s00198-005-1876-6. [DOI] [PubMed] [Google Scholar]
- 17.Compston JE. Skeletal actions of intermittent parathyroid hormone: effects on bone remodelling and structure. Bone. 2007;40(6):1447–52. doi: 10.1016/j.bone.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 18.File E, Deal C. Clinical update on teriparatide. Curr Rheumatol Rep. 2009;11(3):169–76. doi: 10.1007/s11926-009-0023-3. [DOI] [PubMed] [Google Scholar]
- 19.Bilezikian JP. Anabolic therapy for osteoporosis. Int J Fertil Womens Med. 2005;50(2):53–60. [PubMed] [Google Scholar]
- 20.McClung M. Parathyroid hormone for the treatment of osteoporosis. Obstet Gynecol Surv. 2004;59(12):826–32. doi: 10.1097/01.ogx.0000146584.12831.c9. [DOI] [PubMed] [Google Scholar]
- 21.Hodsman AB, Fraher LJ, Watson PH, et al. A randomized controlled clinical trial to compare the efficacy of cyclical parathyroid hormone versus cyclical parathyroid hormone and sequential calcitonin to improve bone mass in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 1997;82:620–28. doi: 10.1210/jcem.82.2.3762. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook PN. Anabolic therapy in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357(20):2084–86. doi: 10.1056/NEJMe0706770. [DOI] [PubMed] [Google Scholar]
- 23.Kurland ES, Cosman F, McMahon DJ, et al. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab. 2000;85:3069–76. doi: 10.1210/jcem.85.9.6818. [DOI] [PubMed] [Google Scholar]
- 24.Rosen CJ, Bilezikian JP. Anabolic therapy for osteoporosis. J Clin Endocrinol Metab. 2001;86:957–64. doi: 10.1210/jcem.86.3.7366. [DOI] [PubMed] [Google Scholar]
- 25.Lukaszkiewicz J, Karczmarewicz E, Pludowski P, et al. EPOLOS Group. Feasibility of simultaneous measurement of bone formation and bone resorption markers to assess bone turnover rate in postmenopausal women: an EPOLOS study. Med Sci Monit. 2008;14(12):PH65–70. [PubMed] [Google Scholar]
- 26.Baczyk G. Quality of life of women with osteoporosis-review of literature. Ortop Traumatol Rehabil. 2009;11:291–303. [PubMed] [Google Scholar]
- 27.Lips P, Cooper C, Agnusdei D, et al. Quality of life as outcome in the treatment of osteoporosis: the development of a questionnaire for quality of life by the European Foundation for Osteoporosis. Osteopor Int. 1997;7:36–38. doi: 10.1007/BF01623457. [DOI] [PubMed] [Google Scholar]
- 28.Lips P, Cooper C, Agnusdei D, et al. Quality of life in patients with vertebral fractures: validation of the quality of life questionnaire of the European Foundation for Osteoporosis (QUALEFFO) Osteopor Int. 1999;10:150–60. doi: 10.1007/s001980050210. [DOI] [PubMed] [Google Scholar]
- 29.Dobnig H, Sipos A, Jiang Y, et al. Early changes in biochemical markers of bone formation correlate with improvements in bone structure during teriparatide therapy. J Clin Endocrinol Metab. 2005;90:3970–77. doi: 10.1210/jc.2003-1703. [DOI] [PubMed] [Google Scholar]
- 30.Chen P, Satterwhite JH, Licata AA, et al. Early changes in biochemical markers of bone formation predict BMD response to Teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005;20:962–70. doi: 10.1359/JBMR.050105. [DOI] [PubMed] [Google Scholar]
- 31.Body JJ, Gaich GA, Scheele WH, et al. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1-34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2002;87:4528–35. doi: 10.1210/jc.2002-020334. [DOI] [PubMed] [Google Scholar]
- 32.Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of recombinant human parathyroid hormone (1-84) on vertebral fractures and bone mineral density in postmenopausal women with osteoporosis. Ann Intern Med. 2007;146:416–24. doi: 10.7326/0003-4819-146-5-200703060-00005. [DOI] [PubMed] [Google Scholar]
- 33.Boivin GY, Chavassieux PM, Santora AC, et al. Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone. 2000;27:687–94. doi: 10.1016/s8756-3282(00)00376-8. [DOI] [PubMed] [Google Scholar]
- 34.Fraenkel L, Gulaski B, Wittink D. Patient willingness to take teriparatide. Patient Educ Couns. 2007;65:237–44. doi: 10.1016/j.pec.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crans GG, Silverman SL, Genant HK, et al. Association of severe vertebral fractures with reduced quality of life: reduction in the incidence of severe vertebral fractures by teriparatide. Arthritis Rheum. 2004;50:4028–34. doi: 10.1002/art.20671. [DOI] [PubMed] [Google Scholar]
- 36.Gold DT, Solimeo S. Osteoporosis and depression: a historical perspective. Curr Osteoporos Rep. 2006;4:134–39. doi: 10.1007/s11914-996-0021-6. [DOI] [PubMed] [Google Scholar]