Summary
Background
To investigate the expression levels of importin13 (IPO13), c-kit, CD146, telomerase, caspase-3, bcl-2 and bax in endometrial polyps (EPs).
Material/Methods
We detected the mRNA expression levels of IPO13, c-kit, bcl-2 and bax in endometrial polyps (EPs) using real-time PCR. We detected the protein expression levels of IPO13, telomerase, CD146, caspase-3, bcl-2 and bax in EPs using S-P (Streptavidin-Peroxidase) immunohistochemistry. Western blotting was performed to determine the levels of importin13 and bcl-2 proteins in EPs.
Results
The expression levels of IPO13, c-kit, telomerase, caspase3, and bax were lower in the EP tissue compared to normal endometrial tissue during the proliferation and secretion phases of the menstrual cycle (p<0.05). The expression of CD146 was decreased in the EP tissue compared to the normal endometrial tissue during the proliferation phase of the menstrual cycle (p<0.05). The expression of bcl-2 was increased in the EP tissue compared to the normal endometrial tissue during the proliferation and secretion phases of the menstrual cycle (p<0.05).
Conclusions
The expression levels of IPO13, c-kit, telomerase, caspase3, and bax were decreased; however, the expression of bcl-2 was increased in the EP tissue compared to the normal endometrial tissue. These findings suggest that the development of EPs is associated with the deregulated activities of the endometrial stem/progenitor cells and the decreased apoptosis of endometrial cells, with the latter being the major factor involved in the development of EPs.
Keywords: endometrial polyps, endometrial stem cell, telomerase, C-kit, caspase-3, bcl-2
Background
Endometrial polyps (EPs) are common lesions that often lead to abnormal uterine bleeding. Most EPs are benign neoplasms; however, some EPs can be precancerous lesions or even cancer [1]. However, the pathogenesis and etiology of EPs remain unclear.
The deregulated expression levels of estrogen receptor (ER) and progesterone receptor (PR) may lead to the development of EPs [2]. In post-menopausal patients, the expression of ER receptor in EP tissue is increased compared to that in normal endometrial tissue, resulting in the development of post-menopausal Eps [3]. Uterine fibroids and endometriosis are the most common complications of EPs, suggesting that EPs are associated with estrogen [4]. Hormone replacement therapy in post-menopausal patients is also linked to the development of EPs [5,6].
The balance between cell mitosis and apoptosis regulates the periodic repair and shedding of endometrial cells [7]. Ki-67 is a nuclear antigen associated with cell proliferation and mitosis, and its expression is increased in endometrial hyperplasia diseases [8]. Bcl-2 is a proto-oncogene that inhibits apoptosis; its expression is upregulated in ectopic endometrial and endometrial hyperplasia tissues [9,10]. Many reports have found that upregulation of Bcl-2 is associated with EPs; however, the relationship between Ki-67 and EP development remains unclear [2,11–14].
In recent years, endometrial stem and progenitor cells have been thought to be responsible for the development of endometrial diseases such as endometriosis and endometrial cancer [15–18]. However, there is no data on the pathogenesis of EPs with regard to endometrial stem cells/progenitor cells. We assessed the expression levels of the adult stem cell markers IPO13, c-kit, and CD146, as well as the expression of the apoptosis-related genes caspase3, bcl-2, and bax in EPs, to explore the pathogenesis of EPs.
Material and Methods
Material
Patients who were admitted to the First Affiliated Hospital of Chongqing Medical University from October 2008 to January 2010, and who were pathologically diagnosed with EP or other benign endometrial disease, and who received a hysteroscopy, were randomly selected. The study was approved by the Ethics Commission of Chongqing Medical University, and informed patient consent was obtained. EP tissues from 40 patients were harvested by a 5.07-mm continuous-perfusion examination and treatment hysteroscopy (Stryker Corporation, USA); these patients were the observation group (20 cases were at proliferation phase, and 20 cases were at secretion phase). Forty cases of normal endometrium were harvested as the control group (20 cases were at proliferation phase, and 20 cases were at secretion phase). The patients were ages 22 to 60 years, with a mean age of 38 years. All pathologies were confirmed by pathological examination. Endometrial polyp tissue (i.e., pathologically altered tissue) was included in the histological sections. Patients who had additional endometrial complications, including adenomyosis, dysfunctional uterine bleeding, polycystic ovary syndrome, and other hormone-dependent diseases, were excluded. All of the patients had regular menstrual cycles, did not receive hormone therapy during the 3 months before surgery, and were not pregnant or lactating during the study.
Methods
Immunohistochemistry
The tissues were embedded in paraffin and cut into 5-μm sections. The sections showing typical endometrial structures by hematoxylin and eosin (H&E) staining were included in this study. Immunohistochemistry was performed according to the SP kit instructions (Bioss Biotechnology, Beijing, China). After dewaxing and hydration, the sections were heated in citrate buffer (pH 6.0, Sigma-Aldrich, USA) in a microwave oven for 20 min for antigen retrieval. The sections were then cooled naturally to room temperature. The sections were washed for 3 min × 3 cycles. The sections were then incubated in 3% H202 for 15 min at room temperature and washed with PBS for 3 min × 3 cycles. The sections were blocked in 5% donkey serum (ab7475 Abcam Company) for 30 min at 37°C. Anti-IPO13 goat polyclonal antibody (1:100, NB100-1369, Novus Biologicals), anti-telomerase rabbit polyclonal antibody (1: 100, ZA-0239, Beijing Golden Bridge Biotechnology Co., Ltd.), anti-CD146 mouse monoclonal antibody (1:50, ZM-0299, Beijing Golden Bridge Biotechnology Co., Ltd.), anti-caspase3 rabbit polyclonal antibody (1:100, BS1518, Bioworld Technology, Inc.), anti-bax rabbit polyclonal antibody (1:100, BS1725, Bioworld Technology, Inc.) and anti-bcl-2 rabbit monoclonal antibody (1:100, bs-0522R, Bioworld Technology, Inc.) were incubated with the sections overnight at 4°C. Negative controls included omission of primary antibody and use of irrelevant primary antibodies. The corresponding secondary antibodies, which were conjugated to horseradish peroxidase (Bioss Biotechnology), were incubated with the sections for 1 h at room temperature. The sections were washed in PBS for 3 min × 3 cycles. The sections were incubated in horseradish enzyme-labeled chain avidin solution (Bioss Biotechnology) for 30 min at 37°C and washed in PBS for 3 min × 3 cycles. The proteins were visualized by diaminobenzidine (DAB). All of the sections were observed by 3 independent pathologists using a light microscope. A total of 22 representative high power fields (40×) were chosen, and the positively-stained cells were counted for each sample. Slides were evaluated independently by 3 pathologists for distribution and intensity of signal as described by De Falco et al. [19]. Intensity was scored from 0 to 3:0 (absent immunopositivity); 1 (low immunopositivity); 2 (moderate immunopositivity); 3 (intense immunopositivity). An average of 22 fields was observed for each tissue. All values are presented as the mean ±standard error (mean ±SEM).
Detection of marker gene expression by real-time PCR
Total RNA was isolated using TRIZOL (Invitrogen). The first-strand cDNA was generated using SuperScript (Invitrogen). The primers used for amplifying IPO13, c-kit, bax, bcl-2, and β-Actin were synthesized by Guangzhou Funeng Co., Ltd. The real-time PCR kit was purchased from Guangzhou Funeng Co., Ltd. PCR conditions were 95° for 10 s, 60° for 20 s, 72° for 10 s. The experiments were performed in triplicate for every sample.
Detection of marker protein expression by Western blotting
Tissues were lysed with a RIPA buffer containing protease inhibitors. Aliquots of the lysates containing 25 μg of total protein were run on a SDS-polyacrylamide gel. The proteins were transferred to a PVDF membrane. The membrane was incubated overnight at 4°C in TTBS containing 5% non-fat milk powder (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% Tween 20). Anti-IPO13 goat polyclonal antibody (1:500) or anti-bcl-2 rabbit monoclonal antibody (1:600) was incubated with the membrane for 2 h at 37°C. Secondary antibodies that were conjugated to horseradish peroxidase were incubated with the membrane for 1 h at 37°C. The proteins that were revealed by Western blotting were visualized by chemiluminescence (Biyuntian Company). The densities of bands were analyzed by a gel imaging system and calculated compared to the internal control.
Statistical analysis
SPSS 11.5 software was used to perform the statistical analysis. The difference between the 2 groups was compared using the independent t-test. A p-value of <0.05 was considered to be statistically significant.
Results
Immunohistochemistry
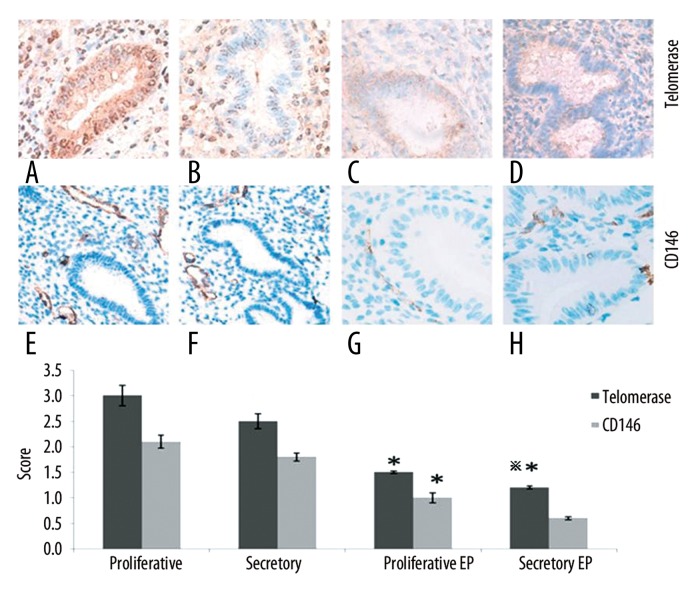
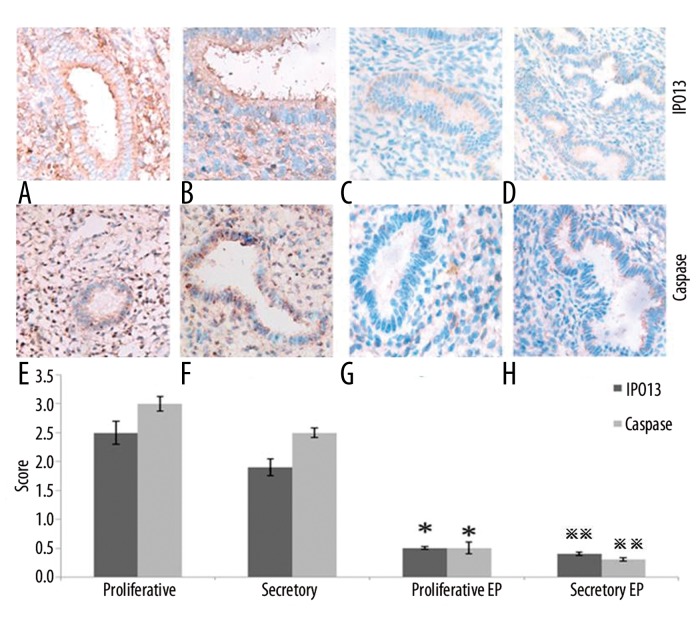
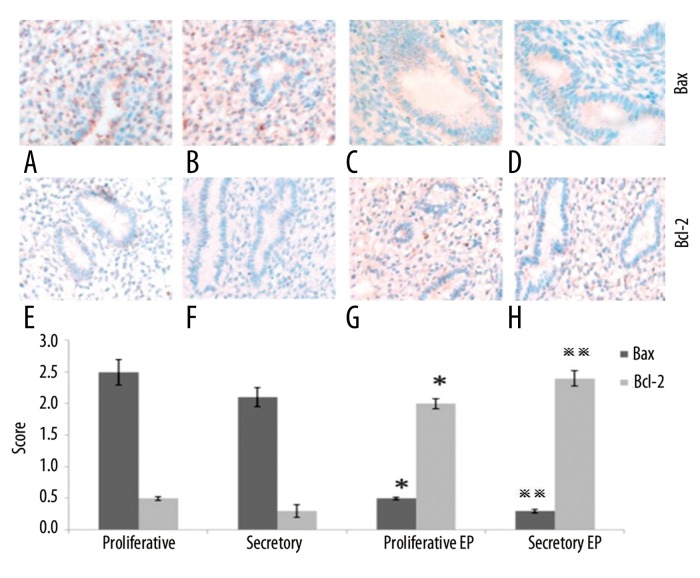
IPO13, telomerase, caspase3, bcl-2 and bax were expressed both in the cytoplasm and nucleus of the epithelial and stromal cells. CD146 was mainly expressed in the cytoplasm of the stromal vascular endothelial cells (Figure 1). Telomerase was strongly expressed in the endometrium during the proliferation or secretion phase in the control group; however, it was weakly expressed or undetectable in the EP patients. The telomerase staining seems to indicate that in the secretory phase diffuse glandular staining is decreased compared to the proliferative phase. CD146 was strongly expressed in the control group; however, it was weakly expressed in the EP patients (Figure 1). IPO13 was strongly expressed in the endometrium in the control group; however, it was weakly expressed or could not be detected in the EP patients (Figure 2). Caspase3 showed very strong expression in the endometrium during the proliferation phase and strong expression during the secretion phase in the control group; however, it was weakly expressed or could not be detected in the EP patients (Figure 2). Bax was strongly expressed in the control group; however, it was weakly expressed or could not be detected in the EP patients (Figure 3). Bcl-2 was weakly expressed in the control group; however, it was strongly expressed in the EP patients. Bcl2 staining was stronger in the stroma compared to the glands (Figure 3).
Figure 1.
The expression of telomerase and CD146 in the endometrial tissues was detected by immunohistochemistry (400×). (A) Control endometrium at the proliferation phase. (B) Control endometrium at the secretion phase. (C) EP at the proliferation phase. (D) EP at the secretion phase. (E) Control endometrium at the proliferation phase. (F) Control endometrium at the secretion phase. (G) EP at the proliferation phase. (H) EP at the secretion phase. * p<0.05 (vs. control endometrium at the proliferation phase); ** p<0.05 (vs. control endometrium at the secretion phase); error bars, SEM.
Figure 2.
The expression levels of IPO13 and caspase3 in the endometrial tissues were detected by immunohistochemistry (400×). (A) Control endometrium at the proliferation phase. (B) Control endometrium at the secretion phase. (C) EP at the proliferation phase. (D) EP at the secretion phase. (E) Control endometrium at the proliferation phase. (F) Control endometrium at the secretion phase. (G) EP at the proliferation phase. (H) EP at the secretion phase. * p<0.05 (vs. control endometrium at the proliferation phase); ** p<0.05 (vs. control endometrium at the secretion phase); error bars, SEM.
Figure 3.
The expression of bax and bcl-2 in the endometrial tissues was detected by immunohistochemistry. (A) Control endometrium at the proliferation phase (400×). (B) Control endometrium at the secretion phase (400×). (C) EP at the proliferation phase (400×). (D) EP at the secretion phase (400×). (E) Control endometrium at the proliferation phase (200×). (F) Control endometrium at the secretion phase (200×). (G) EP at the proliferation phase (200×). (H) EP at the secretion phase (200×). * p<0.05 (vs. control endometrium at the proliferation phase); ** p<0.05 (vs. control endometrium at the secretion phase); error bars, SEM.
Results of real-time PCR
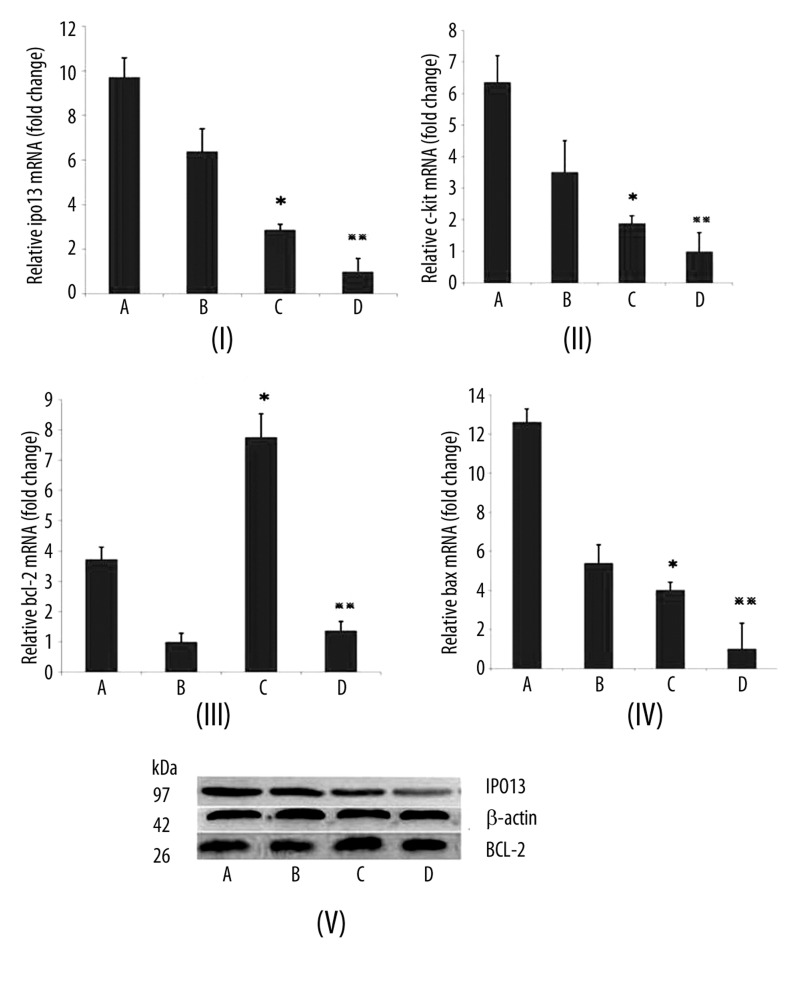
IPO13 mRNA expression was significantly increased in the proliferative endometrium of the control group compared to that in the EP patients (p<0.05). IPO13 mRNA expression was significantly increased in the secretory endometrium of the control group compared to that in the EP patients (p<0.05) (Figure 4I). The c-kit mRNA expression was increased about 1.5-fold in the proliferative endometrium of the control group compared to that in the EP patients (p<0.05). The c-kit mRNA expression was increased about 3-fold in the secretory endometrium of the control group compared to that in the EP patients (p<0.05) (Figure 4II). The bcl-2 mRNA expression was significantly increased in the proliferative endometrium of the EP patients compared to that in the control group (p<0.05). The bcl-2 mRNA expression was significantly increased in the secretory endometrium of the EP patients compared to that in the control group (p<0.05) (Figure 4III). The bax mRNA expression was significantly increased in the proliferative endometrium of the control group compared to that in the EP patients (p<0.05). The bax mRNA expression was significantly increased in the secretory endometrium of the control group compared to that in the EP patients (p<0.05) (Figure 4IV).
Figure 4.
The mRNA expression of IPO13, c-kit, bcl-2 and bax was detected by real-time PCR. (A) Control endometrium at the proliferation phase. (IB) Control endometrium at the secretion phase. (IC) EP at the proliferation phase. (ID) EP at the secretion phase. (IIA) Control endometrium at the proliferation phase. (IIB) Control endometrium at the secretion phase. (IIC) EP at the proliferation phase. (IID) EP at the secretion phase. (IIIA) Control endometrium at the proliferation phase. (IIIB) Control endometrium at the secretion phase. (IIIC) EP at the proliferation phase. (IIID) EP at the secretion phase. (IVA) Control endometrium at the proliferation phase. (IVB) Control endometrium at the secretion phase. (IVC) EP at the proliferation phase. (IVD) EP at the secretion phase. ** p<0.05 (vs. control endometrium at the secretion phase); error bars, SEM.
The protein expression levels of IPO13 and bcl-2 detected by Western blotting
IPO13 protein expression was higher in the proliferative endometrium of the control group compared to that in the EP patients (0.91±0.10 vs. 0.43±0.06, p<0.05). IPO13 protein expression was higher in the secretory endometrium of the control group compared to that in the EP patients (0.85±0.18 vs. 0.22±0.09, p<0.05). Bcl-2 protein expression was higher in the proliferative endometrium of the EP patients compared to that in the control group (0.95±0.13 vs. 0.64±0.13, p<0.05). Bcl-2 protein expression was higher in the secretory endometrium of the EP patients compared to that in the control group (0.90±0.08 vs. 0.50±0.16, p<0.05) (Table 1).
Table 1.
The relative expression levels of IPO13 and bcl-2 proteins in normal endometrial and EP tissues.
| Cases | IPO13 | bcl-2 | |||
|---|---|---|---|---|---|
| Proliferation phase | Secretion phase | Proliferation phase | Secretion phase | ||
| Control | 20 | 0.91±0.10 | 0.85±0.18 | 0.64±0.13 | 0.50±0.16 |
| EP | 20 | 0.43±0.06* | 0.22±0.09** | 0.95±0.13* | 0.90±0.08** |
(Data given as mean ±SEM. * p<0.05, vs. control endometrium at the proliferation phase and ** p<0.05, vs. control endometrium at the secretion phase; Error bars, SEM.).
Discussion
Recent studies have indicated that endometrial stem cells contribute to endometrial repair physiologically; however, the deregulated proliferation and differentiation of these stem cells lead to endometrial diseases such as endometriosis and endometrial cancer [15,20]. IPO13 is a marker of corneal epithelial stem cells, and it plays important roles in maintaining features of corneal stem cells such as cell shape, high proliferation potential, and a poorly differentiated state [21]. Furthermore, we found that IPO13, c-kit and CD146 were expressed in the endometrium.
The expression of IPO13 in the EP patients was lower than that in the normal endometrium, both during the secretory and proliferation phases. IPO13 can promote proliferation of the stem cells [21], so the decreased expression of IPO13 may lead to reduced activities of endometrial stem cells. Ki-67 could promote cell proliferation in the G1/S phase because it is regularly expressed during menstrual cycles. In 1 study, Ki-67 was downregulated in EP patients, and cell proliferation was reduced compared to that in normal endometrial cells [14], which was consistent with our findings.
C-kit is the stem cell factor receptor, and it is expressed in hematopoietic stem cells, multipotent stem cells [22], and the label-retaining cells (LRC) in the endometrium [23]. Therefore, c-kit is considered to be a stem cell marker in the endometrium [14]. In our studies, we found that the expression of c-kit was decreased in the EP patients compared to that in the normal endometrial tissue during the proliferation and secretion phases, suggesting that there is a deregulation of endometrial stem cell activities in EP patients. CD146 is a candidate marker of endometrial stem cells [24]. Our findings revealed that the expression of CD146 was decreased in the EP patients compared to that in the normal endometrial tissue during the proliferation phase, indicating that the endometrial stem cells were reduced.
The expression of CD146 was decreased in the EP patients compared to that in the normal endometrial tissue at the proliferation phase. This suggests that the endometrial stem cells were reduced in the EP patients. However, in endometriosis and endometrial cancers, the expression of c-kit was increased compared to that in the normal endometrial tissues; higher cell proliferation was also observed. Therefore, from the perspective of the endometrial stem cell, the pathogenesis of EPs, endometriosis, and endometrial cancer may be different [25,26]. The expression levels of IPO13 and c-kit in the EPs were low, but they were high in the endometrial cancer; therefore, they can be used to determine if an EP is cancerous.
Telomerase is a reverse transcriptase that is associated with the proliferation and differentiation of embryonic stem cells, bone marrow mesenchymal stem cells, induced pluripotent stem cells (IPS cells) and tumor stem cells [27,28]. The different telomerase activities during the menstrual cycle may reflect the different activities of the endometrial stem cells [29]. Our studies show that the expression of telomerase was decreased in the EP patients compared to the controls during both the proliferation and secretion phases, suggesting a lower proliferation of endometrial stem cells in EP patients. The expression of telomerase in EPs is high in endometrial cancer and endometriosis; therefore, it can be used to determine if an EP is cancerous.
Bcl-2 is an oncogene, and it can inhibit apoptosis [30–32]. It can reduce oxygen free radicals, block the intracellular Ca2+ influx into cell organelles, inhibit p53-dependent apoptosis, and antagonize c-myc [33–35]. The function of bax is opposite to that of bcl-2 [36]; it can accelerate programmed cell death. The ratio of bcl-2: bax determines if a cell lives or dies. During the proliferation phase of the menstrual cycle, the expression levels of bcl-2 and bax are upregulated, and the 2 proteins form a heterogeneous complex. Therefore, mitochondrial-induced apoptosis of the endometrial epithelial cells is reduced, and the endometrial cells continue proliferating, thereby making the endometrial tissue thicker. After entering the secretory phase, bcl-2 expression is decreased; however, bax expression is increased, and the ratio of bcl-2/bax decreases. The bax proteins form more bax/bax homodimers, which lead to cytochrome C release and caspase activation, which induces apoptosis of the endometrial epithelial cells [2,37–39]. Under physiological conditions, bax could induce a low level of apoptosis in endometrial tissues, which is called “housekeeping apoptosis” [2]. In our study, we found bcl-2 to be strongly expressed during the proliferation phase, but weakly expressed during the secretion phase of the menstrual cycle. However, the weak expression of bcl-2 was not significant, unlike that from previous reports [2,40]. This may have resulted from the use of different samples. Maia et al collected samples from curettage surgery, and the polyps could have been mixed with surrounding endometrial tissues [40]. The samples in a study by Taylor et al were limited to the proliferation or secretion phases [2].
Caspases are a family of cysteine proteases that play essential roles in apoptosis and have been termed “executioner” proteins for their roles in the cell. The caspase3 protein plays a central role in the execution-phase of cell apoptosis [41,42]. Our study found that the expression of caspase3 was decreased in the EP patients compared to that of the normal endometrial tissues during both the proliferation and secretion phases (Figure 1). This further supports the notion that the reduced apoptosis plays important roles in the development of EPs.
The overexpression of bcl-2 in the proliferative EP tissues led to a higher ratio of bcl-2/bax; therefore, more bcl-2/bax heterodimers were formed. Thus, in these tissues, less “housekeeping apoptosis” was observed. In the secretory EP tissues, bcl-2 expression remained at a high level, and the expression of bax was significantly lower than that in the normal endometrium. Therefore, a high ratio of bcl-2/bax persisted, and apoptosis was inhibited. We speculate that the increased expression of bcl-2 during the proliferation phase, and the decreased expression of bax during the secretion phase, led to less cell apoptosis and, ultimately, the formation of EPs.
Conclusions
In summary, our study reveals that the proliferation of endometrial stem cells was decreased in EP tissues compared to that of the normal endometrium. Apoptosis of the endometrial cells was also reduced. These factors likely led to the development of EPs. The expression levels of IPO13, telomerase, and c-kit are low in EPs, but they are high in endometrial cancer; thus, these biomarkers may be used for differential diagnosis. The finding of reduced stem cell marker expression in the endometrium of patients with endometrial polyps may indicate that, compared to endometrial cancer, the tissue in this benign disease is more differentiated.
Footnotes
Conflict of interest statement
The authors have no conflict of interest regarding this manuscript.
Source of support: This work was supported by a project of the Natural Science Foundation of Chongqing (CSTC: 2008BB5239)
References
- 1.Dal Cin P, Vanni R, Marras S, et al. Four cytogenetic subgroups can be identified in endometrial polyps. Cancer Res. 1995;55:1565–68. [PubMed] [Google Scholar]
- 2.Taylor LJ, Jackson TL, Reid JG, Duffy SR. The differential expression of oestrogen receptors, progesterone receptors, Bcl-2 and Ki67 in endometrial polyps. BJOG. 2003;110:794–98. [PubMed] [Google Scholar]
- 3.de S, Almeida EC, Nogueira AA, Candido dRFJ, et al. Immunohistochemical expression of estrogen and progesterone receptors in endometrial polyps and adjacent endometrium in postmenopausal women. Maturitas. 2004;49:229–33. doi: 10.1016/j.maturitas.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Kim MR, Kim YA, Jo MY, et al. High frequency of endometrial polyps in endometriosis. J Am Assoc Gynecol Laparosc. 2003;10:46–48. doi: 10.1016/s1074-3804(05)60233-2. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Medina T, Bajo-Arenas J, Haya J, et al. Tibolone and risk of endometrial polyps: a prospective, comparative study with hormone therapy. Menopause. 2003;10:534–37. doi: 10.1097/01.GME.0000064815.74043.32. [DOI] [PubMed] [Google Scholar]
- 6.McGurgan P, Taylor LJ, Duffy SR, O’Donovan PJ. An immunohistochemical comparison of endometrial polyps from postmenopausal women exposed and not exposed to HRT. Maturitas. 2006;53:454–61. doi: 10.1016/j.maturitas.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Stewart CJ, Campbell-Brown M, Critchley HO, Farquharson MA. Endometrial apoptosis in patients with dysfunctional uterine bleeding. Histopathology. 1999;34:99–105. doi: 10.1046/j.1365-2559.1999.00599.x. [DOI] [PubMed] [Google Scholar]
- 8.Pungal A, Balan R, Cotutiu C. [Hormone receptors and markers in endometrial hyperplasia. Immunohistochemical study]. Rev Med Chir Soc Med Nat Iasi. 2010;114:180–84. [PubMed] [Google Scholar]
- 9.Nunobiki O, Nakamura M, Taniguchi E, et al. Adrenomedullin, Bcl-2 and microvessel density in normal, hyperplastic and neoplastic endometrium. Pathol Int. 2009;59:530–36. doi: 10.1111/j.1440-1827.2009.02403.x. [DOI] [PubMed] [Google Scholar]
- 10.Park JS, Lee JH, Kim M, et al. Chang, Endometrium from women with endometriosis shows increased proliferation activity. Fertil Steril. 2009;92:1246–49. doi: 10.1016/j.fertnstert.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Jones RK, Bulmer JN, Searle RF. Immunohistochemical characterization of proliferation, oestrogen receptor and progesterone receptor expression in endometriosis: comparison of eutopic and ectopic endometrium with normal cycling endometrium. Hum Reprod. 1995;10:3272–79. doi: 10.1093/oxfordjournals.humrep.a135901. [DOI] [PubMed] [Google Scholar]
- 12.Dreisler E, Sorensen SS, Lose G. Endometrial polyps and associated factors in Danish women aged 36–74 years. Am J Obstet Gynecol. 2009;200:147.e1–6. doi: 10.1016/j.ajog.2008.08.065. [DOI] [PubMed] [Google Scholar]
- 13.Risberg B, Karlsson K, Abeler V, et al. Dissociated expression of Bcl-2 and Ki-67 in endometrial lesions: diagnostic and histogenetic implications. Int J Gynecol Pathol. 2002;21:155–60. doi: 10.1097/00004347-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Cho NH, Park YK, Kim YT, et al. Lifetime expression of stem cell markers in the uterine endometrium. Fertil Steril. 2004;81:403–7. doi: 10.1016/j.fertnstert.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Maruyama T, Masuda H, Ono M, et al. Human uterine stem/progenitor cells: their possible role in uterine physiology and pathology. Reproduction. 2010;140:11–22. doi: 10.1530/REP-09-0438. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard SA, Gargett CE. A cancer stem cell origin for human endometrial carcinoma. Reproduction. 2010;140:23–32. doi: 10.1530/REP-09-0411. [DOI] [PubMed] [Google Scholar]
- 17.Hubbard SA, Friel AM, Kumar B, et al. Gargett, Evidence for cancer stem cells in human endometrial carcinoma. Cancer Res. 2009;69:8241–48. doi: 10.1158/0008-5472.CAN-08-4808. [DOI] [PubMed] [Google Scholar]
- 18.Starzinski-Powitz A, Zeitvogel A, Schreiner A, Baumann R. [Endometriosis – a stem cell disease?]. Zentralbl Gynakol. 2003;125:235–38. doi: 10.1055/s-2003-42276. [DOI] [PubMed] [Google Scholar]
- 19.De Falco M, Fedele V, Cobellis L, et al. Pattern of expression of cyclin D1/CDK4 complex in human placenta during gestation. Cell Tissue Res. 2004;317:187–94. doi: 10.1007/s00441-004-0880-z. [DOI] [PubMed] [Google Scholar]
- 20.Gotte M, Wolf M, Staebler A, et al. Increased expression of the adult stem cell marker Musashi-1 in endometriosis and endometrial carcinoma. J Pathol. 2008;215:317–29. doi: 10.1002/path.2364. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Tao T, Tang J, et al. Importin 13 serves as a potential marker for corneal epithelial progenitor cells. Stem Cells. 2009;27:2516–26. doi: 10.1002/stem.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson KA, Majka SM, Wang H, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cervello I, Martinez-Conejero JA, Horcajadas JA, et al. Identification, characterization and co-localization of label-retaining cell population in mouse endometrium with typical undifferentiated markers. Hum Reprod. 2007;22:45–51. doi: 10.1093/humrep/del332. [DOI] [PubMed] [Google Scholar]
- 24.Gargett CE, Schwab KE, Zillwood RM, et al. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136–45. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slomovitz BM, Broaddus RR, Schmandt R, et al. Expression of imatinib mesylate-targeted kinases in endometrial carcinoma. Gynecol Oncol. 2004;95:32–36. doi: 10.1016/j.ygyno.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 26.Kao LC, Germeyer A, Tulac S, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–81. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- 27.Boggess JF, Zhou C, Bae-Jump VL, et al. Whang, Estrogen-receptor-dependent regulation of telomerase activity in human endometrial cancer cell lines. Gynecol Oncol. 2006;103:417–24. doi: 10.1016/j.ygyno.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 28.Hapangama DK, Turner MA, Drury JA, et al. Endometriosis is associated with aberrant endometrial expression of telomerase and increased telomere length. Hum Reprod. 2008;23:1511–19. doi: 10.1093/humrep/den172. [DOI] [PubMed] [Google Scholar]
- 29.Williams CD, Boggess JF, LaMarque LR, et al. A prospective, randomized study of endometrial telomerase during the menstrual cycle. J Clin Endocrinol Metab. 2001;86:3912–17. doi: 10.1210/jcem.86.8.7729. [DOI] [PubMed] [Google Scholar]
- 30.Havelka P, Oborna I, Brezinova J, Lichnovsky V. Apoptosis and expression of Bcl-2 in human endometrium in natural and artificial cycles. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149:303–7. doi: 10.5507/bp.2005.047. [DOI] [PubMed] [Google Scholar]
- 31.Jia L, Huang XL, Zhao Y, et al. Vitamin E succinate (VES) inhibits cell growth and induces apoptosis by mitochondrial-derived ROS in SGC-7901 cells. Med Sci Monit. 2010;16(5):BR131–39. [PubMed] [Google Scholar]
- 32.Zhang D, Wang W, Zhou D, et al. Ghrelin inhibits apoptosis induced by palmitate in rat aortic endothelial cells. Med Sci Monit. 2010;16(12):BR396–403. [PubMed] [Google Scholar]
- 33.Amstad PA, Liu H, Ichimiya M, et al. BCL-2 is involved in preventing oxidant-induced cell death and in decreasing oxygen radical production. Redox Rep. 2001;6:351–62. doi: 10.1179/135100001101536535. [DOI] [PubMed] [Google Scholar]
- 34.He H, Lam M, McCormick TS, Distelhorst CW. Maintenance of calcium homeostasis in the endoplasmic reticulum by Bcl-2. J Cell Biol. 1997;138:1219–28. doi: 10.1083/jcb.138.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eischen CM, Packham G, Nip J, et al. Cleveland, Bcl-2 is an apoptotic target suppressed by both c-Myc and E2F-1. Oncogene. 2001;20:6983–93. doi: 10.1038/sj.onc.1204892. [DOI] [PubMed] [Google Scholar]
- 36.Pluciennik E, Krol M, Nowakowska M, et al. Breast cancer relapse prediction based on multi-gene RT-PCR algorithm. Med Sci Monit. 2010;16(3):CR132–36. [PubMed] [Google Scholar]
- 37.Garcia-Velasco JA, Mulayim N, Kayisli UA, Arici A. Elevated soluble Fas ligand levels may suggest a role for apoptosis in women with endometriosis. Fertil Steril. 2002;78:855–59. doi: 10.1016/s0015-0282(02)03320-4. [DOI] [PubMed] [Google Scholar]
- 38.Konno R, Yamakawa H, Utsunomiya H, et al. Expression of survivin and Bcl-2 in the normal human endometrium. Mol Hum Reprod. 2000;6:529–34. doi: 10.1093/molehr/6.6.529. [DOI] [PubMed] [Google Scholar]
- 39.Otsuki Y. Apoptosis in human endometrium: apoptotic detection methods and signaling. Med Electron Microsc. 2001;34:166–73. doi: 10.1007/s007950100011. [DOI] [PubMed] [Google Scholar]
- 40.Maia H, Jr, Maltez A, Studart E, et al. Ki-67, Bcl-2 and p53 expression in endometrial polyps and in the normal endometrium during the menstrual cycle. BJOG. 2004;111:1242–47. doi: 10.1111/j.1471-0528.2004.00406.x. [DOI] [PubMed] [Google Scholar]
- 41.Liang Y, Nylander KD, Yan C, Schor NF. Role of caspase 3-dependent Bcl-2 cleavage in potentiation of apoptosis by Bcl-2. Mol Pharmacol. 2002;61:142–49. doi: 10.1124/mol.61.1.142. [DOI] [PubMed] [Google Scholar]
- 42.Zhang D, Wang W, Zhou D, et al. Ghrelin inhibits apoptosis induced by palmitate in rat aortic endothelial cells. Med Sci Monit. 2010;16(12):BR396–403. [PubMed] [Google Scholar]